Abstract
Previous study has shown human serum albumin (HSA) coated liposomes can deliver bcl-2 antisense oligodeoxyribonucleotide (ODN) into KB carcinoma cells, and decrease bcl-2 mRNA and protein expression level. In the current study, cell growth inhibition and chemosensitization of KB cells were evaluated. Liposomes composed of dimethyldioctadecyl ammonium bromide/egg phosphatidylcholine/α-tocopheryl polyethylene glycol 1000 succinate (58:40:2 molar ratio) complexed with bcl-2 antisense ODN and coated with HSA were examined for cell growth inhibition and sensitization to a commonly used chemotherapeutic drug, doxorubicin. HSA-coated liposome–ODN complexes effectively inhibited cell growth in the range of ODN concentration of 0.45–7.2 µM. Upon posttreatment with doxorubicin, the cytotoxicity was further significantly increased compared to the ODN complexes alone. The cytotoxicity was dependent on antisense ODN concentration, incubation time and doxorubicin concentration, and relatively independent on HSA concentration. This study suggests that HSA-coated liposomes are effective delivery vehicles for antisense ODN with potential therapeutic application and can be effectively combined with doxorubicin.
Introduction
Cancer is one of the most deadly diseases. Conventional treatments such as radiation therapy and chemotherapy have side-effects. Gene-based therapy is a new approach that selectively targets cancer cells, while reducing toxicity on other cells (CitationPagnan et al., 2000). A potential strategy is to use short stretches of single-stranded oligodeoxyribonucleotides (ODNs) to block unwanted gene expression. The specificity of this strategy has been explored as an alternative therapy with potential clinical application (CitationHerrington et al., 2011; CitationSchimmer et al., 2011).
One of main cell death machinery is regulated by the opposing action of bcl-2 family member (CitationIgney and Krammer, 2002; CitationGreen, 2005). Bcl-2 protein is a target for cancer gene therapy because it exhibits antiapoptotic activity and is overexpressed in many types of cancers. Bcl-2 protein can block the release of cytochrome c into the cytosol after the initiation of apoptosis, which prevents the downstream propagation of the death signal, thereby promoting cell survival (CitationBeck et al., 2002). Overexpression bcl-2 also causes the resistance of cancer cells to a variety of anticancer agents (CitationSartorius and Krammer, 2002; CitationZangemeister-Wittke, 2003). Preclinical and clinical studies have established that downregulation of bcl-2 protein leads to an increase in apoptosis and improved response to chemotherapy (CitationChi et al., 2000; CitationKirkwood et al., 2005). Therefore, targeted inhibition of bcl-2 expression has the potential to facilitate tumor cell apoptosis and sensitize cells to a broad spectrum of anticancer agents (CitationGeorge et al., 2009; CitationGarbuzenko et al., 2010).
Several antisense oncology trials could not fulfill the initial goals, which have raised doubts about the clinical potential of this technology (CitationGleave and Monia, 2005). A major problem of antisense ODN administration, which limits its therapeutic activity, is low cellular uptake (CitationArima et al., 1997; CitationLe Doan, 2001). Patients with locally advanced breast cancer received bcl-2 antisense ODN by continuous intravenous infusion had limited bcl-2 down regulation in the tumors, related with insufficient delivery to the intact tumors (CitationMoulder et al., 2008).
Improving the efficacy of therapy, drug delivery systems based on lipids, such as cationic liposomes, have potential usefulness to improve the intracellular delivery of antisense ODNs (CitationPastorino et al., 2008). For example, a cholesterol-derived cationic lipid for delivery of plasmid DNA (CitationKim et al., 2009), membrane sensitive peptide-derived cationic lipid for delivery of antisense ODN (CitationJääskeläinen et al., 2000), and amino acid-derived cationic lipid has been used for delivery of siRNA (CitationSuh et al., 2009).
Our previous study has investigated human serum albumin (HSA)-coated liposomes could deliver bcl-2 antisense ODN into KB carcinoma cells and decrease bcl-2 mRNA and protein expression. (CitationWeecharangsan et al., 2009). In current study, we examined cell growth inhibition and sensitization induced by a commonly used chemotherapeutic drug, doxorubicin, after transfecting bcl-2 antisense ODN by HSA-coated liposomes.
