Abstract
Nerve growth factor (NGF) has been proved with the potential of promoting neurogenesis in adult mammalians. This study was aimed to investigate the effect of intranasal (IN) NGF on striatal neurogenesis and functional recovery in adult rats with focal cerebral ischemia. Rats were subjected to middle cerebral artery occlusion (MCAO) for 2 h, and then reperfused. NGF or vehicle was intranasally administered 24 h after cerebral reperfusion, and the treatments continued for 6 consecutive days there after. All animals were injected with 5-bromodeoxyuridine (BrdU) twice daily for 5–7 days after MCAO, and sacrificed 1 day and 28 days, respectively, after the last BrdU injection. Neural cell proliferation and survival in different brain regions were analyzed. Functional tests and immunohistochemical staining were also performed. The results showed that treatment with IN NGF failed to increase cell proliferation but improved survival of newly generated cells in ipsilateral striatum and subventricular zones (SVZ). Double immunofluorescence with BrdU and neuronal nuclei protein, a mature neuronal marker, were increased in striatum and SVZ in rats treated with IN NGF. The functional recovery was also observed at time of neurogenesis enhancement in striate. In conclusion, IN NGF may enhance neurogenesis and survival of newly generated cells, which may result in improved functional recovery after cerebral ischemia.
| Abbreviations | ||
| BBB | = | blood–brain barrier |
| BrdU | = | bromodeoxyuridine |
| CNS | = | central nervous system |
| DG | = | dentate gyrus |
| IN | = | intranasal |
| MCAO | = | middle cerebral artery occlusion |
| NeuN | = | neuronal nuclei |
| NGF | = | nerve growth factor |
| SVZ | = | subventricular zone. |
Introduction
Stroke is the leading cause of long-term disability and a major cause of death. The limited repair potential of the adult brain makes functional recovery after stroke extremely difficult. Some studies demonstrated that neural progenitor cells located in the subventricular zone (SVZ) and subgranular zone of dentate gyrus (DG) have the potential to differentiation and migration in adult mammalians (CitationGould & Gross, 2002; CitationGonzalez-Perez & Quinones-Hinojosa, 2010). The neurogenesis in SVZ may be triggered by cerebral ischemia, and the newborn neurons may migrate to the damaged striatum where neurogenesis is less possible (CitationJin et al., 2001; CitationArvidsson et al., 2002). Theoretically, the neurogenesis may initiate self-repair in the central nervous system (CNS) after insult, and may be of therapeutic value. However, this spontaneous regeneration can not reach the quantity requirement for functional recovery. It is, therefore, necessary to take some pharmacological measures for enhancing the effect of stroke-induced neurogenesis, including proliferation, survival, and neuronal maturation.
Nerve growth factor (NGF) was initially discovered as a neurotrophic factor that could promote survival and differentiation of developing neurons in the peripheral nervous system. In CNS, NGF is produced throughout adult life and targets basal forebrain and striatal neurons (CitationSofroniew et al., 2001). Evidence suggested that NGF was involved in neurogenesis in adult CNS. Some in vitro studies demonstrated that NGF enhances the survival of neural stem cells isolated from the embryonic forebrain or striatum and promotes neuronal differentiation (CitationLachyankar et al., 1997; CitationNakajima et al., 2007). It has been shown that endogenous NGF is up-regulated after cerebral ischemia, and then educes a neuroprotective effect (CitationLee et al., 1998). Therefore, it is worthwhile to explore the effect of exogenous NGF in treating striatum damage after focal cerebral ischemia.
However, NGF can not cross the blood–brain barrier (BBB) freely because of its large molecular profile (CitationSaragovi & Gehring, 2000). Intracerebroventricular or intracerebral injection of NGF or grafting of NGF-producing cells may be less practical due to invasiveness and other safety concerns. The intranasal (IN) route was proved to be one of the most effective routes for distributing drugs to systemic circulation (CitationAlsarra et al., 2008). Our previous studies demonstrated that IN administration offers a non-invasive and safe alternative that can bypass BBB and deliver neurotrophic factors directly into CNS (CitationLiu et al., 2001; CitationMa et al., 2008). Intranasal delivery of neurotrophic factors such as brain-derived neurotrophic factor (BDNF), ciliary neurotrophic factor (CNTF), and neurotrophin-4 were also proved to cross the blood–brain barrier (CitationAlcalá-Barraza SR et al., 2010). Several examinations showed that a significant amount of NGF can be delivered into CNS through the intranasal pathway (CitationChen et al., 1998; CitationCapsoni et al., 2009; CitationShi et al., 2010). It has also been shown by CitationChen et al. (1998) that a significant amount of NGF can be delivered into CNS through the intranasal pathway. In this study, we evaluated the effect of IN NGF on striatal neurogenesis and neurological functional recovery.
