Abstract
Buccoadhesive wafers (BW) containing Nimodipine (N) were prepared using the solvent casting method. Chitosan lactate was used as the mucoadhesive polymer with different ratios to PVP K-30 in preparation of the sustained release layer. Sodium carboxymethyl cellulose low viscosity (SCMC LV) in different ratios with Eudragit-RS30D was used in preparation of the immediate release layer of (BW). The wafers were tested for their physical characteristics, ex vivo mucoadhesion strength, ex vivo mucoadhesion time, in vitro drug release, and in vitro permeation and in vivo bioavailability studies. Wafers exhibited controlled release for 7 h. The mechanism of drug release was found to be non-Fickian diffusion and followed by first-order kinetics. Thus, BW of N could be an alternative route to bypass the hepatic first-pass metabolism and to improve the bioavailability of N.
Introduction
Buccal mucosa is an attractive route for systemic delivery of drugs, particularly in overcoming limitations associated with the oral mode of administration. It has several advantages; such as bypassing first pass metabolism, avoiding degradation in the harsh gastrointestinal environment, offering safer method of drug utilization, and more patient compliance Citation(Hoogstraate & Wertz, 1998; CitationWong et al., 1999; CitationMiller et al., 2005). Mucoadhesive buccal dosage form can be solid buccoadhesive formulations, semi-solid adhesive formulations, and liquid dosage forms. An ideal buccal wafer should be flexible, elastic, and soft; moreover, it must possess good bioadhesive properties so that it can remain in the oral cavity for the pre-determined period (CitationEouani et al., 2001; CitationPatel et al., 2007). In addition, it should release the drug in a controlled manner to get the required therapeutic response. The limitations are associated with the conventional oral buccal delivery system as they may not have uniform adhesiveness, leading to the weak adhesion, and the release of the drug may not be unidirectional (CitationGoud et al., 2004). The present study was an attempt to prepare and evaluate novel buccoadhesive wafer BW. Flexible buccal wafer for controlled delivery of nimodipine was prepared. Nimodipine was selected for the study as it undergoes first pass metabolism with oral bioavailability of 13%. The oral dose is 60 mg/4 h. Since a buccal route would bypass the pre-systemic metabolism it will help in dose reduction and improve patient compliance. Eudragit-RS30D was the base matrix for immediate release layer. Sodium carboxymethylcellulose low viscosity (SCMC LV) with good mucoadhesive property and high hydrophilic nature which dissolved fast when came in contact with dissolution medium was incorporated into the Eudragit-RS30D wafer in order to achieve immediate drug release profile and for adequate mucoadhesion chitosan lactate, a non-toxic, biocompatible, and biodegradable polysaccharide (CitationSandri et al., 2005) was used as a base matrix in sustained release layer; -oluble polymer PVP K-30 was incorporated into the chitosan lactate layer in order to modify the drug release profile and mucoadhesiveness.
Experimental
Materials
Nimodipine was received as gift sample from USV (Mumbai, India), Chitosan lactate (CL) was received from Bio-gen Extracts Pvt. Ltd. (Bangalore, India). Sodium carboxymethylcellulose low viscosity (SCMC LV.) and PVP K-30 were purchased from CDH Ltd. (Mumbai, India). Eudragit-RS30D (Eu-RS30D) was purchased from Rohm GmbH (Bermstadt, Germany), Ethyl cellulose was purchased from Loba Chemie Co. (Mumbai, India), glacial acetic acid was procured from Qualigens Fine Chemicals (Mumbai, India), triethanolamine and propylene glycol were procured from S.D. Fine Chem. Ltd. (Mumbai, India). All other chemicals and reagents used were of analytical reagent grade and were used as received.
