Abstract
For the treatment of ocular keratitis acyclovir, as a highly specific inhibitor of herpes virus replication, is applied topically into the eye. The objective of this study was to design and evaluate freeze-dried, bioadhesive and biodegredable acyclovir ocular minitablets for prolonged local drug action. The sponge-like nature of the lyophilized ocular minitablets ensures rapid hydration and gelation of these tablets in the eye and thus would reduce the foreign body sensation. The polymers used were sodium carboxymethylcellulose (NaCMC), hydroxypropylmethylcellulose (HPMC), xanthan gum, chitosan and Carbopol 943P. The minitablets were evaluated for drug content, weight variation, bioadhesion, water uptake and in vitro drug release. In addition, the rheological characteristics of the polymers solutions were investigated. Rheological data revealed that all tested polymers exhibited pseudoplastic behaviour which is required to minimize interference with blinking. Drug release was found to be affected by the type and concentration of polymer. The order of sustainment was chitosan > xanthan > HPMC > Carbopol > NaCMC. Water uptake study, dissolution rate of the polymers and viscosity measurements could explain the different release profiles of the drug from the polymers. Chitosan minitablet was chosen for its significant sustained release and good bioadhesive property for in vivo study in rabbits. The tablet showed a good permeation into the cornea in comparison to the commercially available Zovirax® eye ointment. In conclusion, chitosan ocular minitablets containing acyclovir could be considered as a promising sustained drug delivery system for ocular keratitis treatment.
Introduction
The eye as a portal for drug delivery is generally used for local therapy against systemic therapy in order to avoid the risk of eye damage from high blood concentration of the drug, which is not intended. Most ocular treatments like eye drops and suspensions call for topical administration of ophthalmically active drugs to the tissues around the ocular activity. These dosage forms are easy to instill but suffer from poor bioavailability, because the majority of the medications they contain are immediately diluted in the tear film as soon as the eye drop solution is instilled into the cul-de-sac and is rapidly drained away from the pre-corneal cavity by constant tear flow and lacrimo-nasal drainage (CitationLee & Robinson, 1986). Therefore, daily frequent instillation of the solution is necessary to achieve a therapeutic effect. However, the frequent use of concentrated solutions may damage the ocular surface. Moreover, systemic absorption of the drug drained through the nasolacrimal duct systems can cause side-effects (CitationBaudouin, 1996; CitationTopalkara et al., 2000).
Various methods were investigated to increase the drug bioavailability by prolonging the contact time between drug and corneal–conjunctival epithelium. The first strategy developed was the viscosity increase of the vehicle by addition of viscolyzers to the formulation. Only a small improvement of the retention time of the drug in the fornix was obtained (CitationLudwig & Van Ooteghem, 1992; CitationLehr et al., 1994). Highly viscous gels and ointments, on the other hand, provided a sustained contact with the eye surface, but caused a sticky sensation and blurring of vision and induced reflex blinking due to irritating properties (CitationSintzel et al., 1996). Another approach to optimizing bioavailability was the implementation of the mucoadhesive concept. In this method, suitable polymers interact with the mucous layer that coats the external surface of the eye (CitationHui & Robinson 1985; CitationSaettone & Salminen, 1995). The use of films or inserts was proposed to allow drug release over a long period (CitationGurtler et al., 1995). Inserts and collagen shields were very effective because of less frequent administration and absence of additives, which often cause adverse effects on corneal epithelial wound healing, leading to toxic keratitis manifested as punctuate lesions with a persistent epithelial defect. Insoluble inserts had the disadvantage that they had to be removed manually, as they are not eliminated naturally (e.g. Ocusert; Akorn, Buffalo Grove, IL). Soluble inserts that dissolve or erode gradually after administration offer several advantages (i.e. a simple design), employing ingredients that are well adapted for ophthalmic use and an easy manufacturing process (e.g. direct compression).
Nevertheless, despite the advantages of ocular inserts, they have not been so far widely used in ocular therapy, which might be due either to the foreign body sensation that these solid devices are likely to cause in the patients’ eye or to the movement of these inserts around on the ocular surface causing more irritation.
Corneal epithelial and stromal keratitis caused by herpes simplex virus is a common opportunistic ocular infection and a leading cause of blindness. Acyclovir is a highly specific inhibitor of herpes virus replication. The topical application of Acyclovir is widely used for the treatment of various herpes simplex infections (CitationJabs, 1998).