Materials and methods
Dimethyldioctadecyl ammonium bromide (DDAB), α-tocopheryl polyethylene glycol 1000 succinate (TPGS), and HSA were purchased from Sigma-Aldrich (St. Louis, MO, USA). Egg phosphatidylcholine (PC) was obtained from Lipoid GMBH (Ludwigshafen, Germany). Doxorubicin was purchased from Pfizer (Bentley, Australia). Bcl-2 antisense ODN, fully phosphorothioated 18-mer oliogonucleotide (Sequence 5′–3′: TCT CCC AGC GTG CGC CAT) was purchased from Alpha DNA (Quebec, Canada). Lipofectamine™ 2000 reagent, MEM media and fetal bovine serum (FBS) were purchased from Invitrogen (Grand Island, NY, USA). 96-well plates were purchased from Corning Inc. (Corning, NY, USA).
Preparation of cationic liposomes and HSA liposome–ODN complexes
Cationic liposomes were prepared from DDAB, PC, and TPGS by ethanol dilution as described previously (CitationMaurer et al., 2001) with minor modification. Briefly, DDAB, PC, and TPGS were dissolved in ethanol at a molar ratio of 58:40:2 and injected into a stirring HEPES buffered solution (20 mM HEPES, pH 7.4) at room temperature. Cationic liposome–ODN complexes were prepared by mixing cationic liposomes with an equal volume of ODN at a lipid-to-ODN ratio of 15:1 in MEM and incubated at room temperature for 15 min. HSA was directly added to the liposome–ODN complexes at molar ratios to lipid of 0.15:100, 0.75:100, 1.5:100, 3:100, 4.5:100, and 9:100, and incubated for 15 min before use in transfection. The concentration of ODN was 0.45, 0.9, 1.8, 3.6, and 7.2 µM.
Cell culture
KB human oral carcinoma cells were obtained from Natural Products Research Section, Reasearch Division, National Cancer Institute, Bangkok, Thailand. KB cells have been reported to display HeLa markers and thus are possibly a subline of HeLa cervical cancer cells. The cells were grown in MEM containing 10% FBS, 100 µg/mL streptomycin, 100 U/mL penicillin, and 1% amphotericin B. The cells were maintained at 37°C in a humidified incubator with 5% CO2.
Growth inhibition
Evaluation of growth inhibition was performed by 3-(4,5-dimethylthiazol-2-yl)-2,5- diphenyl tetrazolium bromide (MTT) assay. KB cells were seeded in a 96-well plate at a density of 7 × 103 cells/cm2 in 100 µL of growth medium and incubated for 16 h at 37°C under 5% CO2 atmosphere. Prior to treatment, the medium was removed and the cells were rinsed with PBS. The cells were incubated with 62.5 µL of HSA liposome–ODN complexes and blank liposomes for 6 h at 37°C under 5% CO2 atmosphere. Untreated cells and cells transfected with free ODN and Lipofectamine™ 2000 (0.23 µL per well)–ODN complexes were used as controls. After transfection, the cells were washed with PBS and continued to culture in 100 µL of fresh growth medium at 37°C under 5% CO2 atmosphere for 72 h. After incubation the viability of cell was determined by the MTT assay. Relative growth inhibition (%) was calculated based on absorbance at 570 nm using a microplate spectrophotometer (Zenyth 200 rt; Anthos Labtech Instruments GmbH, Salzburg, Austria). The viability of untreated control cells was arbitrarily defined as 100%.