Preparation of novel buccoadhesive wafers
Wafers were prepared by solvent casting method as three layers, the protective backing layer, prepared by casting ethanolic solution of ethyl cellulose (CitationRemunan-Lopez et al., 1998), sustained release layer (drug and Chitosan lactate polymer with different ratios to PVP K-30) and immediate release layer (drug and Eu-RS30D with different proportion to SCMC LV). One gram of CL was dissolved in 100 ml of 1.5 % v/v acetic acid solution under magnetic stirring for 24 h, resulting in a viscous solution which was filtered through nylon mesh to remove the suspended particles. To improve wafer performance and release characteristics, a water-soluble hydrophilic polymer PVP K-30 was added in different concentrations. The drug and PVP K-30 were added into the CL solution under constant stirring. Propylene glycol (5% v/v) was added into the solution as a plasticizer under constant stirring. This viscous solution was left overnight at room temperature to ensure a clear solution. The solution was poured onto ethyl cellulose layer on glass Petri dish and was allowed to dry at 37°C till a complete, flexible layer was formed. The immediate release layer consisted of Eu-RS30D polymer (40%) in water, plasticizer (10%) triethanolamine was added under constant stirring, then an aqueous solution of the mucoadhesive polymer SCMC LV (2%) at three different ratios (wafer forming solution/mucoadhesive polymer solution) 5:1, 5:5, 5:10 and the drug was added (CitationPerioli et al., 2004), the mixtures were prepared with a magnetic stirrer for 24 h, the solution was left overnight to remove air bubbles entrapped, the solution was casted onto the already prepared layer (backing layer and sustained release layer) on a petri dish and allowed to dry at 37°C till a flexible wafer was formed. Dried wafers were carefully removed, checked for any imperfections or air bubbles, and cut into wafers of 1.25 cm2 surface area containing 12 mg of drug per sustained release layer and 3 mg of drug per immediate release layer. shows the composition of different buccoadhesive wafers immediate and sustained release layers. The wafers were packed in aluminum foil and stored in airtight glass containers to maintain the integrity and elasticity of the wafers.
Table 1. Composition of buccoadhesive wafers of Nimodipine.
Drug loading calculation
The wafer was casted in the area of 12.5 cm2 this area was divided into smaller wafers of 1.25 cm2. Nimodipine oral dose is 60 mg/4 h. Nimodipine undergoes first pass metabolism with oral bioavailability of 13%. The nimodipine dose for buccal route was calculated to be 15 mg, which was further divided into 12 mg sustained release dose and 3 mg immediate release dose.
Surface pH study
The surface pH of the wafers was determined in order to investigate the possibility of any irritation in vivo. The method adopted by CitationBottenberg et al. (1991) was used to determine the surface pH of the wafers. The wafers were allowed to swell by keeping them in contact with 1 ml of distilled water for 2 h at room temperature in specially fabricated glass tubes, and the surface pH was noted by bringing the electrode in contact with the surface of the wafer, and allowing it to equilibrate for 1 min.
Weight variation, thickness, and folding endurance
Weight variation was tested in 20 different randomly selected wafers from each batch, and patch thickness was measured at five different randomly selected spots using a micrometer screw gauge.
Folding endurance of the wafer was determined by repeatedly folding a small strip of wafer (∼ 2 cm × 2 cm) at the same place till it broke or was folded up to 200 times if not broken (CitationKhanna et al., 1997). The number of times the wafer could be folded at the same place, without breaking, gave the value of folding endurance.
Swelling study
Wafers were weighed individually (W1) and placed separately in petri dishes containing 5 ml of citro-phosphate buffer (pH 6.5) (CitationNakhat et al., 2007). At regular intervals (0.5, 1, 2, 3, 4, 5, and 6 h) the wafers were removed from the petri dishes and excess surface buffer was removed carefully using the filter paper. The swollen wafers were then reweighed (W2) and swelling index (SI) was calculated using formula SI = W2 − W1/W1. The experiments were carried out in triplicate and average values were reported.
Drug content
Drug content was determined by homogenization of wafer in 100 ml of citro-phosphate buffer pH 6.5 for 8 h. The 5 ml solution was taken and diluted with citro-phosphate buffer pH 6.5 up to 20 ml, and the resulting solution was filtered through a 0.45 µm Whatman filter paper. The drug content was then determined after proper dilution at 238 nm using UV-spectrophotometer (Shimadzu corporation, Tokyo, Japan). The experiments were carried out in triplicate and average values were reported.