Acyclovir formulations do not allow suitable drug levels at target sites following oral, local or parenteral administration, due to the low water solubility of the drug. Therefore, Acyclovir is formulated as an ophthalmic ointment (Zovirax®), which could cause transient vision disturbance, thus reducing compliance.
It was therefore the aim of this work to formulate Acyclovir as biodegradable, mucoadhesive and extremely small ocular minitablets that could be well tolerated by the patients. Preparing the minitablets by lyophilization showed many advantages over ordinary compressed minitablets. First it is extremely small and light, which would increase the patient’s compliance. Moreover, the sponge-like nature of the lyophilized ocular minitablets ensures rapid hydration and gelation of these tablets in the eye and thus would reduce the foreign body sensation.
Materials and methods
Materials
Acyclovir and xanthan gum were kindly supplied from Kahira Pharmaceuticals & Chemical Industries Company (Cairo, Egypt). The other materials used in the study were chitosan (high molecular weight, 85% degree N-deacetylation, Fluka, Steinheim, Germany), Carbopol 934 (CP 934, Goodrich Chemical Company, Brecksville, OH, USA), hydroxypropylmethyl cellulose (HPMC 400 CPS, Aqualon Dartford, UK), carboxymethylcellulose sodium salt (SCMC, medium viscosity, Fluka) and glacial acetic acid (United Company for Chemical and Medical Preparations, Cairo, Egypt). All other reagents were of analytical grade.
Preparation of acyclovir ocular minitablets
Aqueous solutions of 1% NaCMC, 2% NaCMC, 1% HPMC, 1% xanthan gum, 1% Carbopol, 1% chitosan and a 1:1 mixture of 0.5% chitosan and 1% NaCMC (mix) were prepared. In the case of chitosan the polymer was dissolved in 1% glacial acetic acid and for carbopol solution the gelation was achieved by the addition of 50 μl of triethanolamine.
After complete dissolution of the polymers, the drug was dispersed in the polymer solution in a concentration of 5.7 mg/ml. The medicated polymer solution was then cast into specially designed tablet moulds (length: 4.0 mm, diameter: 2.0 mm) and frozen at −22°C for 24 h. The frozen tablets were then placed in a lyophilizer (Novalyphe-NL 500; Savant Instruments Corp., Holbrook, NY, USA) for 24 h with a condenser temperature of −45°C and a pressure of 7 × 10−2 mbar. The formulated sponge-like minitablets containing the therapeutic dose (CitationMartindale, 2007) of 0.15 mg acyclovir/tablet were stored in a desiccator until use.
In-vitro evaluation of the prepared minitablets
Weight uniformity
Ten randomly selected minitablets from each formulation were weighed individually (Precisa Balances, Dietikon, Switzerland) and average weight was determined.
Content uniformity
One minitablet was dissolved in 10 ml phosphate buffered saline (PBS). The absorbance of the solution was then measured spectrophotometrically (UV-1601, Shimadzu, Japan) at 252 nm. The test was done on 10 individual tablets.
Water uptake
The water uptake at room temperature was measured gravimetrically (CitationWeyenberg et al., 2003) (Precisa Balances). A small filter paper (d = 55 mm, Schleicher & Schuell GmbH, Dassel, Germany) was placed on a 1% w/v agar gel plate. This experimental set-up was equilibrated for 60 min. The accurately weighed minitablet (md) was then placed on the upper side of the filter paper in the covered Petri dish and the weight of the swollen tablet (mw) was determined at different time intervals. In order to calculate the percent of water uptake of the minitablet (w) the following equation was used.
Rheological characterization
Measurements of viscosity were carried out on gels before casting into minitablets moulds. The viscosity of each gel was determined using a cone and plate viscometer (Brookfield Co., Model HBDV-I+ CP, Middleboro, MA, USA). The flow behavior of the different gel bases was studied according to equation:
where D is the shear rate in s−1; S is the shear stress in dyne/cm2; η is the viscosity in cp; and N is Farrow’s constant (CitationFarrow et al., 1928).
Bioadhesion potential of inserts
A modified balance method (CitationParodi et al., 1996) was used to determine the bioadhesive performance of the ocular minitablets by measuring the force required to detach the tablets from a mucosal surface. The instrument is broadly composed of a modified two arm physical balance in which the right pan had been replaced by a glass plate (4 × 4 cm). The tablets were glued to the lower side of the glass plate with α-cyanoacrylate glue. This was followed by tarring the balance. A piece of rabbit’s intestine was dissected and placed in normal saline after being washed of all food debris. The intestine, cut into 2 cm lengths, was adhered to a moving platform with α-cyanoacrylate glue. A volume of 0.1 ml of phosphate buffered saline (PBS) was slowly added using a plastic syringe over the mucosal membrane. The platform was slowly raised until the tablet touched the mucosa. The tablet and mucosa were left in contact for 2 min, after which weights were added to the left pan. Addition was stopped upon detachment of the tablet from the mucosa. The equivalent adhesion force was then calculated in N. The experiment was performed in triplicate.
