Abstract
To decrease the initial burst release of protein entrapped in PLGA microspheres, bovine serum albumin was entrapped into microspheres and a one-step modified method was evaluated. Low level of alginate was added into the internal aqueous phase with protein together; meanwhile calcium chloride and chitosan were put into the external, and all the other processes kept unvaried. After modification, the initial release was inhibited markedly, and the entrapment efficiency was increased slightly, while the particle size remained unaltered. An attracting result is that almost all the little pores at the surface of microspheres had been closed with the modification one under SEM.
Keywords::
Introduction
With the rapid growth in the biotechnological field, more and more peptide or protein drugs such as hormones, cytokines, enzymes, and vaccines have been extensively studied for therapeutic availability (CitationGiteau et al., 2008; CitationTang & Singh, 2009). However, they have many disadvantages, including physical and chemical instabilities relevant with degradation by pH of the gastrointestinal tract or enzyme existed, low oral bioavailability because of their high molecular weights, and frequent injection protocols for their short half-lives in vivo. As a result, high doses and repeated administration is required and ultimately lead to side-effects or even toxicity. Such problems become a bottleneck for clinical application of protein therapeutics. During the last three decades, there has been continuous interest in the use of biodegradable polymers for the development of peptide and protein delivery systems able to prolong their therapeutic effects (CitationSchoubben et al., 2009). The natural biodegradable polymers are mostly hydrophilic, such as glutin, dextran, albumin, starch, alginate, and chitosan, whereas the synthetic ones are usually hydrophobic, e.g. poly (lactic-co-glycolic acid) (PLGA), poly (carboxylic anhydride), poly (amino acid), and poly (phosphazene). PLGA has gained much attention and research focus in controlled release of therapeutic proteins (CitationEstey et al., 2006). The most common emulsification technique for protein microencapsulation in PLGA microspheres is the W/O/W emulsion method (CitationMao et al., 2007; CitationWu et al., 2008). Initial burst is one of the major challenges in protein-encapsulated microparticle systems (CitationYeo & Park, 2004). The main problem is that the initial burst release is usually at undesirably high levels. For example, 20–50% of drugs will be released within 24 h in vitro release (CitationLagarce et al., 2005; CitationLuzardo-Alvarez et al., 2005; CitationLee & Lee, 2008), and in some cases, even up to 60–70% (CitationAl-Azzam et al., 2005; CitationTahara et al., 2008). Controlled-release drug delivery systems have been becoming increasingly important, especially in biomedicine, in order to achieve an optimal therapeutic level and reduce side-effects. To obtain ideal release patterns, many strategies have been developed, for instance employing different emulsion methods (CitationWu et al., 2008) and modifying the encapsulated drugs directly (CitationAl-Azzam et al., 2005; CitationWang et al., 2005; CitationTahara et al., 2008). Among these, PLGA microsphere/hydrogel combination systems could not only provide entrapped proteins a more ‘friendly’ environment, but also retard the initial burst release. Alginate and chitosan are the two most commonly used hydrophilic polymers, but an obvious limitation was that the size of composite microspheres was too large, for example, alginate particles were embedded into PLGA microparticles and the resulting composite microparticles had a dmv of ∼ 65 µm (CitationSchoubben et al., 2009), alginate-PLGA composite microspheres of 19–73 µm diameter (CitationLuzardo-Alvarez et al., 2005), and alginate-chitosan-PLGA composite ones of 30 µm diameter (Zheng et al., 2004a), compared with the convenient PLGA microspheres of ∼ 5 µm. To maintain the size of the microparticles, we put more attention on those slightly modified methods. Some PLGA nanospheres could be modified by adding chitosan solution into the outer aqueous phase of PVA solution. Protonation of amino groups allows the chitosan polymer to coat nanoparticles by interacting with negatively-charged materials (CitationKim et al., 2008; CitationTahara et al., 2008). However, for PLGA microspheres, different from nanoparticles, no direct cross-linking method can be used. Herewith alginate was introduced to the internal aqueous phase to produce ionic interaction with external chitosan solution during preparation of the PLGA microspheres to obtain expected reduced initial burst release of entrapped protein in microspheres. To confirm this idea, both the non-modified and the modified composite PLGA microspheres were prepared and their initial in vitro release behaviors were studied. Moreover, to observe their surface and cross-sectional characteristics more clearly, both the morphologies of the non-modified microspheres and the modified ones with large sizes were observed.