Chemosensitization
KB cells were seeded into 96-well plates at a density of 7 ×103 cells/cm2 in 100 µL of growth medium and incubated for 16 h. Prior to treatment, the medium was removed and the cells were rinsed with PBS. The cells were incubated with 62.5 µL of HSA liposome–ODN complexes for 6 h at 37°C under 5% CO2 atmosphere. Untreated cells and cells transfected with free ODN were used as controls. After transfection, the medium was replaced with 100 µL of fresh growth medium, and the cells were incubated for 24 h at 37°C under 5% CO2 atmosphere. Cells were then incubated with medium containing doxorubicin at 0.1, 0.25, 0.5, or 0.75 µM. After another 24, 48, or 72 h, cytotoxicity was determined by the MTT assay. Relative cytotoxicity (%) was calculated based on absorbance at 570 nm using a microplate spectrophotometer (Zenyth 200 rt; Anthos Labtech Instruments GmbH, Salzburg, Austria). The cytotoxicity of untreated control cells was arbitrarily defined as 0%.
Statistical analysis
The results are represented as the mean ± SD of three repeat studies. Statistical significance of differences in growth inhibition and cytotoxicity were examined using one-way ANOVA followed by an LSD post hoc test.
Results
HSA-coated liposome–ODN complexes on KB cell growth inhibition
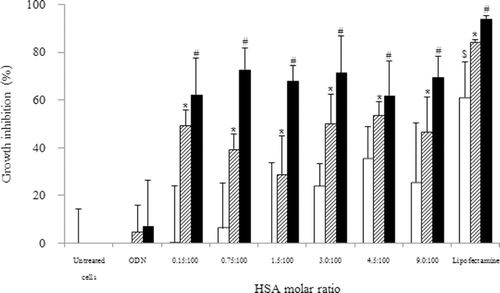
The growth inhibition on KB cells following 72 h incubation with HSA-coated liposome–ODN complexes, Lipofectamine-ODN complexes, free ODN at ODN concentration of 1.8 and 3.6 µM, and liposome alone is shown in . HSA-coated liposome–ODN complexes inhibited cell growth at ODN concentration of 1.8, and extensively at 3.6 µM. The cell growth inhibition level was relatively independent on the molar ratio of HSA-to-lipid of 0.15:100 to 9:100 in ODN complex formulation. HSA-coated liposome–ODN complexes inhibited cell growth to 29.0 ± 15.7 to 53.5 ± 5.6% and 61.8 ± 14.4 to 72.8 ± 8.8% that of the control at ODN concentrations of 1.8 (p < 0.05) and 3.6 µM (p < 0.001), respectively. This finding shows that bcl-2 downregulation of HSA-coated liposome–ODN complexes was effective in inhibiting cancer cell growth with increasing ODN concentration. Our previous study showed that HSA enhanced the uptake liposome–ODN complexes (CitationWeecharangsan et al., 2009), however, the cytotoxicity relatively independent on the concentrations of HSA. HSA-coated liposomes at the molar ratios of HSA of 3:100 to 9:100 slightly inhibited cell growth to 23.9 ± 9.5 to 35.5 ± 13.3% (p > 0.05). HSA-coated liposomes at the molar ratios of HSA of 0.15:100 to 1.5:100 had no effect on the level of cell growth. We, therefore, selected HSA-coated liposome at the HSA of molar ratio of 1.5:100 for further study. Lipofectamine-ODN complexes, as a positive control, at ODN concentrations of 1.8 and 3.6 µM caused cell growth inhibition of 84.4 ± 0.7 (p < 0.05) and 94.1 ± 1.1% (p <0.001), respectively. Lipofectamine alone inhibited cell growth to 61.1 ± 14.9% (p < 0.05).
Figure 1. Effect of HSA concentration of HSA-coated liposome/ODN complexes on the growth of KB cells. Cells were incubated with HSA-coated liposome/ODN complexes for 6 h and in growth medium for 72 h. White bar: HSA-coated liposome; dash bar: ODN concentration of 1.8 µM; dark bar: ODN concentration of 3.6 µM. *p < 0.05; #p < 0.001 compared with cells treated with ODN; $p < 0.05 when compared with untreated cells. HSA, human serum albumin; ODN, oligodeoxyribonucleotide.