In vitro drug release study
In vitro release studies of lyophilized acyclovir minitablets were performed in bottles with 20 ml PBS pH 7.4 containing 1 minitablet. The bottles were placed in oscillating water bath (Stuart SBS 40, Staffordshire, UK) at 35°C ± 1. Aliquots each of 1 ml were withdrawn at different time intervals and replaced each time with fresh PBS. The withdrawn samples were analyzed for acyclovir by measuring the absorbance at 252 nm (UV-1601, Shimadzu, Kyoto, Japan).
Kinetic analysis of in vitro release data
In order to describe the release model which describes the pattern of drug release, the in vitro release data were analyzed according to zero-order, first-order, and diffusion controlled mechanism according to the simplified Higuchi model. The preference of a certain mechanism was based on the determination coefficient (R2) for the parameters studied, where the highest determination coefficient is preferred for the selection of the order of release (CitationDangprasirt & Pongwai, 1998).
To understand the release mechanisms of acetaminophen from suppositories, we described the release rate using the following equations (CitationPeppas, 1985):
where Mt/M∞ is the fraction of drug release at time t, K denotes the constant incorporating structural and geometrical characteristics of the drug/polymer system and n is the diffusion exponent related to the mechanism of the drug release.
In vivo study on rabbits
In vivo study was carried out on New Zealand rabbits weighing 1.5–2 kg (all experiments were approved by the Institutional Animal Ethics Committee, Cairo University, Egypt). The method applied to test the corneal absorption of the drug was done after CitationLaw et al. (2000) and CitationSasaki et al. (2003).
One minitablet (chitosan 1%) was inserted in the lower conjunctival sac of one eye of each rabbit. Commercially available Zovirax® eye ointment containing 3% acyclovir was applied in an equivalent dose to that of minitablets in the lower conjunctival sac of the other eye of the rabbit. During application care was taken not to irritate the eye or touch the corneal surface.
At time intervals of 1.5, 3, 5, and 7 h after insertion of minitablets or application of Zovirax® eye ointment rabbits were sacrificed. The ocular surface was irrigated with isotonic phosphate buffered saline and dried with soft tissue. The cornea from each eye was separated and collected in pre-weighed tubes.
HPLC assay
Chromatographic conditions.
A modified HPLC assay of CitationLaw et al. (2000) for determination of acyclovir was adopted. The mobile phase was composed of acetonitrile:0.05 M ammonium acetate with a pH adjusted to 5.4 using acetic acid (3:97, v/v). Separation was performed on a 4.6 mm × 150 mm, 5 μm, Thermo BDS Hypersil C18 column (Electron Co., Runcorn, UK). The mobile phase flow rate was 1 ml/min. The system was operated at ambient temperature and the detection wavelength was 252 nm (La Chrom, UV/VIS detector, L- 7420, La Chrom and interface module, D-7000, Merk Hitachi, Darmstadt, Germany). The sample volume was 50 μl.
Sample preparations.
Excised corneas were homogenized in 1 ml of a chilled 50:50 mixture of isotonic PBS and a chilled 4:5 mixture of acetonitrile and methanol for ∼ 5 min in a tissue homogenizer. Subsequently, the corneal homogenates were centrifuged at 12,500 rpm for 25 min at 4°C to remove cellular debris, and the supernatant was used for analysis.
The degree of drug penetration was measured in μg/g cornea tissues. However, it should be noted that the weight of cornea is less than 1 g (ranging from 56–89 mg) so that values expressed in the units given represent data extrapolated from small quantities. Cmax and the area under curve (AUC) were determined. The AUC was calculated following trapezoidal rule during the experimental period (AUC0–7). All data were expressed as means value ± SD. Statistical analysis was performed using one way analysis of variance ANOVA test. Differences between treatments were considered statistically significant at a level p < 0.05.
In vivo study on pigeons
The study was done on 12 Bokhara Trumpeter Pigeons diagnosed by Department of Poultry Diseases, Animal Health Research Institute, Dokki, Giza as a bilateral ocular herpal infection manifested by redness in the eyes and increased lacrimation. The pigeons were divided into two groups, each of six pigeons. A single minitablet of the chosen formula (1% chitosan) was inserted into both eyes of the first group. The other group received no treatment. The pigeons were observed for relief of symptoms.