Materials and methods
Chitosan (≥ 80% deacetylation, Mw 80,000) was obtained from Yuhuan Oceanic Biochemistry Co. Ltd. (China). Alginate (6 mps for 1% at 25°C) and poly (vinyl alcohol) (PVA, average Mw 30,000–70,000) were procured from Shanghai Chemical Reagent Company of Chinese Medicine (China). PLGA, lactic-to-glycolic acid molar ratios of 50:50 and 70:30 were purchased from Shandong Medical Instrumental Institute (China). Bovine serum albumin (BSA) was bought from Sino-American Biotechnology Co. (China). O.C.T Compound (Tissue-Tek 4583) was procured from RMEB RNC, Philadelphia, USA. Bicinchoninic acid (BCA) protein assay kits were supplied by Beyotime Co. (China). Sorbitan trioleate (Span 80), polyoxyethylene sorbitan trioleate (Tween 80), iso-octane, isopropyl alcohol, calcium chloride, and all other reagents were of analytical grade and supplied by Huadong Medical Co. (China).
Preparation of PLGA microspheres
PLGA microspheres of BSA were fabricated by the double-emulsion solvent evaporation method that we previously reported (Zheng et al., Citation2004b). Briefly, 30 mg of BSA was dissolved in 200 μl of distilled water, and emulsified with 4 ml methylene chloride containing 7.5% (w/v) PLGA. This primary emulsion was injected into 40 ml of 1.0% poly (vinyl alcohol) (PVA) with stirring, and then maintained at a high speed stirring for 5 min to form the double w/o/w emulsion. After evaporation of methylene chloride for 60 min under the reduced pressure, the solidified microspheres were centrifuged and washed with distilled water. The resulting BSA-loaded microspheres were collected after lyophilization.
In order to obtain more clearly the surface and interior characteristics by SEM imaging, large size microspheres were prepared. The preparation procedure was described above, and a modified procedure of 3-min high speed stirring at 12,000 rpm using a homogenizer was replaced by 5-min magnetic stirring.
Preparation of PLGA microspheres modified by alginate and chitosan
About 0.33 g of PLGA was dissolved in 4 ml of methylene chloride. Thirty milligrams of BSA was dissolved in 100 µl of distilled water and then added into 100 µl of 1% alginate solution as an internal aqueous phase. The aqueous phase solution was mixed with the PLGA oil phase and sonicated for 20 s at 50 W. The resulting emulsion was further injected into 40 ml solution containing 20 ml of 2% PVA, 20 ml of 2% chitosan solution, and 1 g of CaCl2, followed by stirring for 5 min at 12,000 rpm. After evaporation under the reduced pressure, the solidified microspheres were centrifuged and washed three times with water. The resulting BSA-loaded modified microspheres were collected after lyophilization.
Large particles of modified PLGA microspheres were prepared by a similar method, described as large particles of PLGA microspheres.
Morphology observation
The surface and cross-sectional morphologies of large PLGA microspheres with or without modification were observed using an optical microscope and a scanning electron microscope (SEM, XL30, Philips). Cross-sectional sample was prepared with a freezing ultramicrotome (Microm HM 560, Germany). Microspheres were immobilized with an appropriate amount of O.C.T Compound. After being frozen for 1–2 min at −20°C, cryosections were prepared from the convex to the plane, with a thickness of 2 µm. The slides were observed under SEM.