Sensitization of KB cells to doxorubicin treatment by HSA-coated liposome–ODN complexes
Chemoresistance reversion and cell sensitization to anticancer chemotherapy drug by bcl-2 downregulation was studied in KB cells with a commonly used anticancer drug, doxorubicin. KB cells were pretreated with HSA-coated liposome–ODN complexes for six following in growth medium for 24 h, prior to treatment with doxorubicin for another 48 h (). When the bcl-2 ODN was delivered as HSA-coated liposome complexes, KB cells became more susceptible to the cytotoxic effect of doxorubicin compared to the cells treated with doxorubicin and bcl-2 ASO. HSA-coated liposome–ODN complexes significantly improved cytotoxicity at ODN concentration of 1.8 µM (p < 0.05), and extensively at 3.6 µM (p < 0.001) in combination with 0.5 μM doxorubicin.
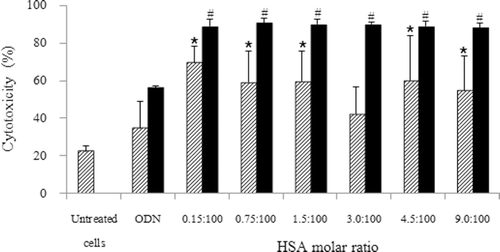
Figure 2. Effect of HSA concentration of HSA-coated liposome/ODN complexes on the sensitization to doxorubicin in KB cells. Cells were incubated with HSA-coated liposome/ODN complexes for 6 h, in growth medium for 24 h, and in growth medium containing 0.5 µM doxorubicin for 48 h. Dash bar: ODN concentration of 1.8 µM; dark bar: ODN concentration of 3.6 µM. *p < 0.05; #p < 0.001 when compared with cells treated with 0.5 µM doxorubicin. HSA, human serum albumin; ODN, oligodeoxyribonucleotide.
Effect of HSA concentration
Treatment with doxorubicin alone had a slight cytotoxicity of 22.3 ± 2.9%, while pretreatment with free ODN at a concentration of 1.8 μM followed by doxorubicin had a slight cytotoxicity of 34.7 ± 14.0% (p > 0.05) in KB cells. The combination of pretreatment with HSA-coated liposome–ODN complexes at the molar ratio of HSA of 0.15:100 to 9:100 followed by doxorubicin resulted in an improvement of cytotoxicity on KB cells from 42.2 ± 14.4 to 69.6 ± 8.3%. The cytotoxicity level was relatively independent on the ratio of HSA-to-lipid from 0.15:100 to 9:100 in the liposome formulation ().
Pretreatment with free ODN at a concentration of 3.6 μM followed by doxorubicin had a moderate cytotoxicity of 56.2 ± 1.0% (p > 0.001), while the combination of pretreatment with HSA-coated liposome–ODN complexes followed by doxorubicin treatment resulted in a significant improvement of cytotoxicity on KB cells from 88.2 ± 2.6 to 90.7 ± 2.5% (p < 0.001). The different ratio of HSA in HSA-coated liposome–ODN complexes had no effect on the level of cytotoxicity ().
Effect of ODN concentration
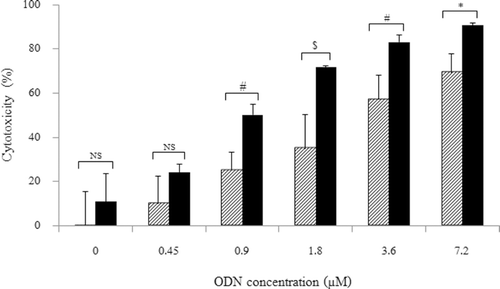
The cytotoxicity of HSA-coated liposome–ODN complexes was studied following treatment with and without doxorubicin at a concentration of ODN of 0–7.2 μM (). Treatment the cells with HSA-coated liposome–ODN complexes followed by doxorubicin treatment slightly improved the cytotoxicity at ODN concentration from 0.45 μM to 9.9 ± 12.4% (p > 0.05). Increasing the concentration of ODN of 0.9–7.2 μM in HSA-coated liposome formulation dramatically increased the cytotoxicity on KB cells from 25.0 ± 8.3 to 69.4 ± 8.6%. The cytotoxicity on KB cells treated with HSA-coated liposome–ODN complexes followed by doxorubicin significantly improved from 50.2 ± 4.7 to 90.7 ± 1.0% at ODN concentrations of 0.9–7.2 μM, respectively. This shows that the cytotoxicity level was dependent on the concentration of ODN in the liposome formulation and the KB cells treated with doxorubicin was significantly sensitized by HSA-coated liposome–ODN complexes at a concentration of ODN of 0.9–7.2 μM, respectively. The IC50 of KB cells treated with HSA-coated liposome–ODN complexes was 3.2 ± 1.3 μM, and reduced to 0.9 ± 0.1 μM in the cells treated with HSA-coated liposome–ODN complexes followed by doxorubicin treatment.