In vitro mucoadhesive measurement
Texture analyzer TA-XT+ (Stable Micro Systems, Surrey, England) was used to determine the mucoadhesive strength of the wafers. Bovine buccal mucosa membrane was used as a model membrane. It was hydrated with simulated saliva solution pH 6.5 (dehydrated disodium hydrogen orthophosphate 2.38 g, anhydrous potassium dihydrogen orthophosphate 0.18 g, sodium chloride 8.0 g, and demineralized water up to 1000 ml), and tied to the lower probe of the assembly. The wafer was stuck to the upper probe of the assembly using an adhesive. The upper probe was allowed to fall on the lower probe with a test speed of 0.5 mm/s and post-test speed of 1 mm/s. The wafer was allowed to adhere to the bovine buccal mucosa membrane with applied force of 150 g, return distance 10 mm, and contact time 15 s (CitationPeh & Wong, 1999). Mucoadhesive force was than calculated according to the following equation:
Ex vivo mucoadhesion time
The ex vivo mucoadhesion time was evaluated after application of the wafers onto freshly cut bovine buccal mucosa (CitationHan et al., 1999). The fresh bovine buccal mucosa was fixed in the inner side of the beaker, above 2.5 cm from the bottom, with cyanoacrylate glue. One side of each wafer was wetted with one drop of citro-phosphate buffer pH 6.5 and pasted to the bovine buccal mucosa by applying a light force with a fingertip for 30 s. The beaker was filled with 500 ml of citro-phosphate buffer pH 6.5 and was kept at 37 ± 1°C. After 2 min, a 50 rpm stirring rate was applied to simulate the buccal cavity environment, and wafer adhesion was monitored up to 12 h. The time required for the wafer to detach from the bovine buccal mucosa was recorded as the mucoadhesion time.
In vitro release study
The Rotating paddle method (USP 23, 2000) was used to study the drug release from the buccoadhesive wafers. The dissolution medium consisted of 900 ml citro-phosphate buffer pH 6.5 containing 1% Sodium lauryl sulfate (CitationHe et al., 2004). The release was performed at 37 ± 0.5°C, at a rotation speed of 50 rpm. Teflon block with depression was used to attach the wafer from one side with instant adhesive. The Teflon block was put in the bottom of the dissolution vessel so that the wafer remained on the upper side of the block. Samples (5 ml) were withdrawn at pre-determined time intervals (15, 30, 60, 120, 180, 240, 300, 360, and 420 min) and replaced with fresh medium (CitationOkamoto et al., 2001). The samples were filtered through 0.45 µm Whatman filter paper and were analyzed spectrophotometrically at 238 nm (CitationCanlica & Islimyeli, 2005).
In vitro buccal permeation study
The in vitro study of nimodipine permeation through the bovine buccal mucosa was performed using locally fabricated Franz diffusion cell by Vinod Scientific works (New Delhi, India). The assembly consisted of an upper chamber (donor compartment), which was open from above and harbored the bovine buccal mucosa at the base. The lower chamber (receptor compartment) was closed on the bottom side. The lower chamber also contained a sampling port and had a Teflon-coated needle at the base. The junction between the two compartments was designed to hold the buccal mucosa in such a manner that the mucosa did not shift from its place once the dosage form was attached to it. The capacity of the lower compartment was 20 ml. The hooks were secured with rubber bands on the sides of both compartments, so that the two compartments formed one single unit without leakage. This was surrounded by a water jacket for temperature regulation. Water at 37 ± 1°C was circulated in the jacket using a peristaltic pump. Bovine buccal mucosa was obtained from a local slaughterhouse. Freshly obtained bovine buccal mucosa was mounted between the donor and the receptor compartments so that the smooth surface of the mucosa faced the donor compartment. The buccal mucosa was allowed to stabilize for 24 h by continuously replacing the buffer in the lower chamber with fresh buffer until no absorbance was obtained at λmax of the drug. This was done to allow the removal of soluble components in the membrane as they may interfere with the analysis of the drug. The wafer was placed on the mucosa and the compartments clamped together. The donor compartment was filled with 1 ml of citrate buffer pH 6.5. The receptor compartment (20 ml) capacity was filled with isotonic phosphate buffer pH 7.4. The hydrodynamics in the receptor compartment was maintained by stirring with a magnetic bead at 100 rpm. One milliliter sample was withdrawn from the receptor compartment at pre-determined time intervals (15, 30, 60, 120, 180, 240, 300, 360, and 420 min) (CitationCeschel et al., 2006) and were always replaced with fresh IPB of pH 7.4, and analyzed for drug content spectrophotometrically at 238 nm.