Results and discussion
Physical characteristics
The ocular minitablets were successfully prepared by the lyophilization of the medicated polymer solutions. The inserts were cylindrical in shape () and the weight of the inserts ranged from 0.62–0.81 mg with a weight variation not exceeding 10% for each formulation. Average drug content was 95.5% ± 7.2 and the results of the content uniformity test proved that there was homogenous drug distribution.
Water uptake
The uptake of water by in situ gelling inserts is a crucial step for the transformation into the gel and for adhesion to the mucosa. The ability of hydrogels to absorb water is due to the presence of hydrophilic groups such as -OH, -COOH, and -OSO3H. The hydration of these functional groups results in water entry into the polymer network, which leads to expansion and consequently an ordering of the polymer chains. The swelling equilibrium (maximum water uptake) is reached, when the osmotic forces of the functional groups are balanced by the restrictive forces of the higher ordering of the polymer chains (CitationPeppas & Khare, 1993).
The water uptake of inserts depends on the type of polymer used. The swelling index after 1 h of study was in the following order () Carbapol > NaCMC (2%) > NaCMC (1%) > xanthan > HPMC > mix > chitosan.
Table 1. Physical characteristics of minitablets prepared from different polymers (M ± SD, n = 3).
It was expected that the charged polymers would lead to a higher extent of water uptake compared to neutral polymers. This was true for Carbapol, NaCMC, and xanthan gum with a good correlation to their charge densities (CitationBertram & Bodmeier, 2003).
HPMC, being an uncharged polymer, showed a water uptake index less than the previously mentioned negatively charged polymers. Moreover, CitationMichailova et al. (2000) stated that the elastic structure of HPMC gels and their long mean relaxation time determine the low velocity of water penetration.
On the other hand, chitosan is a charged polymer, yet it showed the least swelling. Swelling of chitosan in acidic or basic media is due to the free negative charges of the polyacid or free positive charges of the ammonium groups, respectively. Their mutual repulsion and the entry of water together with counterions to neutralize these charges cause swelling. Chitosan in neutral medium has neither free negative charges of the polyacid, nor free positive charges of the ammonium groups. So, this leads to decrease the entry of water and decrease swelling (CitationChu et al., 1996).
The water uptake profile of Carbopol minitablet was biphasic with a fast initial phase followed by a reduced water uptake rate (). The first fast phase is probably due to rapid water uptake by capillary forces, which could be explained by ionization of the carboxylic group causing the ionic repulsion of the polymer, which uncoils the polymer into an extended structure. This was followed by a reduced water uptake rate, which may be attributed to the formation of a gel layer and thus a slower hydration.
Figure 2. Water uptake of minitablets prepared from different polymers. Error bars represent SD (n = 3).
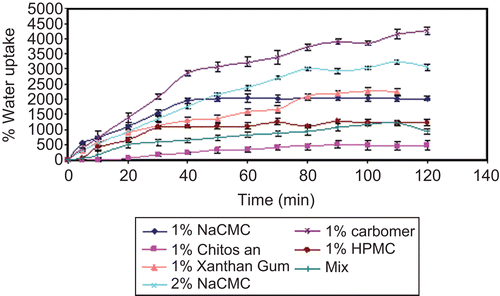
Increasing the concentration of NaCMC from 1% to 2% initially slightly decreased the water sorption probably due to formation of a highly viscous gel layer at the contact area between the dry insert and the medium. However, 2% NaCMC insert with its greater polymer content showed a greater capacity for water sorption, as it continued swelling after 1% NaCMC had probably reached a swelling equilibrium.
The mixture [1(1% NaCMC):1(0.5% chitosan)] showed a considerable slow water uptake. The interaction of the two oppositely-charged polymers (CitationRosca et al., 2005; CitationLiuyun et al., 2009) resulted probably in an increased polymer cross-linking density, which in turn decreased the rate of water diffusion into the polymer network, thus causing an insufficient swelling of the polymer. This is in accordance with CitationSalamat-Miller et al. (2005), who reported that the degree of swelling at equilibrium has an inverse relationship with the degree of cross-linking of the polymer.