Figure 3. Effect of ODN concentration of HSA-coated liposome/ODN complexes on the sensitization to doxorubicin in KB cells. Cells were incubated with HSA-coated liposome/ODN complexes at HSA to liposome molar ratio of 1.5:100 for 6 h, in growth medium for 24 h, and in growth medium containing 0.5 µM doxorubicin for 48 h. Dash bar: without 0.5 µM doxorubicin; dark bar: with 0.5 µM doxorubicin. NS, not significant; *p < 0.05; #p < 0.005; $p < 0.001 when compared with untreated cells. HSA, human serum albumin; ODN, oligodeoxyribonucleotide.
Effect of incubation time
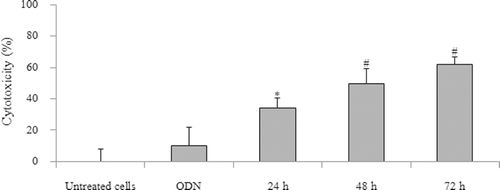
Effect of incubation time of doxorubicin of 24, 48, and 72 h on KB cytotoxicity after pretreatment with HSA-coated liposome–ODN complexes at an ODN concentration of 0.9 μM is shown in . Cytotoxicity after pretreatment with HSA-coated liposome–ODN complexes significantly increased with increased incubation time of doxorubicin from 24 to 72 h.
Figure 4. Effect of incubation time of doxorubicin treatment on KB cell after treatment with HSA-coated liposome/ODN complexes. Cells were incubated with HSA-coated liposome/ODN complexes at HSA to liposome molar ratio of 1.5:100 and ODN concentration of 0.9 µM for 6 h, in growth medium for 24 h, and in growth medium containing 0.75 µM doxorubicin for 24, 48 and 72 h. *p < 0.005; #p < 0.001 compared with cells treated with ODN. HSA, human serum albumin; ODN, oligodeoxyribonucleotide.
Effect of doxorubicin concentration
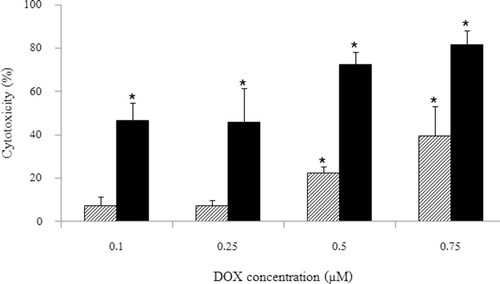
The cytotoxicity of KB cells pretreated with HSA-coated liposome–ODN complexes at an ODN concentration of 0.9 μM after 24 h ODN treatment following 48 h incubation with doxorubicin at concentrations of 0.1, 0.25, 0.5, and 0.75 μM is shown in . The cytotoxicity of KB cells pretreated with HSA-coated liposome–ODN complexes significantly improved following 48 h incubation with doxorubicin at the concentrations of 0.5 and 0.75 μM (p < 0.001). The IC50 of KB cells treated with doxorubicin was >0.75 μM, and reduced to 0.25 ± 0.14 μM in the cells treated with HSA-coated liposome–ODN complexes following doxorubicin treatment.
Figure 5. Effect of doxorubicin concentration on KB cells after treatment with HSA-coated liposome/ODN complexes. Cells were incubated with HSA-coated liposome/ODN complexes at HSA to liposome molar ratio of 1.5:100 and ODN concentration of 0.9 µM for 6 h, in growth medium for 24 h, and in growth medium containing 0.1, 0.25, 0.5, and 0.75 µM doxorubicin for 48 h. Dash bar: untreated cells; dark bar: HSA liposome/ODN complexes. *p < 0.001 when compared with untreated cells. HSA, human serum albumin; ODN, oligodeoxyribonucleotide.