In vivo studies
Approval to carry out in vivo study was obtained from CPCSEA (Committee for the purpose of controlled and supervision of experimentation animals) of Shriram Institute for Industrial Research, New Delhi (Reg. No: 4117742) and their guidelines were followed for the study. These studies were performed on optimized buccoadhesive wafer and marketed oral tablet. Switzerland white rabbits were kept in a central animal house facility for 7 days prior to the start of the study. The temperature and relative humidity of the animal house facility were 25 ± 5°C and 55 ± 5%, respectively. A dose equivalent to 5 mg of nimodipine per kg of body weight of rabbit was given in the case of buccoadhesive wafer and marketed tablet Nimodip® marketed by USV Mumbai (India) (Batch number: 23000492, Exp date 6/2010) (CitationBabu et al., 2002) and nimodipine solution as control (nimodipine was dissolved in a minimum quantity of 95% v/v ethanol). There were three groups of rabbits, each containing four rabbits. Animals were lightly anesthetized by an i.m. injection of a 1:5 mixture of xylazine (1.9 mg/kg) and ketamine (9.3 mg/kg). Following induction of anesthesia, a catheter was placed in the marginal ear vein for blood sample collection. Blood samples were obtained at 0, 15, 45, 60, 180, 240, 360, 480 min, and 12 h after administration of the drug. Nimodipine in plasma was determined by the reported validated HPLC method (CitationQian & Gallo, 1992). A Shimadzu model HPLC equipped with binary LC-10A VP pumps, variable wavelength programmable UV /VIS detector SPD-10AVP column oven (Shimadzu), SCL 10AVP system controller (Shimadzu), and Rheodyne injector fitted with a 10 µl loop software was used for analysis. Analysis was performed on a C18 RP-HPLC column. The mobile phase consisted of methanol:water (65:35 v/v). The mobile phase was delivered at the flow rate of 1 ml/min. The detection was performed at 238 nm. Injection volume was 10 µl and the concentration of plasma samples was calculated from the calibration curve plotted between peak areas of nimodipine against corresponding nimodipine concentrations.
To 100 µl of plasma sample onto an amber microcentrifuge tube, ethyl acetate (1 ml) was added to each tube which was shaken on a horizontal shaker for 3 min, followed by centrifugation at 10,000 rpm for 10 min. The organic layer was transferred to a clean amber microcentrifuge tube, and evaporated to dryness at 40–50°C. The resultant residue was reconstituted with 200 µl of mobile phase and a 10 µl aliquot was injected into the HPLC system.
Data analysis
The plasma concentration of nimodipine at different time intervals was subjected to pharmacokinetic analysis in order to calculate various parameters like maximum plasma concentration (Cmax), time to reach maximum concentration (Tmax), and area under the plasma concentration–time curve (AUC0◊12. The values of Cmax and Tmax were read directly from an arithmetic plot of time and plasma concentration of nimodipine. The AUC0◊12 was calculated by using software WinLin.
The relative bioavailability of nimodipine after buccal administration vs the oral administration was calculated as follows:
The pharmacokinetic data was compared with the control used for statistical significance by one-way analysis of variance (ANOVA) followed by Tukey-Kramer multiple comparisons test using GraphPadInstat software (GraphPad Software Inc., CA).
Stability in human saliva
The stability of BW was tested in normal human saliva using the optimized formulation (C4 + C7) as given in , which was selected based on the results of swelling, drug release, and mucoadhesion strength studies. BWs were placed in separate petri dishes containing 5 ml of human saliva and placed in a temperature controlled oven at 37 ± 2°C. At regular time intervals (1, 2, 3, 4, 5, and 6 h), the wafers were examined for change in color and shape, collapse, diameter, thickness, and nimodipine content was determined using assay method reported in USP 28. The experiments were repeated in triplicate and average values were reported.
Accelerated stability testing according to ICH Q1A(R2) guidelines
The optimized wafers were freshly prepared, packed, and sealed in aluminum pouches. For the stability evaluation of the buccoadhesive wafers the samples were stored at a temperature of 40 ± 2°C and humidity of 75 ± 5% RH. The samples were withdrawn at 0, 30, 60, and 90 days and the physical characteristics and drug content were determined (). The zero time samples were used as controls.
Table 2. Important physical characteristics of buccoadhesive wafers after storage at 40 ± 2°C and 75 ± 5% RH.