Materials and methods
Animals and focal cerebral ischemia
A total of 24 adult male Sprague-Dawley rats weighing 250–280 g were obtained from the Model Animal Research Center of Nanjing University and housed at room temperature with access to food and water ad libitum. Animal care and all procedures were carried out in accordance with the NIH Guide for the Use and Care of Laboratory Animals. The study was approved by the local ethical review committee.
Focal cerebral ischemia was induced by right middle cerebral artery occlusion (MCAO) as described previously (CitationLonga et al., 1989). Briefly, rats were anesthetized with 10% chloral hydrate (350 mg/kg, intraperitoneally). The right common carotid artery (CCA), the right external carotid artery (ECA) and the internal carotid artery (ICA) were isolated. The ECA was ligated and dissected distally. A 4-0 monofilament nylon suture with rounded tip was introduced from the ECA stump into the ICA to occlude the origin of the right middle cerebral artery (MCA), ~ 18 ± 1 mm past the carotid bifurcation. Two hours after occlusion, the filament was withdrawn to allow reperfusion. The rectal temperature was maintained at 37°C with a heating pad at all times during surgical procedures.
IN administration
Rats subjected to 2 h of MCAO were randomized into control (vehicle) and NGF groups (n = 12 in each group). NGF (Haite Inc, Wuhan, China) was dissolved at a concentration of 0.6 mg/ml in phosphate buffered saline (PBS). IN administration was performed as described previously with minimal modifications (CitationThorne et al., 1995). Rats were re-anesthetized with 10% chloral hydrate (350 mg/kg, intraperitoneally) and placed on their back. A total volume of 100 μl solution per rat was given and 10 μl was given to the left and right naris alternately within 30 min. The mouth and the opposite naris were shut during administration. Once-daily treatment was initiated 24 h after reperfusion and continued for 6 consecutive days.
5-Bromodeoxyuridine (BrdU) administration
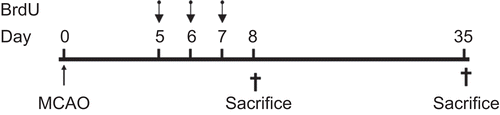
All animals received BrdU injections (50 mg/kg, Sigma, St. Louis, MO) intraperitoneally twice daily with an interval of 8 h for 5–7 days after MCAO. To assess cell proliferation, one set of each group was sacrificed 1 day after the last injection (8 days after MCAO), while the other set was sacrificed 28 days after the last injection (35 days after MCAO) to evaluate cell survival and neuronal differentiation ().
Functional tests
For the animals that survived at 35 days after MCAO, modified Neurological Severity Score (mNSS) was performed to evaluate neurological function before and 1, 7, 14, 21, 28, and 35 days after MCAO by a researcher who was blind to randomization. mNSS is a neurological functional evaluation including motor, sensory, reflex, and balance tests (CitationChen et al,. 2001). Neurological function is graded on a scale of 0–18 (normal score, 0; maximal deficit score, 18).
Tissue preparation and measurement of infarct volume
One day or 28 days after the final BrdU dose, rats were transcardially perfused with 150 ml saline, followed by 250 ml 4% paraformaldehyde (PFA) in 0.01 M PBS at 4°C. The rat brains were post-fixed in PFA for 6 h and cryo-protected in 30% sucrose solution overnight. Brain tissues encompassing the SVZ were cut into 20 μm coronal slices spaced 200 μm apart and stored at −80°C.
For the rats sacrificed at 35 days after MCAO, seven sections equally spaced 2 mm from each brain were stained with hematoxylin and eosin for testing the volume of cerebral infarction using the NIH Image program. The infarct volumes were calculated and presented as a percentage of the intact hemisphere (CitationChen et al., 2001).