Determination of entrapment efficiency
Determination of BSA content in PLGA non-modified microspheres was according to our previously reported method 2004a. The quantification of BSA in modified PLGA microspheres was operated as follows: 10 mg of PLGA microspheres was precisely weighed and dispersed in 1.0 ml of acetonitrile. The suspension was centrifuged and the supernatant containing polymers was discarded. To remove the polymer more thoroughly, the step of dissolution and centrifugation was repeated and then the pellet was dried under vacuum overnight. Subsequently the pellet was dispersed in PBS (pH 7.4, 0.05 mol/L) and vibrated in a shaking water bath at 37°C, 100 rpm for 6 h. The supernatant was stored at the refrigerator of 4°C, meanwhile the sediment was further dispersed into 0.1 mol/L NaOH, vibrated in a shaking water bath at 37°C, 100 rpm overnight. The supernatant was collected again after centrifugation and the sediment was further dispersed into 0.1 mol/L HCl with equal volume of NaOH above, the process was repeated finally. All supernatants were combined and used for the determination of BSA contents in both PLGA microspheres with and without modification, using BCA protein assay kits (Beyotime Biotechnology, China). The entrapment efficiency was calculated by the ratio of actual BSA content to theoretical BSA content.
In vitro release study
The release study was carried out in saline containing 0.01% sodium azide as a preservative. Accurately weighted microspheres were placed in eppendorf tubes containing 3 ml of the release medium. The tubes were then incubated at 37°C with the rotation speed of 70 rpm. At pre-determined time intervals, the tubes were centrifuged at 2000 rpm for 5 min; 1.0 ml of the supernatant was taken out and 1.0 ml of fresh solution replenished. BSA concentration was determined by the BCA kit.
Results
Morphology observation
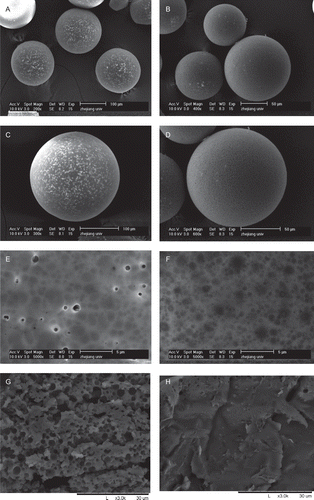
The PLGA microspheres with or without modification displayed the similar spherical, smooth, and non-aggregated micrographs under an optical microscope. To observe their morphologies more clearly, two kinds of microspheres with much larger sizes were made correspondingly and observed by a SEM (). Light pores bestrewed on the surface of PLGA microspheres were made by the conventional method (). However, elimination of such light pores was observed on the surface of the composite PLGA microspheres modified by alginate and chitosan (). Furthermore, the interior structure was compact according to the cross-sectional SEM images of the modified PLGA microspheres (), whereas the characteristic was found in the conventional microspheres ().
Figure 1. The scanning electron micrographs of the non-modified PLGA microspheres and PLGA composite microspheres modified by alginate and chitosan (a, c, e, g: the non-modified PLGA microspheres; b, d, f, h: the modified composite microspheres; (e) and (f) presented the surface morphologies, meanwhile (g) and (h) presented the cross-sectional images).
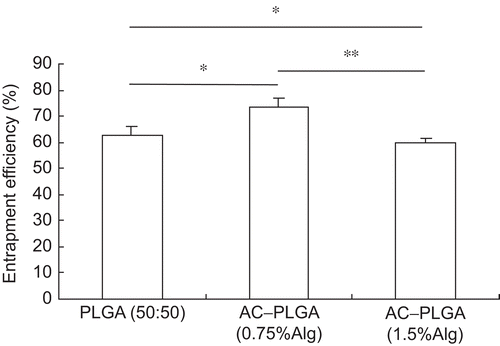
Entrapment efficiency determination
The BSA entrapment efficiency of composite microspheres modified with 1.5% alginate was significantly lower than that with 0.75% alginate (p < 0.05). However, both the modified microspheres displayed no statistical differences from the non-modified PLGA microspheres in entrapment efficiency (, p > 0.05).
Effects of modification on release
The initial burst release within 24 h was high in PLGA microspheres prepared by the double-emulsion solvent evaporation method. However, the burst release effect could be significantly retarded by modification with alginate and chitosan. The release amounts of 0.5 h, 1 h, and 24 h were presented in . Compared with the release rates of BSA from the non-modified PLGA microspheres, 30.65% at 0.5 h, the release rates at 0.5 h from both of the modified microspheres were markedly delayed (p < 0.01), and the release rate at 1 h of modified microspheres by 1.5% alginate and 0.5% chitosan was also obviously retarded (p < 0.05).