Rheological characterization
In situ gelling ocular inserts are supposed to take up fluid from the mucosa and to form a gel. The viscosity of this gel and, thus, respectively, the viscosity of the polymer solution for insert preparation are of utmost importance for the performance of inserts with respect to drug release, water uptake, and corresponding polymer mass loss, as well as bioadhesion. The solutions of polymers in the previously mentioned concentrations were therefore investigated concerning their rheological behavior (). The balance of water uptake and the molecular weight of the polymer defined the viscosity of the resulting gel. The viscosity of the polymers followed the order: Carbopol > mix > chitosan > NaCMC > HPMC > xanthan gum. All tested polymers exhibited pseudoplastic behavior associated with thixotropy, which is required to minimize interference with blinking.
Increasing the concentration of NaCMC from 1% to 2% resulted in an expected increase in viscosity. Concerning the mixture, the interaction between the oppositely charged polymers was obviously seen in the considerable increase in the viscosity of the polymer mixture when compared with each polymer individually, indicating the increased cross-linking density of the polymer mixture.
Bioadhesion potential of inserts
Hydration is required for a mucoadhesive polymer to expand and create a proper macromolecular mesh of sufficient size and also to induce mobility in the polymer chains in order to enhance the interpenetration process between polymer and mucin. Polymer swelling permits a mechanical entanglement by exposing the bioadhesive sites for hydrogen bonding and/or electrostatic interaction between polymer and mucous network. The great bioadhesive force revealed by Carbopol thus correlates with the great swelling capacity exhibited by the polymer (). Furthermore, CitationPeppas and Buri (1985) have demonstrated that strong anionic charge on the polymer is one of the required characteristics for mucoadhesion. This may explain the good bioadhesive characteristics shown by NaCMC, xanthan gum in addition to Carbopol which possesses the greatest charge density (CitationBertram & Bodmeier, 2003).
Additionally, a good bioadhesive potential was also shown by chitosan. Regarding the structure of the polymer, this finding is probably due to the high capability of the polymer to form hydrogen bonding. Moreover, the cationic nature of the polymer may allow an electrostatic attraction to mucin. This result agrees with CitationLehr et al. (1992), who reported that some cationic high-molecular-weight polymers such as chitosan were shown to possess good adhesive properties.
HPMC as a non-ionic polymer revealed a significant small degree of adhesion compared to all other charged polymers; this may be attributed to the inability of the polymer to interact electrostatically with mucin (CitationBertram & Bodmeier, 2003; CitationSankalia et al., 2008).
As expected, an obviously greater force of adhesion was shown by NaCMC 2% relative to NaCMC 1% () as, when the concentration of the polymer increases, the number of penetrating polymer chains per unit volume of the mucus is increased and the interaction between polymer and mucus become more stable (CitationPeppas & Buri, 1985).
In vitro drug release study
When hydrophilic polymers come into contact with a liquid hydrate, a gel layer is formed, essential for sustaining and controlling drug release from polymer solid dosage form. The thickness of this hydrate layer determines the diffusion of the drug molecules through the polymer mass into the liquid medium. Moreover, the rate and extent of the drug release also depends on the swelling and erosion of the hydrate polymer preparation as well as polymer–polymer interactions and the viscosity of the hydrated inserts resulting additionally from the polymer molecular weight.
As revealed in , % chitosan obviously showed the least release rate among the tested polymers despite its low viscosity (). This was in correlation with the very slow hydration process of chitosan shown in the water sorption study ().
Figure 3. Release of acyclovir from minitablets prepared from different polymers. Error bars represent SD (n = 3).
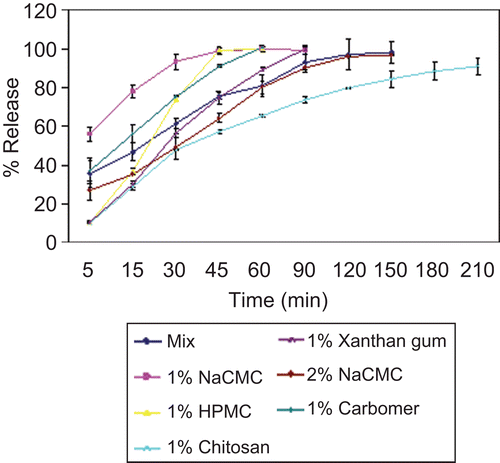
In contrast, a fast drug release was revealed by NaCMC 1% and HPMC 1% which is probably due to their low viscosities. The higher extent of water uptake of the anionic NaCMC as well as its lower viscosity compared to the neutral polymer HPMC are probably behind the faster release profile of the drug from NaCMC relative to HPMC.