Discussion
Our previous study showed that HSA-coated liposomes are effective delivery vehicle for bcl-2 antisense ODN and induced downregulation of bcl-2 in KB cells (CitationWeecharangsan et al., 2009). In the current study, we further evaluated bcl-2 antisense ODN delivered by HSA-coated liposomes on KB cell growth and chemosensitization with a commonly used anticancer chemotherapy drug, doxorubicin. We used KB oral carcinoma cells as a representative of human carcinoma cells. We found that HSA-coated bcl-2 antisense ODN–liposome complexes could effectively inhibit cell growth and sensitize KB cells treated with doxorubicin. The cytotoxicity was dependent on the ODN concentration, incubation time, doxorubicin concentration, and relatively not on the HSA concentration. In order to exclude unspecific toxic effects, we tested HSA-coated liposomes alone on the cytotoxic effect. We found that HSA-coated liposomes at the molar ratios of HSA from 3:100 to 9:100 slightly inhibited cell growth. HSA-coated liposomes at the molar ratios of HSA from 0.15:100 to 1.5:100 had no effect on the level of cell growth.
Our study showed that HSA-coated liposome–ODN complexes inhibited KB cell growth at an ODN concentration of 1.8, and extensively at 3.6 µM. CitationDoi et al. (2004) showed that the cell viability of H69 small cell lung cancer cells treated with bcl2-ODN in the form of lipoplexes was dose-dependent. Cytotoxicity in B16 (F10) melanoma cells transfected with cationic liposomes loaded with proapoptotic peptide and bcl-2 ODN increased with increasing ODN concentration (CitationKo et al., 2009).
Our preliminary study on ODN treatment (24 and 48 h) following doxorubicin treatment showed that 24 h ODN treatment following doxorubicin treatment was the most effective cytotoxic effect. Therefore, we investigated the chemosensitization of KB cells after 24 h ODN treatment following 24–72 h doxorubicin treatment. Doxorubicin treatment slightly reduced the cell growth. The combination of HSA-coated liposome–ODN complexes with a subsequent treatment with doxorubicin substantially increased the cytotoxicity. Our result showed that treatment of KB cells with low concentration of 0.45 µM by HSA-coated liposome–ODN complexes did not significantly inhibit cell growth following doxorubicin treatment. This suggests that a low concentration of bcl-2 ODN might not be adequate to initiate cell death. Increased ODN concentrations of 0.9–7.2 µM in HSA-coated liposome–ODN complexes were therefore much more effective. The high ODN concentration itself may have potential cytotoxic effect and may not be applicable in clinical use; therefore, the study on the effect of incubation time and doxorubicin concentration was used with ODN concentration of 0.9 µM.
The cytotoxicity of KB cells after pretreatment with HSA-coated liposome–ODN complexes increased with increased incubation time of doxorubicin from 24 to 72 h. This result showed that optimal incubation time after ODN treatment is necessary. CitationLima et al. (2004) showed that the effective reduction in MCF-7 human breast cancer cell proliferation was 48–96 h cytotoxic drug treatment after siRNA transfection. Growth inhibition of GI-LI-N human neuroblastoma cells treated by c-myb antisense oligodeoxynucleotides encapsulated in anti-disialoganglioside GD liposomes increased with increasing postincubation period and decreasing c-myb protein expression (CitationPagnan et al., 2000).
The cytotoxicity of KB cells after pretreatment with HSA-coated liposome–ODN complexes increased with increased doxorubicin concentration from 0.1 to 0.75 µM. CitationHussain et al. (2006) showed that cytotoxicity increased with increasing concentration of doxorubicin in SW2 small cell lung cancer and MCF-7 breast adenocarcinoma cells pretreated with bcl-2 and bcl-xL antisense ODN-loaded immunoliposomes. Cytotoxicity on CD133+ cells after silencing of MDR1 by MDR1 siRNA PEI-lipid complexes increased with increasing paclitaxel concentration (CitationLiu et al., 2009). Apoptosis of U138MG and U251MG human glioblastoma cells treated with taxol was dose-dependent in combination with bcl-2 siRNA duplex (CitationGeorge et al., 2009).