Immunohistochemistry
For the animals who survived at 28 days after BrdU injection, fluorescence double staining was used for visualizing BrdU and neuronal nuclei (NeuN). Brain sections were treated with 50% formamide in 30 mM sodium citrate at 65°C for 2 h, and then incubated in 2 M HCl at 37°C for 30 min, rinsed in 0.1 M boric acid (pH 8.5) at room temperature for 10 min. After incubation in 3% H2O2 for 30 min, sections were blocked with 2% goat serum for 1 h, followed by incubated with sheep polyclonal anti-BrdU antibody (1:800, Biodesign, Saco, ME) and mouse monoclonal anti-NeuN antibody (1:600, Chemicon, Temecula, CA) at 4°C overnight. Sections were then incubated for 2 h with fluorescein isothiocyanate-conjugated goat anti-mouse IgG (1:100, Jackson ImmunoResearch, West Grove, PA), and rhodamine-conjugated donkey anti-sheep IgG (1:100, Jackson ImmunoResearch). Finally, sections were mounted with Vectashield mounting medium H-1000 (Vector Laboratories, Burlingame, CA).
For BrdU single labeling, slices were pre-treated for fluorescence double staining as previously described. After being blocked with 2% goat serum for 1 h, sections were incubated with mouse monoclonal anti-BrdU antibody (1:800; Sigma) at 4°C overnight. Biotinylated goat anti-mouse secondary antibody (1:500; Jackson ImmunoResearch) was applied for 2 h at room temperature, then washed and incubated with peroxidase-conjugated streptavidin solution (1:500; Jackson ImmunoResearch) for 30 min. Reaction products were detected with 3, 3’-diaminobenzidine- tetrahydrochloride (Sigma).
Cell Counting
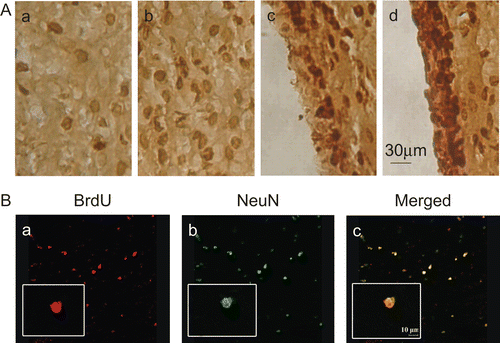
Five DAB-stained sections per animal were taken to evaluate the number of BrdU-labeled cells. All of the BrdU-positive nuclei in SVZ and striatum ipsilateral to the injury were counted under high power (200×) on an Olympus BX51 microscope with Nikon digital camera. Images were visualized with a computer monitor. Results were presented as average number of BrdU-immunoreactive cells per section. Co-localization of BrdU and NeuN was determined with a confocal laser-scanning microscope (Leica TCS SP2, Germany). Results were expressed as mean number of BrdU-labeled cells co-expressing NeuN per section.
Statistical analysis
All data were expressed as mean ± SE. Student’s t-test was used to evaluate differences between groups in terms of infarct volume and cell population. mNSS scores were analyzed by two-way repeated ANOVA, followed by Student’s t-test for pair-wise comparison. Results were considered significant at p < 0.05.
Results
Neurological outcome and infarct volume
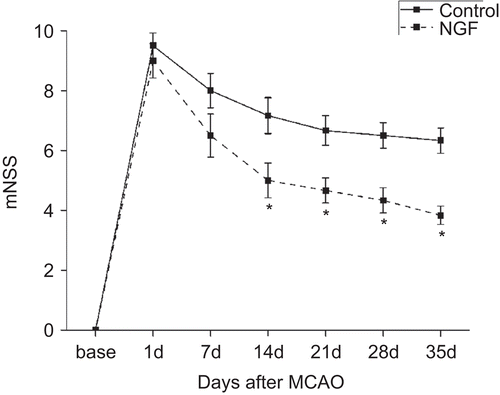
The outcomes of mNSS test revealed that all animals exhibited moderate neurological deficits. Treatment with IN NGF significantly improved neurological functions 14 days after MCAO, which continued for at least 3 weeks (p < 0.05) (). No significant reduction in lesion volumes was observed in rats treated with IN NGF compared with the control rats (35.8 ± 2.1% vs 40.3 ± 3.2% of intact contralateral hemispheric volume, respectively, p = 0.25).