Table 1. The initial release of PLGA microspheres and that modified by alginate and chitosan.
Discussion
In the present study, BSA, a model protein drug, was entrapped in PLGA by a double emulsion solvent evaporation method. Although we optimized the formula of the BSA-PLGA microspheres prepared by conventional methods, the burst release of BSA at 0.5 h from PLGA microspheres still remained (≥ 30%) high. The high initial release of protein drug from PLGA microspheres is usually caused by the surface-adsorbed drug and the fast drug diffusion from interconnecting network of pores (CitationIbrahim et al., 2005). To modulate the initial release rate, a kind of composite microsphere was developed by modifying the conventional PLGA microspheres with alginate and chitosan. Low level of alginate was added into the internal aqueous phase with protein together to reduce these unwanted initial release amounts. Meanwhile calcium chloride and chitosan were put into the external PVA solution, and the other preparation process kept invariability with that in non-modified PLGA microspheres.
Drug molecules adsorbed on the surface or near the surface of the microspheres release directly or firstly from millipores dispersed there, leading to the majority of initial release. These small pores are observed clearly on the cross-sectional image of non-modified PLGA microspheres. For the composite microspheres designed here, the protein entrapped in microspheres had a relative hydrophilic alginate or alginate-chitosan microenvironment, and the protein near the surface was in the same situation, partly covered by alginate and/or chitosan that combined with each other by electrostatic interaction, so the initial release could be inhibited markedly. According to the SEM imaging, significant structural improvement was found in the modified microspheres featured by non-porous surface and compact interior structure, which presumably contributed to the retardation of burst release. Moreover, for the described composite microspheres, the incorporated alginate/chitosan may form thin films through electrostatic interaction. Loaded proteins in microspheres had a relative hydrophilic alginate or alginate/chitosan microenvironment, which actively interplayed with the entrapped proteins, and, subsequently, retarded their release. Meanwhile, the electrostatic interaction between alginate and chitosan was not so strong that the release amounts would not be affected except the initial phase, and the incompleted release, another tough problem, would not appear.
The entrapment efficiency, which in the modified microspheres with 0.75% alginate was slightly higher than that in the non-modified and that in the modified microspheres with 1.5% alginate, was a little lower than that in the non-modified. However, the entrapment efficiencies in both modified microspheres had no statistical significance with that in non-modified. It should be noted that the entrapment efficiency in microspheres modified with 1.5% alginate was lower than that with 0.75% alginate (p < 0.05). This indicated that the entrapment efficiency was obviously influenced by the alginate concentration in the internal aqueous phase. Furthermore, it was hard to the formation of W/O emulsion when the viscosity of alginate in the internal aqueous phase increased to a certain degree. So the modification process was closely related to the level of internal alginate solution.
Conclusions
The described one-step modified method exhibited significant efficacy in retarding the burst initial release of PLGA microspheres. Compared with the non-modified microspheres, almost all of the little pores which showed on the surfaces and cross-sectional images were eliminated after the modification process with alginate and chitosan, only some shallow umbilications on the surface could be observed under SEM. The burst protein amount in composite PLGA microspheres at 0.5 h was reduced to 1/6 and 1/3 when modified with 1.5% and 0.75% alginate solution, respectively. There were no statistical differences between BSA entrapment efficiency in non-modified PLGA microspheres and that of the modified ones when the concentration of the alginate was controlled to some degree.
It remained an urgent problem to solve the rapid burst initial release, especially for those protein drugs with high toxicity. Moreover, the attractive merits of this modification method are that the preparation process is simple and doesn’t cause any significant change in size of the modified microspheres and entrapment efficiency.
Acknowledgements
Declaration of interest
This work got the financial support of Zhejiang Provincial Natural Science Foundation of China (No. Y2080185). The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Al-Azzam, W., Pastrana, E.A., King, B., Méndez, J., Griebenow, K. (2005). Effect of the covalent modification of horseradish peroxidase with poly(ethylene glycol) on the activity and stability upon encapsulation in polyester microspheres. J Pharm Sci. 94:1808–19.