Conclusion
In the present study, bcl-2 antisense ODN delivered with HSA-coated liposomes effectively reduced the growth of KB oral carcinoma cells and significantly increased the chemosensitivity to doxorubicin. This study suggests that HSA-coated liposomes are effective delivery vehicles for antisense ODN with potential therapeutic application and can be effectively combined with doxorubicin, and have potential to apply with other antisense ODN and carcinoma cells.
Declaration of interest
This study was financially supported by Srinakharinwirot University (No. 069/2554). The authors report no declarations of interest.
References
- Pagnan G, Stuart DD, Pastorino F, Raffaghello L, Montaldo PG, Allen TM, Calabretta B, Ponzoni M. (2000). Delivery of c-myb antisense oligodeoxynucleotides to human neuroblastoma cells via disialoganglioside GD(2)-targeted immunoliposomes: antitumor effects. J Natl Cancer Inst 92:253–261.
- Schimmer AD, Herr W, Hänel M, Borthakur G, Frankel A, Horst HA, Martin S, Kassis J, Desjardins P, Seiter K, Fiedler W, Noppeney R, Giagounidis A, Jacob C, Jolivet J, Tallman MS, Koschmieder S. (2011). Addition of AEG35156 XIAP antisense oligonucleotide in reinduction chemotherapy does not improve remission rates in patients with primary refractory acute myeloid leukemia in a randomized phase II study. Clin Lymphoma Myeloma Leuk 11:433–438.
- Herrington WG, Talbot DC, Lahn MM, Brandt JT, Callies S, Nagle R, Winearls CG, Roberts IS. (2011). Association of long-term administration of the survivin mRNA-targeted antisense oligonucleotide LY2181308 with reversible kidney injury in a patient with metastatic melanoma. Am J Kidney Dis 57:300–303.
- Green DR. (2005). Apoptotic pathways: ten minutes to dead. Cell 121:671–674.
- Igney FH, Krammer PH. (2002). Death and anti-death: tumour resistance to apoptosis. Nat Rev Cancer 2:277–288.
- Beck MT, Peirce SK, Chen WY. (2002). Regulation of bcl-2 gene expression in human breast cancer cells by prolactin and its antagonist, hPRL-G129R. Oncogene 21:5047–5055.
- Sartorius UA, Krammer PH. (2002). Upregulation of Bcl-2 is involved in the mediation of chemotherapy resistance in human small cell lung cancer cell lines. Int J Cancer 97:584–592.
- Zangemeister-Wittke U. (2003). Antisense to apoptosis inhibitors facilitates chemotherapy and TRAIL-induced death signaling. Ann N Y Acad Sci 1002:90–94.
- Chi KC, Wallis AE, Lee CH, De Menezes DL, Sartor J, Dragowska WH, Mayer LD. (2000). Effects of Bcl-2 modulation with G3139 antisense oligonucleotide on human breast cancer cells are independent of inherent Bcl-2 protein expression. Breast Cancer Res Treat 63:199–212.
- Kirkwood JM, Bedikian AY, Millward MJ, Conry RM, Gore ME, Pehamberger HE, Sterry W, Pavlick AC, Deconti RC, Itri LM. (2005). Long-term survival results of a randomized multinational phase 3 trial of dacarbazine (DTIC) with or without Bcl-2 antisense (oblimersen sodium) in patients (pts) with advanced malignant melanoma (MM). J Clin Oncol ASCO Ann Meet Proc 23:7506.
- George J, Banik NL, Ray SK. (2009). Bcl-2 siRNA augments taxol mediated apoptotic death in human glioblastoma U138MG and U251MG cells. Neurochem Res 34:66–78.
- Garbuzenko OB, Saad M, Pozharov VP, Reuhl KR, Mainelis G, Minko T. (2010). Inhibition of lung tumor growth by complex pulmonary delivery of drugs with oligonucleotides as suppressors of cellular resistance. Proc Natl Acad Sci USA 107:10737–10742.