Cell proliferation
We defined proliferation rate as the number of newly formed cells measured 1 day after the last injection of BrdU. The BrdU immunoreactive cells were mainly present in the ipsilateral striatum adjacent to the SVZ. There was no significant difference in the number of BrdU-positive cells in the striatum between the NGF and control groups (p = 0.45). We also examined BrdU staining in the ipsilateral SVZ and reached similar results ().
Table 1. Cell proliferation and survival in ipsilateral striatum and SVZ (mean ± SE).
Cell survival
In the rats sacrificed at 28 days after the last BrdU administration, the number of BrdU-positive cells was significantly higher in the NGF group compared with that in the control group (p < 0.05) (, ). Furthermore, we explored the survival rate of progenitors, which was based on the ratio of the labeled cells at 28 days after BrdU injection to that 1 day after the injection. In comparison with the control group, treatment with IN NGF enhanced the survival rate in both the ipsilateral striatum and SVZ ().
Neuronal differentiation
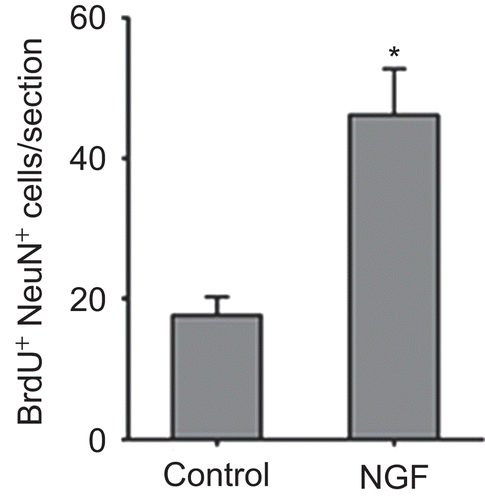
The presence of newly generated neurons in the ipsilateral striatum was determined by double labeling of BrdU and NeuN at 28 days after the final BrdU dose, a time point when the labeled cells had finished differentiation (CitationBiebl et al., 2000). A representative BrdU-positive cell co-expressing NeuN was illustrated in . The number of BrdU-positive cells co-expressing NeuN was higher in the NGF group than that in the control group (p < 0.05) ().
Discussion
In this study, we provided the first evidence that intranasally administered NGF may enhance survival of neural progenitors and lead to neuronal differentiation in the striatum of adult rats with focal cerebral ischemia. The results indicated that treatment with IN NGF improves neurological functions, which is simultaneous to the increased neurogenesis in the striatum.
The IN pathway has been used to administer NGF in some previous studies. CitationChen et al. (1998) demonstrated that a significant amount of NGF could enter CNS including striatum via the intranasal pathway. In addition, IN NGF has shown its therapeutic effect in treating Alzheimer’s disease in mice (CitationDe Rosa et al., 2005). In this study, we have also attempted to facilitate NGF brain delivery by using the IN pathway and showed for the first time the effect of IN NGF on stroke-induced striatal neurogenesis. The present study has also suggested that IN administration may be an effective strategy for delivering NGF into CNS. In this study, the infarct size was unchanged, but significant neuronal formation was observed after IN NGF. We think NGF may not change the acute ischemic cerebral lesion which develops during a relatively short time, but NGF may improve neuronal formation which is a chronic process compared with infarction. Besides, neurogenesis is merely one of many reasons for this functional improvement. NGF could improve functional recovery via many other mechanisms.
The striatum is one of the most vulnerable regions to ischemia. Increased neurogenesis in the injured striatum may be beneficial for stroke rehabilitation. In this study, we investigated several phases separately: cell proliferation, progenitor cell survival, and neuronal maturation. BrdU, which incorporates DNA of dividing cells during DNA synthesis in S-phase, is now regarded as a basic technique of labeling newly generated cells (CitationKnapp, 1992; CitationDolbeare, 1995). Although it has pitfalls and limitations, such as toxicity and mutagenic substance (CitationTaupin, 2007), BrdU was used as a tracer for investigating the effect of IN NGF on stroke-induced neurogenesis in this study. The time point when animals received BrdU administration was chosen on the basis of prior studies showing maximal increase of proliferating cells in SVZ 7 days after focal cerebral ischemia (CitationZhang et al., 2001; CitationGotts & Chesselet, 2005). The time choice is also made for the purpose of avoiding BrdU integration during DNA repair in the earlier period after MCAO (CitationArvidsson et al., 2002).