Results and discussion
Development and evaluation of optimized formula
The aim of the present study was to develop and evaluate novel buccoadhesive wafer (NBW). Both immediate release layer and sustained release layer of the wafer were evaluated by swelling index, in vitro drug release, and mucoadhesive strength to select the optimized formula for further study. Wafers for delivery of nimodipine were developed using CL as the base matrix in the sustained release layer. This layer was prepared using different ratios of CL to PVP-K30 (), 1:0.1, 1:0.15, 1:0.2, and 1:0.25 using formula C1 to C4, respectively. PVP-K30 was added to improve the drug release by polymer swelling, and film forming properties. Propylene glycol (5%) was added as plasticizer. On the basis of mucoadhesive strength (10.2 ± 0.03 g) and in vitro drug release (98.2%) from the buccal films, formula C4 was selected as optimized sustained release formula for further study. Eu-RS30D was used as base matrix in the immediate release layer. Sodium carboxymethylcellulose low viscosity (SCMC LV) was added in different ratios to improve drug release from this layer, Eu-RS30D:SCMC LV (), 5:1, 5:5, and 5:10. Triethanolamine (10%) was added as plasticizer. On the basis of mucoadhesive strength (6.6 ± 0.05 g) and in vitro drug release (95.55%) for immediate release layer, formula C7 was selected as optimized immediate release formula for further study.
The prepared films were smooth in appearance, uniform in thickness, weight, and drug content and showed no visible cracks. The films exhibited good folding endurance (). The thickness of films ranged from 0.48–0.57 mm and weight ranged from 34–37 mg. Films had a surface pH of 5.6–6.3. The drug content in the buccal films ranged from 99.01–100.38%, indicating the effective drug loading and films uniformity with respect to drug content.
Table 3. Parameter of different formulae for nimodipine buccoadhesive films (± SD).
Swelling behavior
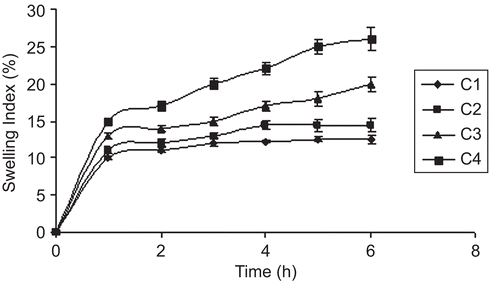
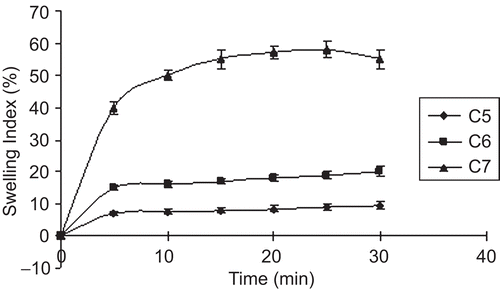
Swelling behavior of a buccoadhesive system is the essential property for uniform and prolonged release of the drug and effective mucoadhesion (CitationChen & Cyr, 1970). The swelling study indicated that the swelling index was higher in films containing a higher amount of PVP-K30. Addition of the hydrophilic polymer PVP-K30 increased the surface wettability and consequently water penetration within the matrix. The low aqueous solubility of the cationic polymer CL reduced the swelling of the wafers. The optimized sustained release formula C4 showed a swelling index of 26, due to the presence of PVP-K30. The swelling behavior of the sustained release films as a function of time is shown in . The swelling index of immediate release formula was due to the high hydrophilic nature of SCMC LV, which dissolved fast when it came into contact with dissolution medium. The rate and extent of swelling increased with an increasing concentration of SCMC LV, maximum swelling was seen with formulations containing a low proportion of Eu-RS30D as compared to SCMC LV. The optimized immediate release formula C7 showed a swelling index of 58 within 30 min. Swelling behavior of the immediate release films as a function of time is shown in .
Bioadhesion/mucoadheson study
Mucoadhesion is considered to occur in three stages: wetting, interpenetration, and mechanical interlocking between mucus and polymer. The strength of mucoadhesion is affected by various factors such as molecular weight of the polymers, contact time with mucus, swelling rate of the polymer, and the biological membrane used in the study. Incorporation of PVP-K30, a water-soluble hydrophilic polymer, reduced significantly the mucoadhesive strength of buccoadhesive wafers. Mucoadhesive strength of the optimized formula C7 + C4 was found to be 12.5 g. Addition of Eu-RS30D exhibited good physical and mechanical properties, but with short adhesion time and poor bioadhesive strength. Eu-RS30D appeared to be useful, in low concentrations for increasing the mechanical strength of wafers. SCMC LV had good bioadhesive strength, so wafers formulated with Eu-RS30D along with SCMC LV (C5 to C7) exhibited satisfactory bioadhesive and physical properties.