- Gleave ME, Monia BP. (2005). Antisense therapy for cancer. Nat Rev Cancer 5:468–479.
- Arima H, Aramaki Y, Tsuchiya S. (1997). Effects of oligodeoxynucleotides on the physicochemical characteristics and cellular uptake of liposomes. J Pharm Sci 86:438–442.
- Le Doan T. (2001). Cell binding and internalisation of oligonucleotides. STP Pharma Sci 11:75–82.
- Moulder SL, Symmans WF, Booser DJ, Madden TL, Lipsanen C, Yuan L, Brewster AM, Cristofanilli M, Hunt KK, Buchholz TA, Zwiebel J, Valero V, Hortobagyi GN, Esteva FJ. (2008). Phase I/II study of G3139 (Bcl-2 antisense oligonucleotide) in combination with doxorubicin and docetaxel in breast cancer. Clin Cancer Res 14:7909–7916.
- Pastorino F, Mumbengegwi DR, Ribatti D, Ponzoni M, Allen TM. (2008). Increase of therapeutic effects by treating melanoma with targeted combinations of c-myc antisense and doxorubicin. J Control Release 126:85–94.
- Kim BK, Doh KO, Nam JH, Kang H, Park JG, Moon IJ, Seu YB. (2009). Synthesis of novel cholesterol-based cationic lipids for gene delivery. Bioorg Med Chem Lett 19:2986–2989.
- Jääskeläinen I, Peltola S, Honkakoski P, Mönkkönen J, Urtti A. (2000). A lipid carrier with a membrane active component and a small complex size are required for efficient cellular delivery of anti-sense phosphorothioate oligonucleotides. Eur J Pharm Sci 10:187–193.
- Suh MS, Shim G, Lee HY, Han SE, Yu YH, Choi Y, Kim K, Kwon IC, Weon KY, Kim YB, Oh YK. (2009). Anionic amino acid-derived cationic lipid for siRNA delivery. J Control Release 140:268–276.
- Weecharangsan W, Yu B, Zheng Y, Liu S, Pang JX, Lee LJ, Marcucci G, Lee RJ. (2009). Efficient delivery of antisense oligodeoxyribonucleotide g3139 by human serum albumin-coated liposomes. Mol Pharm 6:1848–1855.
- Maurer N, Wong KF, Stark H, Louie L, McIntosh D, Wong T, Scherrer P, Semple SC, Cullis PR. (2001). Spontaneous entrapment of polynucleotides upon electrostatic interaction with ethanol-destabilized cationic liposomes. Biophys J 80:2310–2326.
- Doi S, Soda H, Oka M, Tsurutani J, Kitazaki T, Nakamura Y, Fukuda M, Yamada Y, Kamihira S, Kohno S. (2004). The histone deacetylase inhibitor FR901228 induces caspase-dependent apoptosis via the mitochondrial pathway in small cell lung cancer cells. Mol Cancer Ther 3:1397–1402.
- Ko YT, Falcao C, Torchilin VP. (2009). Cationic liposomes loaded with proapoptotic peptide D-(KLAKLAK)(2) and Bcl-2 antisense oligodeoxynucleotide G3139 for enhanced anticancer therapy. Mol Pharm 6:971–977.
- Lima RT, Martins LM, Guimarães JE, Sambade C, Vasconcelos MH. (2004). Specific downregulation of bcl-2 and xIAP by RNAi enhances the effects of chemotherapeutic agents in MCF-7 human breast cancer cells. Cancer Gene Ther 11:309–316.
- Hussain S, Plückthun A, Allen TM, Zangemeister-Wittke U. (2006). Chemosensitization of carcinoma cells using epithelial cell adhesion molecule-targeted liposomal antisense against bcl-2/bcl-xL. Mol Cancer Ther 5:3170–3180.
- Liu C, Zhao G, Liu J, Ma N, Chivukula P, Perelman L, Okada K, Chen Z, Gough D, Yu L. (2009). Novel biodegradable lipid nano complex for siRNA delivery significantly improving the chemosensitivity of human colon cancer stem cells to paclitaxel. J Control Release 140:277–283.