To study proliferation, rats were sacrificed 1 day after the last BrdU injection, which was regarded as a sufficient duration for labeling cells in the first period (CitationCiaroni et al., 2002). Two possible mechanisms have been proposed as the source of the neural progenitors in the damaged striatum after MCAO (CitationYamashita et al., 2006). One is the SVZ where progenitors have been proved to migrate toward the ischemic striatum (CitationArvidsson et al., 2002; CitationZhang et al., 2004). The other is the striatal parenchyma which may also contain latent progenitor cells that might be activated by IN NGF (CitationPencea et al., 2001). Therefore, to evaluate striatal neurogenesis, we firstly investigated cell proliferation in the SVZ, the main pool of neural progenitors. It was found that NGF did not influence the number of BrdU-positive cells in the SVZ, which was consistent with previous studies (CitationCalza et al., 2003; CitationFrielingsdorf et al., 2007). We further examined the BrdU-positive cells in the striatum and obtained results similar to those achieved in the SVZ.
As previously reported (CitationCiaroni et al., 2002), this study indicated that the number of BrdU-labeled cells decreased within 4 weeks in both groups. Interestingly, IN NGF did not affect cell proliferation in the ipsilateral striatum and SVZ, but the number of lost BrdU-labeled cells was lower in the NGF group compared with control, which indicated that NGF enhanced the survival of newly formed cells. Furthermore, we observed that treatment with IN NGF increased the survival rate, which confirmed the effect of IN NGF on progenitor survival. Apoptosis is regarded as the main cause of cell death during neurogenesis (CitationBengzon et al., 1997; CitationBiebl et al., 2000). NGF can activate tyrosine kinase A receptor and increase progenitor survival, and further educe anti-apoptotic effects (CitationSofroniew et al., 2001). These effects may partly explain the results observed in this study. Actually, besides the enhanced survival by IN NGF, another possible explanation for the observed increase in the number of striatal BrdU-positive cells following IN NGF treatment is the SVZ cell migration to the striatum, which might be promoted by IN NGF. However, evidence about NGF in the context of in vivo migration of neural progenitors is very limited. The precise role of NGF on migration needs future study.
To examine the neuronal fate of newly generated cells, double immunostaining for BrdU and NeuN was performed on sections from animals which survived for 35 days after MCAO. The results indicated that IN NGF increased the number of newly generated neurons. Interestingly, in the study by CitationFrielingsdorf et al. (2007), the in vivo administration of NGF failed to alter the phenotype of newly generated cells in the DG of aged rats. There are two plausible explanations for this discrepancy. First, different brain regions were tested in two studies. DG detected in the study of CitationFrielingsdorf et al. (2007) may have different responses to NGF compared with the ischemic striatum, because of different cell compositions between these two regions. Second, CitationFrielingsdorf et al. (2007) evaluated neuronal differentiation 15 days after BrdU administration, while we investigated the neuronal fate 28 days after the final BrdU dose, a more adequate time for neuronal maturation. More importantly, in this work, we did not investigate whether the newly formed neurons expressed a phenotype appropriate for the lesioned striatum. NGF has been demonstrated to respond to only cholinergic neurons in the striatum (CitationSofroniew et al., 2001). Further studies are warranted to evaluate the effect of IN NGF on a sub-set of newly generated striatal neurons.
Conclusion
In summary, IN NGF may enhance survival of newly formed neurons and promotes striatal neurogenensis in adult rats after focal cerebral ischemia. These newly formed neurons may integrate into the lesion circuits and improve neurological functions. IN delivery NGF may provide therapeutic potentials for stroke patients.
Acknowledgements
The authors would like to thank Drs Heng-Hui Ma and Yun Li for their technical assistances.
Declaration of Interest
This work is supported by the National Natural Science Foundation of China (30870848), China Postdoctoral Science Foundation (20080431416) and Jinling Hospital Foundation in Nanjing University (2008023). The authors report no conflicts of interest.