This was in correlation with the study done by CitationMichailova et al. (2000) comparing the water uptake and rheological properties of HPMC and NaCMC.
A considerable reduction of drug release from 1% NaCMC was satisfactorily obtained by forming a 1:1 mixture with 0.5% chitosan. The ionic interaction between the polymers resulted in a considerable increase in the viscosity of the polymer mixture, which was also accompanied with a decreased rate of water sorption. Taking these factors together explained the reduced release pattern of the drug.
Another approach to reduce the rate of drug release from NaCMC was achieved by increasing the concentration of the polymer to 2%. An increased polymer concentration was associated with an increase in polymer density, which led to decreased polymer erosion, thus making the tablet more resistant to dissolution and consequently reducing drug release. Furthermore, as the amount of the polymer in the matrix increases there would be a greater degree of hydration with simultaneous swelling which results in a lengthening of the drug diffusion pathway and reduction in drug release rate (CitationSankalia et al., 2008).
A fast drug release was revealed by carbopol minitablets. As previously mentioned the water uptake profile of carbopol minitablets was biphasic with a fast initial phase due to rapid water uptake by capillary forces, followed by a reduced water uptake rate, which was attributed to the formation of a gel layer and thus a slower hydration. The drug probably diffused rapidly through the matrix during the initial phase and before the formation of the gel barrier. The effect of the pronounced swelling of the polymer on drug release clearly overcame the effect of the high viscosity of the 1% carbopol polymer solution.
Xanthan gum showed a significant slow drug release, though the viscosity of the polymer solution was relatively low. This may be attributed to the semi-rigid chain structure of xanthan gum, when compared to the flexible chains of semi-synthetic polymers, like the cellulose ethers (CitationBaumgartner et al., 2006). This finding was in agreement with CitationUghini et al. (2004), who reported that all studied formulations containing xanthan gum showed prolonged release profiles. Furthermore, xanthan gum was proved to have higher drug-retarding ability than the well known HPMC (CitationTalukdar et al., 1996).
Kinetic analysis of in vitro release data
The kinetic study of the tested polymers is shown in . According to the Power Law when exponent n = 1 the drug release is independent of time. This corresponds to zero-order release kinetics. The mechanism for zero order release is known as Case-II Transport. Water imbibes into the matrix, relaxing the polymers. The relaxed polymers swell significantly and their volume expands. The penetration of water into the polymer (swelling) is the rate-determining step. The values for n > 1 (Super Case II transport), as revealed by HPMC, would be the consequence of a plasticization process in the gel layer (CitationRitger & Peppas, 1987) arising from reduction of the attractive forces among polymeric chains that increases the mobility of macromolecules. Thus, diffusion rate of the drug depends on relaxation rate of polymeric chains (CitationSiepmann et al., 1999).
Table 2. Kinetic parameters of the prepared ocular minitablets.
When n ≤ 0.45, release is diffusion controlled (Fickian diffusion) as in the case of Carbapol, mix and 2% NaCMC. With values of n between 0.45–1.0, as for chitosan and xanthan, the mechanism of drug release is defined as anomalous (non-Fickian) and it represents a superposition of the two mechanisms, where the release is controlled by a combination of diffusion and polymer relaxation (CitationSiepmann & Peppas, 2001). According to determination coefficient R2, 2% NaCMC showed a mechanism of zero order, while its respective n-value (0.4329) indicates a Fickian diffusion. Nevertheless, the value of n seems to be on the border of the anomalous transport mechanism, which probably indicates that the polymer swelling plays a role in the drug release pattern.
In vivo study on rabbits
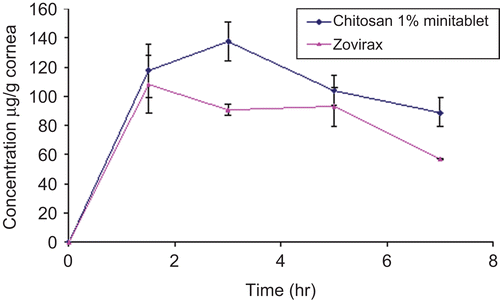
The in vivo study involved chitosan minitablet and the commercial Zovirax® eye ointment. Among all tested polymers chitosan minitablet was chosen for some reasons. First, it showed a significant sustained release of the drug as well as good bioadhesive properties. In addition, the slow swelling rate revealed by chitosan was considered advantageous as it would reduce irritation due to dryness.
shows the in vivo corneal absorption of acyclovir after topical application of chitosan minitablet and Zovirax®. Chitosan tablet showed a significantly higher Cmax of acyclovir in the cornea (p < 0.05) compared to the commercial acyclovir ointment. Moreover, the extent of acyclovir absorption into the cornea from the selected minitablets was higher (p < 0.05) than from the commercial acyclovir ointment, where their respective AUC(0–7) values were 713.74 and 563.88 µg/g.h.