In vitro dissolution sudy
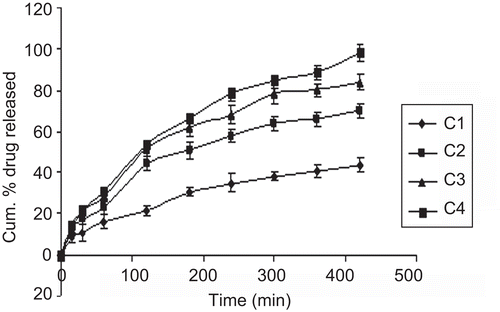
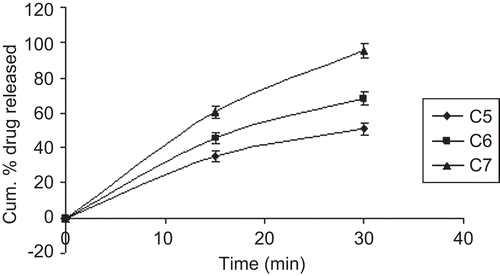
In vitro drug release of nimodipine from different sustained release formulae is shown in ; the drug released increased linearly with increasing concentration of PVP-K30 from films formula (C1 to C4), the maximum in vitro release was found to be 98.2% over a period of 7 h in wafer C4 containing the highest amount of PVP-K30, which could be attributed to its high rate and extent of swelling. This finding was also supported by the results of swelling studies where the highest swelling index was also exhibited by film C4, indicating that the increase in water-soluble polymer PVP-K30 content resulted in faster swelling and release from sustained release layer of the wafers, while in immediate release layer (C5 to C7) SCMC LV played an important role in drug release pattern () due to its high amount of water uptake may lead to considerable swelling of the polymer matrix, allowing drug to diffuse out at a faster rate. Therefore the maximum in vitro release was found to be 95.55% over a period of 30 min. The release profile for both sustained and immediate release formulae was analyzed and found to be significant (p > 0.05) using GraphPadInStat [DATASET1.ISD].
Mechanism of drug release
The release data was analyzed using the well known semi-empirical Korsmeyer-Peppas equation:
where Mt/M∞ is the fractional release of the drug, t denotes the release time, k represents a kinetic constant, incorporating structural and geometrical characteristics of the device, and n is the diffusional exponent and characterizes the type of release mechanism during the dissolution process. For non-Fickian release, the value of n falls between 0.5–1.0, while in the case of Fickian diffusion n = 0.5; for first order release (case II transport) n = 1, and for supercase II transport n > 1. The obtained value of k, n and r2 (coefficient determination) are presented in the values of n were estimated by linear regression of log (Mt/M∞) vs log t, and were between 0.5–1.0, indicating that the release of nimodipine was by non-Fickian diffusion.
Table 4. Value of r2 (correlation coefficient), n (diffusional exponent), k (kinetic constant) following linear regression of log (Mt/M∞) vs log (t) for BW.
Surface pH
The surface pH of the wafers was determined in order to investigate the possibility of any irritation on buccal mucosa in vivo. Since an acidic or alkaline pH may cause irritation to the buccal mucosa, we attempted to maintain the surface pH as close to neutral as possible. The surface pH of all films (C1 to C7) was ∼ 6 and, hence, these films should not cause any irritation in the buccal cavity. Ex vivo mucoadhesion time for the films varied from 420 to 320 min sustained release layer and from 30 to 42 min immediate release layer (). Incorporation of PVP-K30 reduced significantly ex vivo mucoadhesion time of the films. Optimized sustained release formula, C4, showed a 320 min mucoadhesion time on bovine buccal mucosa, while optimized immediate release formula, C7, showed a 30 min mucoadhesion time on bovine buccal mucosa.
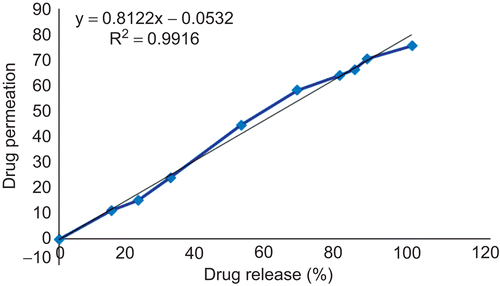
These two optimized films for immediate and sustained release were sandwiched to form buccoadhesive wafer which was characterized by moderate swelling, a convenient residence time as well as adequate drug release along with a backing layer. The wafers were subject to investigation of in vitro drug permeation and stability in human saliva. They showed 75.42% drug permeation in 7 h through bovine buccal mucosa. Good correlation was observed between in vitro drug releases and in ex vivo drug permeation with a correlation coefficient of 0.9916 ().