- Estey, T., Kang, J., Schwendeman, S.P., Carpenter, J.F. (2006). BSA degradation under acidic conditions: a model for protein instability during release from PLGA delivery systems. J Pharm Sci. 95:1626–39.
- Giteau, A., Venier-Julienne, M.C., Aubert-Pouëssel, A., Benoit, J.P. (2008). How to achieve sustained and complete protein release from PLGA-based microparticles? Int J Pharm. 350:14–26.
- Ibrahim, M.A., Ismail, A., Fetouh, M.I., Göpferich, A. (2005). Stability of insulin during the erosion of poly (lactic acid) and poly (lactic-co-glycolic acid) microspheres. J Contr Rel. 106:241–52.
- Kim, B.S., Kim, C.S., Lee, K.M. (2008). The intracellular uptake ability of chitosan-coated poly (D,L-lactide-co-glycolide) nanoparticles. Arch Pharm Res. 31:1050–4.
- Lagarce, F., Faisant, N., Desfontis, J.C., Marescaux, L., Gautier, F., Richard, J., Menei, P., Benoit, J.P. (2005). Baclofen-loaded microspheres in gel suspensions for intrathecal drug delivery: in vitro and in vivo evaluation. Eur J Pharm Biopharm. 61:171–80.
- Lee, J., Lee, K.Y. (2009). Injectable microsphere/hydrogel combination systems for localized protein delivery. Macromol Biosci. 9:671–6.
- Luzardo-Alvarez, A., Blarer, N., Peter, K., Romero, J.F., Reymond, C., Corradin, G., Gander, B. (2005). Biodegradable microspheres alone do not stimulate murine macrophages in vitro, but prolong antigen presentation by macrophages in vitro and stimulate a solid immune response in mice. J Contr Rel. 109:62–76.
- Mao, S., Xu, J., Cai, C., Germershaus, O., Schaper, A., Kissel, T. (2007). Effect of WOW process parameters on morphology and burst release of FITC-dextran loaded PLGA microspheres. Int J Pharm. 334:137–48.
- Schoubben, A., Blasi, P., Giovagnoli, S., Perioli, L., Rossi, C., Ricci, M. (2009). Novel composite microparticles for protein stabilization and delivery. Eur J Pharm Sci. 36:226–34.
- Tahara, K., Sakai, T., Yamamoto, H., Takeuchi, H., Kawashima, Y. (2008). Establishing chitosan coated PLGA nanosphere platform loaded with wide variety of nucleic acid by complexation with cationic compound for gene delivery. Int J Pharm. 354:210–6.
- Tang, Y., Singh, J. (2009). Biodegradable and biocompatible thermosensitive polymer based injectable implant for controlled release of protein. Int J Pharm. 365:34–43.
- Wang, S.H., Zhang, L.C., Lin, F., Sa, X.Y., Zuo, J.B., Shao, Q.X., Chen, G.S., Zeng, S. (2005). Controlled release of levonorgestrel from biodegradable poly(d,l-lactide-co-glycolide) microspheres: in vitro and in vivo studies. Int J Pharm. 301:217–25.
- Wu, J.H., Wu, L., Xu, X.Q., Xu, X.Y., Yin, X.J., Chen, Y.J., Hu, Y.Q. (2008). Microspheres made by w/o/o emulsion method with reduced initial burst for long-term delivery of endostar, a novel recombinant human endostatin. J Pharm Sci. 98:2051–8.
- Yeo, Y., Park, K. (2004). Control of encapsulation efficiency and initial burst in polymeric microparticle systems. Arch Pharm Res. 27:1–12.
- Zheng, C.H., Liang, W.Q., Zhang, Y.P. (2004a). A protein delivery system: biodegradable alginate-chitosan-poly (lactic-co-glycolic acid) composite microspheres. Biochem Biophys Res Comm. 323:1321–7.
- Zheng, C.H., Liang, W.Q., Yu, H.Y., Chen, H.L. (2004b). Evaluation of different analytical methods of proteins in PLGA Microspheres. Pharmazie. 59:232–3.