In vivo study on pigeons
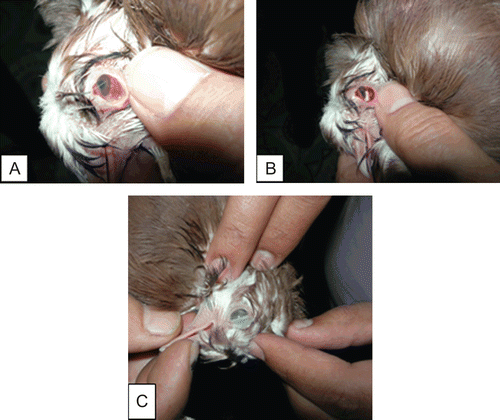
The pigeons of the first group which received the treatment showed reduced lacrimation and redness of eyes in the first 24 h after insertion of chitosan 1% minitablets. Within 48 h complete recovery of symptoms was observed (). Furthermore, a weekly observation of the pigeons revealed no recurrence of infection for the following 3 months. On the other hand, the untreated group showed no change in their condition.
Conclusion
Ocular minitablets containing acyclovir can be considered as a promising new erodible drug delivery system to treat ulcerative viral keratitis, as it offers sustained drug release in the tear film for a prolonged period. Thus, the administration of one minitablet in the fornix of the infected eye in the evening could avoid the frequent instillation of eye drops at night. This increases greatly the comfort of patient and care provider.
Declaration of interest
The authors report no declarations of interest
References
- Baudouin, C. (1996). Side effects of antiglaucomatous drugs on the ocular surface. Curr Opin Ophthalmol. 7:80–6.
- Baumgartner, S., Planisˇek O, SrcˇicˇS Kristl, J. (2006). Analysis of surface properties of cellulose ethers and drug release from their matrix tablets. Eur J Pharm Sci. 27:375–83.
- Bertram, U., Bodmeier, R. (2003). In situ gelling nasal inserts for prolonged drug delivery. Dissertation, Freie University Berlin, Germany.
- Chu, C.H., Kumagai, H., Sakiyama, T., Ikeda, S., Nakamura, K. (1996). Development of a model for analyzing the swelling rate of ionic gels on the basis of the diffusion of mobile ions—application to the pH-sensitive swelling of a polyelectrolyte complex gel prepared from xanthan and chitosan. Biosci Biotech Biochem. 60:1627–32.
- Dangprasirt, P., Pongwai, S. (1998). Development of diclofinac sodium controlled release solid dispersion powders and capsules by freeze drying technique using ethylcellulose and chitosan as carriers. Drug Dev Ind Pharm. 24:947–53.
- Farrow, F., Lowe, G., Neale, S.J. (1928). The flow of starch pastes. Flow at high and low rates of shear. Textile Inst. 19:181.
- Gurtler, F., Kaltsatos, V., Boisramé, B., Deleforge, J., Gex-Fabry, M., Balant, P., Gurny, R. (1995). Ocular availability of gentamicin in small animals after topical administration of a conventional eye drop solution and a novel long acting bioadhesive ophthalmic drug insert. Pharm Res. 12:1791–5.
- Hui, H.W., Robinson, J.R. (1985). Ocular drug delivery of progesterone using a bioadhesive polymer. Int J Pharm. 26:203–13.
- Jabs, D.A. (1998). Acyclovir for recurrent herpes simplex virus ocular disease. N Engl J Med. 339:300–6.
- Law, S.L., Huang, K.J., Chiang, C.H. (2000). Acyclovir-containing liposomes for potential ocular delivery. Corneal penetration and absorption. J Contr Rel. 63:135–40.
- Lee,V.H.L., Robinson, J.R. (1986). Review: topical ocular drug delivery recent developments and future challenges. J Ocul Pharmacol. 2:67–108.
- Lehr, C.M., Bouwstra, J.A., Schacht, E.H., Junginger, H.E. (1992). In vitro evaluation of mucoadhesive properties of chitosan and some other natural polymers. Int J Pharm. 78:43–8.
- Lehr, C.M., Lee, Y.H., Lee, V.H. (1994). Improved ocular penetration of gentamicin by mucoadhesive polymer polycarbophil in the pigmented rabbit. Invest Ophthalmol Vis Sci. 35:2809–14.