In vivo evaluation
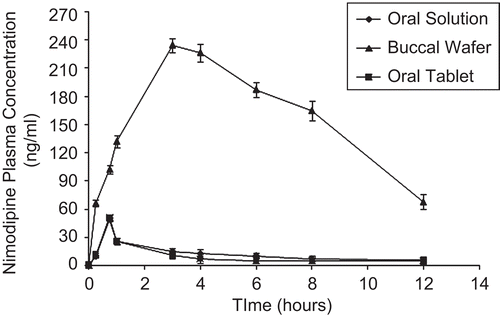
An animal study was carried out to study the absorption behavior of nimodipine from the two formulations (buccoadhesive wafers and marketed oral tablets) compared with oral solution, which were administered to rabbits. shows the plasma drug concentrations as a function of time after oral and buccal administration of these preparations; the nimodipine pharmacokinetic parameters such as Cmax, Tmax, AUC0–12, and % Relative bioavailability are summarized in . Peak plasma concentrations (Cmax) of buccoadhesive wafer, oral tablet, and oral solution were 234.91 ± 36.5 ng/ml, 50.83 ± 10.1 ng/ml, and 57.63 ± 9.2, respectively. Tmax of 3 h was observed for buccoadhesive wafer as compared to 0.75 h for oral marketed tablet and oral solution (CitationBabu et al., 2002) ( and ). (AUC0◊12) in buccoadhesive wafer, oral marketed tablet, and oral solution were 1909.49 ± 83.5, 111.69 ± 30.4, and 147.72 ± 25.3, respectively (). These pharmacokinetic parameters (Cmax, Tmax, and AUC0◊12) obtained with buccoadhesive wafer were significantly different from those obtained with oral tablet formulation (p < 0.05). This indicated that buccal route can significantly modify pharmacokinetics parameter of nimodipine. The significant AUC values observed with buccoadhesive wafer also indicated increased bioavailability of nimodipine comparison with oral formulation (p < 0.05). The buccoadhesive wafer was found to enhance the bioavailability of nimodipine by 12.92-fold with reference to the oral solution formulation (). This increased bioavailability from buccal formulation may be due to the avoidance of hepatic first-pass metabolism of nimodipine.
Table 5. Relative bioavailability and mean pharmacokinetic parameters (± SD, n = 4) of nimodipine from buccoadhesive wafer and oral marketed tablets.
Stability study
Stability studies are usually performed in phosphate buffer solutions pH of the buccal cavity, but stability studies performed in normal human saliva would be more appropriate to mimic the stability of drug and device in the oral cavity. Therefore, the stability study of optimized wafer (C4 + C7) was examined in human saliva and their appearance characteristics, such as color, shape, and drug content in normal human saliva were evaluated (). BW did not exhibited change in color until the end of the study, confirming the sufficient strength of the device. The recovery of the drug from the wafers was found to be 99.9 ± 0.1%, indicating maximum utilization of the drug incorporated.
Table 6. Stability study data of optimized BW in normal human saliva.
Accelerated stability studies were performed according to ICH guidelines at 40 ± 2°C and 75 ± 5% RH. The studies were performed to study the effect on average weight gain/loss, surface pH, bioadhesive performance, folding endurance, and drug content. No significant changes were observed for the physical characteristics of the buccoadhesive wafers ().
Conclusions
From the present investigation, one can conclude that the optimized buccoadhesive wafers of nimodipine with the combinatin of CL and PVP-K30 in sustained release layer and Eu-RS30D and SCMC LV in immediate release layer can meet the ideal requirements for buccal devices, which can be a good way to bypass the extensive hepatic first pass metabolism (CitationAl-Omar, 2005) and to overcome the limitations with conventional buccal adhesive wafers.
Acknowledgements
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Al-Omar, M.A. (2005). Nimodipine. In: Brittain, H. ed. Profiles of drug substances, excipients, and related methodology 31. Boston: Academic Press.
- Babu, G.V.M.M., Kumar, N.R., Sankar, K.H. (2002). In vivo evaluation of modified gum karaya as a carrier for improving the oral bioavailability of a poorly water-soluble drug, nimodipine. AAPS Pharm Sci Tech 3:E12.