References
- Alcalá-Barraza, S.R., Lee, M.S., Hanson, L.R., McDonald, A.A., Frey, W.H., 2nd McLoon, LK. (2010). Intranasal delivery of neurotrophic factors BDNF, CNTF, EPO, and NT-4 to the CNS. J Drug Target. 18:179–90.
- Alsarra, I.A., Hamed, A.Y., Alanazi, F.K. (2008). Acyclovir liposomes for intranasal systemic delivery: development and pharmacokinetics evaluation. Drug Deliv. 15:313–21.
- Arvidsson, A., Collin, T., Kirik, D., Kokaia, Z., Lindvall, O. (2002). Neuronal replacement from endogenous precursors in the adult brain after stroke. Nat Med. 8:963–70.
- Bengzon, J., Kokaia, Z., Elmer, E., Nanobashvili, A., Kokaia, M., Lindvall, O. (1997). Apoptosis and proliferation of dentate gyrus neurons after single and intermittent limbic seizures. Proc Natl Acad Sci USA. 94:10432–7.
- Biebl, M., Cooper, C.M., Winkler, J., Kuhn, H.G. (2000). Analysis of neurogenesis and programmed cell death reveals a self-renewing capacity in the adult rat brain. Neurosci Lett. 291:17–20.
- Calza, L., Giuliani, A., Fernandez, M., Pirondi, S., D’Intino, G., Aloe, L., Giardino, L. (2003). Neural stem cells and cholinergic neurons: regulation by immunolesion and treatment with mitogens, retinoic acid, and nerve growth factor. Proc Natl Acad Sci USA. 100:7325–30.
- Capsoni, S., Covaceuszach, S., Ugolini, G., Spirito, F., Vignone, D., Stefanini, B., Amato, G., Cattaneo, A. (2009). Delivery of NGF to the brain: intranasal versus ocular administration in anti-NGF transgenic mice. J Alzheimers Dis. 16:371–88.
- Chen, J., Li, Y., Wang, L., Zhang, Z., Lu, D., Lu, M., Chopp, M. (2001). Therapeutic benefit of intravenous administration of bone marrow stromal cells after cerebral ischemia in rats. Stroke. 32:1005–11.
- Chen, X.Q., Fawcett, J.R., Rahman, Y.E., Ala, T.A., Frey, W.H. 2nd. (1998). Delivery of nerve growth factor to the brain via the olfactory pathway. J Alzheimers Dis. 1:35–44.
- Ciaroni, S., Cecchini, T., Ferri, P., Cuppini, R., Ambrogini, P., Santi, S., Benedetti, S., Del Grande, P., Papa, S. (2002). Neural precursor proliferation and newborn cell survival in the adult rat dentate gyrus are affected by vitamin E deficiency. Neurosci Res. 44:369–77.
- De Rosa, R., Garcia, A.A., Braschi, C., Capsoni, S., Maffei, L., Berardi, N., Cattaneo, A. (2005). Intranasal administration of nerve growth factor (NGF) rescues recognition memory deficits in AD11 anti-NGF transgenic mice. Proc Natl Acad Sci USA. 102:3811–6.
- Dolbeare, F. (1995). Bromodeoxyuridine: a diagnostic tool in biology and medicine, part I: historical perspectives, histochemical methods and cell kinetics. Histochem J. 27:339–69.
- Frielingsdorf, H., Simpson, D.R., Thal, L.J., Pizzo, D.P. (2007). Nerve growth factor promotes survival of new neurons in the adult hippocampus. Neurobiol Dis. 26:47–55.
- Gonzalez-Perez, O., Quiñones-Hinojosa, A. (2010). Dose-dependent effect of EGF on migration and differentiation of adult subventricular zone astrocytes. Glia. 58:975–83.
- Gotts, J.E., Chesselet, M.F. (2005). Mechanisms of subventricular zone expansion after focal cortical ischemic injury. J Comp Neurol. 488:201–14.
- Gould, E., Gross, C.G. (2002). Neurogenesis in adult mammals: some progress and problems. J Neurosci. 22:619–23.
- Jin, K., Minami, M., Lan, J.Q., Mao, X.O., Batteur, S., Simon, R.P., Greenberg, D.A. (2001). Neurogenesis in dentate subgranular zone and rostral subventricular zone after focal cerebral ischemia in the rat. Proc Natl Acad Sci USA. 98:4710–5.