- Liuyun, J., Yubao, L., Chengdong, X. (2009). Preparation and biological properties of a novel composite scaffold of nano-hydroxyapatite/chitosan/carboxymethyl cellulose for bone tissue engineering. J Biomed Sci. 16:65–71.
- Ludwig, A., Van Ooteghem, M. (1992). Influence of viscolysers on the residence of ophthalmic solutions evaluated by slit lamp fluorophotometry. STP Pharma Sci. 2:81–7.
- Martindale Sweetman SC (2007). The complete drug reference. London and Chicago: The Pharmaceutical Press.
- Michailova, V., Titeva, S.T., Kotsilkova, R., Krusteva, E., Minkov, E. (2000). Water uptake and relaxation processes in mixed unlimited swelling hydrogels. Int J Pharm. 209:45–56.
- Parodi, B., Russo, E., Caviglioni, G., Caffagi, S., Bignardi, G. (1996). Development and characterization of a buccoadhesive dosage from of oxycodone hydrochloride. Drug Dev Ind Pharm. 22:445–50.
- Peppas, N.A. (1985). Analysis of Fickian and non Fickian drug release from Polymers. Pharm Acta Helv. 60:110–1.
- Peppas, N.A., Buri, P.A. (1985). Surface, interfacial and molecular aspects of polymer bioadhesion on soft tissues. J Contr Rel. 2:257–75.
- Peppas, N.A., Khare, A.R. (1993). Preparation, structure and diffusional behavior of hydrogel in controlled release. Adv Drug Deliv Rev. 11:1–35.
- Ritger, P.L., Peppas, N.A. (1987). A simple equation for description of solute release. I. Fickian and non-Fickian release from non-swellable devices in form of slabs, sphere, cylinders or discs. J Contr Rel. 5:23–36.
- Rosca, C., Popa, M.I., Lisa, G., Chitanu, G.C. (2005). Interaction of chitosan with natural or synthetic anionic polyelectrolytes. 1. The chitosan–carboxymethylcellulose complex. Carbohydrate Polymers. 62:35–41.
- Saettone, M.F., Salminen, L. (1995). Ocular inserts for topical delivery. Adv Drug Deliv Rev. 16:94–106.
- Salamat-Miller, N., Chittchang, M., Johnston, T.P. (2005). The use of mucoadhesive polymers in buccal drug delivery. Adv Drug Deliv Rev. 57:1666–91.
- Sankalia, J.M., Sankalia, M.G., Mashru, R.C. (2008). Drug release and swelling kinetics of directly compressed glipizide sustained-release matrices: establishment of level A IVIVC. J Contr Rel. 129:49–58.
- Sasaki, H., Nagano, T., Sakanaka, K., Kawakami, S., Nishida, K., Nakamura, T., Nakashima, M. (2003). One-side coated insert as a unique ophthalmic drug delivery system. J Contr Rel. 92:241–7.
- Siepmann, J., Lecomte, F., Bodmeier, R. (1999). Diffusion controlled drug delivery systems: calculation of the required composition to achieve desired release profiles. J Contr Rel. 60:379–89.
- Siepmann, J., Peppas, N. (2001). Modeling of drug release from delivery systems based on hydroxypropyl methylcellulose (HPMC). Adv Drug Del. 48:139–57.
- Sintzel, M., Bernatchez, S., Tabatabay, C., Gurny, R. (1996). Biomaterials in ophthalmic drug delivery. Eur J Pharm Biopharm. 42:258–374.
- Talukdar, M.M., Michoel, A., Rombaut, P., Kinget, R. (1996). Comparative study on xanthan gum and hydroxypropylmethylcellulose as matrices for controlled-release drug delivery. I. Compaction and in vitro drug release behaviour. Int J Pharm. 129:233–41.
- Topalkara, A., Güler, C., Arici, D.S., Arici, M.K. (2000). Adverse effects of topical antiglaucoma drugs on the ocular surface. Clin Exp Ophthalmol. 28:113–7.
- Ughini, F., Andreazza, I.F., Ganter, J.L.M.S., Bresolin, T.M.B. (2004). Evaluation of xanthan and highly substituted galactomannan from M. scabrella as a sustained release matrix. Int J Pharm. 271:197–205.
- Weyenberg, W., Vermiere, A., Remon, J.P., Ludwig, A. (2003). Characterization and in vivo evaluation of ocular bioadhesive minitablets compressed at different forces. J Contr Rel. 89:329–40.