- Bottenberg, P., Cleymaet, R., Muynek, C.D., Remon, J.P., Coomans, D., Slop, D. (1991). Development and testing of bioadhesive, fluoride-containing slow-release tablets for oral use. J Pharm Pharmacol 43:457–64.
- Canlica, M., Islimyeli, S. (2005). The atomic absorption spectrophotometric method for indirect determination of nimodipine in tablets. Turk J Chem 29:141–6.
- Ceschel, G.C., Bergamante, V., Calabrese, V., Biserni, S. (2006). Design and evaluation in vitro of controlled release mucoadhesive tablets containing chlohexidine. Drug Dev Ind Pharm 32:53–61.
- Chen, J.L., Cyr, G.N. (1970). Composition producing adhesion through hydration. In: Manly, R.S., ed. Adhesion in biological system. New York: Academic press, 163–81.
- Eouani, C., Piccerelle, P.H., Prinderre, P., Bourret, E., Joachim, J. (2001). In vitro comparative study of buccal mucoadhesive performance of different polymeric films. Eur J Pharm Biopharm 52:45–55.
- Goud, K., Desai, H., Kumar, T.M.P. (2004). Preparation and evaluation of a novel buccal adhesive system. AAPS Pharm Sci Tech 5:E35.
- Han, R.Y., Fang, J.Y., Sung, K.C., Hu, O.Y.P. (1999). Mucoadhesive buccal disks for novel nalbuphine prodrug controlled delivery: effect of formulation variables on drug release and mucoadhesive performance. Int J Pharm 177:201–9.
- He, Z., Zhong, D., Chen, X., Liu, X., Tang, X., Zhao, L. (2004). Development of a dissolution medium for nimodipine tablets based on bioavailability evaluation. Eur J Pharm Sci 21:487–91.
- Hoogstraate, J.A.J., Wertz, P.H.W. (1998). Drug delivery via the buccal mucosa. Pharm Sci Tech Today 1:309–16.
- Khanna, R., Agarwal, S.P., Ahuja, A. (1997). Preparation and evaluation of mucoadhesive buccal films of clotrimazole for oral candida infections. Indian J Pharm Sci 59:299–305.
- Miller, N.S., Chittchang, M., Johnston, T.P. (2005). The use of mucoadhesive polymers in buccal drug delivery. Adv Drug Deliv 57:1666–91.
- Nakhat, P.D., Kondawar, A.A., Babla, I.B., Rathi, L.G., Yeole, P.G. (2007). Studies on buccoadhesive tablets of terbutaline sulphate. Ind J Pharm Sci 69:505–10.
- Okamoto, H., Taguchi, H., Iida, K., Danjo, K. (2001). Development of polymer film dosage forms of lidocaine for buccal administration I. penetration rate and release rate. J Contr Rel 77:253–60.
- Patel, V.M., Pajapati, B.G., Patel, M.M. (2007). Effect of hydrophilic polymers on buccoadhesive eudragit patches of propranolol hydrochloride using factorial design. AAPS Pharm Sci Tech 8:E45.
- Peh, K.K., Wong, C.F. (1999). Polymer films as vehicle for buccal delivery: swelling, mechanical, and bioadhesive properties. J Pharm Sci 2:53–61.
- Perioli, L., Ambrogi, V., Angelici, F., Ricci, M., Giovagnoli, S., Capuccella, M., Rossi, C. (2004). Development of mucoadhesive patches for buccal administration of ibuprofen. J Contr Rel 99:73–82.
- Qian, M., Gallo, J.M. (1992). High-performance liquid chromatographic determination of the calcium channel blocker nimodipine in monkey plasma. J Chromatogr 578:316–20.
- Remunan-Lopez, C., Portero, A., Luis, J., Alonso, M.J. (1998). Design and evaluation of chitosan/ethylcellulose mucoadhesive bilayered devices buccal drug delivery. J Contr Rel 55:143–52.
- Sandri, G., Rossi, S., Bonferoni, M.C., Ferrari, F., Zambito, Y., Colo, G.D., Caramella, C. (2005). Buccal penetration enhancement properties of N-trimethyl chitosan: infuence of quaternization degree on absorption of a high molecular weight molecule. Int J Pharm 297:146–55.
- The United Stated Pharmacopeia. (2000). Rockville, MD: United States Pharmacopeial Convention Inc.
- Wong, C.F., Yuen, K.H., Peh, K.K. (1999). Formulation and evaluation of controlled release Eudragit buccal patches. Int J Pharm 178:11–22.