- Knapp, P.E. (1992). The cell cycle of glial cells grown in vitro: an immunocytochemical method of analysis. J Histochem Cytochem. 40:1405–11.
- Lachyankar, M.B., Condon, P.J., Quesenberry, P.J., Litofsky, N.S., Recht, L.D., Ross, A.H. (1997). Embryonic precursor cells that express Trk receptors: induction of different cell fates by NGF, BDNF, NT-3, and CNTF. Exp Neuro. 144:350–60.
- Lee, T.H., Kato, H., Chen, S.T., Kogure, K., Itoyama, Y. (1998). Expression of nerve growth factor and trkA after transient focal cerebral ischemia in rats. Stroke. 29:1687–96.
- Liu, X.F., Fawcett, J.R., Thorne, R.G., Frey, W.H. 2nd. (2001). Non-invasive intranasal insulin-like growth factor-I reduces infarct volume and improves neurologic function in rats following middle cerebral artery occlusion. Neurosci Lett. 308:91–4.
- Longa, E.Z., Weinstein, P.R., Carlson, S., Cummins, R. (1989). Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke. 20:84–91.
- Ma, M., Ma, Y., Yi, X., Guo, R., Zhu, W., Fan, X., Xu, G., Frey, W.H. 2nd, Liu, X. (2008). Intranasal delivery of transforming growth factor-beta1 in mice after stroke reduces infarct volume and increases neurogenesis in the subventricular zone. BMC Neurosci. 10:117.
- Nakajima, M., Ishimuro, T., Kato, K., Ko, I.K., Hirata, I., Arima, Y., Iwata, H. (2007). Combinatorial protein display for the cell-based screening of biomaterials that direct neural stem cell differentiation. Biomaterials. 28:1048–60.
- Pencea, V., Bingaman, K.D., Wiegand, S.J., Luskin, M.B. (2001). Infusion of brain-derived neurotrophic factor into the lateral ventricle of the adult rat leads to new neurons in the parenchyma of the striatum, septum, thalamus, and hypothalamus. J Neurosci. 21:6706–17.
- Saragovi, H.U., Gehring, K. (2000). Development of pharmacological agents for targeting neurotrophins and their receptors. Trends Pharmacol Sci. 21:93–8.
- Shi, C.G., Wang, L.M., Wu, Y., Wang, P., Gan, Z.J., Lin, K., Jiang, L.X., Xu, Z.Q., Fan, M. (2010). Intranasal administration of nerve growth factor produces antidepressant-like effects in animals. Neurochem Res. 35:1302–14.
- Sofroniew, M.V., Howe, C.L., Mobley, W.C. (2001). Nerve growth factor signaling, neuroprotection, and neural repair. Annu Rev Neurosci. 24:1217–81.
- Taupin, P. (2007). BrdU immunohistochemistry for studying adult neurogenesis: paradigms, pitfalls, limitations, and validation. Brain Res Rev. 53:198–214.
- Thorne, R.G., Emory, C.R., Ala, T.A., Frey, W.H. 2nd. (1995). Quantitative analysis of the olfactory pathway for drug delivery to the brain. Brain Res. 692:278–82.
- Yamashita, T., Ninomiya, M., Hernández Acosta, P., García-Verdugo, J.M., Sunabori, T., Sakaguchi, M., Adachi, K., Kojima, T., Hirota, Y., Kawase, T., Araki, N., Abe, K., Okano, H., Sawamoto, K. (2006). Subventricular zone-derived neuroblasts migrate and differentiate into mature neurons in the post-stroke adult striatum. J Neurosci. 26:6627–36.
- Zhang, R., Zhang, Z., Wang, L., Wang, Y., Gousev, A., Zhang, L., Ho, K.L., Morshead, C., Chopp, M. (2004). Activated neural stem cells contribute to stroke-induced neurogenesis and neuroblast migration toward the infarct boundary in adult rats. J Cereb Blood Flow Metab. 24:441–8.
- Zhang, R.L., Zhang, Z.G., Zhang, L., Chopp, M. (2001). Proliferation and differentiation of progenitor cells in the cortex and the subventricular zone in the adult rat after focal cerebral ischemia. Neuroscience. 105:33–41.