Abstract
The purpose of the present study was to evaluate the tissue distribution and antitumor activity of 2-methoxyestradiol (2-ME) nanosuspension compared with 2-ME solution both in vitro and in vivo. 2-ME nanosuspension was made by nanoprecipitation-high-frequency ultrasonication method with the particle size of 168.4 ± 3.2 nm and the zeta potential of −29.79 ± 1.89 mV. The overall targeting efficiency (TEQ) of 2-ME nanosuspension was improved from 28.71 to 51.95% in the lung of rats. MTT assay showed that 2-ME nanosuspension could significantly enhance the in vitro cytotoxicity against lewis lung carcinoma (LLC) cells compared with the 2-ME solution, the IC50 at 72 h was reduced from 6.35 µM for 2-ME solution to 3.56 µM for 2-ME nanosuspension. The antitumor activity in vivo was investigated in C57BL/6 mice bearing LLC, and the results indicated that 2-ME nanosuspension not only exhibited significant suppression of the tumor growth when compared with that of positive group or cyclophosphamide group at the same dose, but also enhanced the spleen indices. Overall, 2-ME nanosuspension could mainly deliver the drug to lungs and made the drug accumulate in the lungs, so 2-ME nanosuspension has a possible lung cancer therapeutic potential.
Introduction
Lung cancer is the most common malignancy in the world and the first leading cause of deaths from cancer. Small cell lung cancer and non-small cell lung cancer are the two types of lung cancer with prevalence rates of 14 and 85%, respectively (Spivey et al., Citation2010). Surgery combined with chemotherapy is main clinical therapy for lung cancer. Conventional lung cancer treatments show poor clinical response, thus it is important to develop novel treatment strategies (Sève et al., Citation2010). 2-Methoxyestradiol (2-ME), chemical structure shown in , is an endogenous metabolite of estrogen. It inhibits growth of cancer cells, including melanoma, prostate cancer, breast cancer, lung cancer, etc (Dobos et al., Citation2004; Garcia et al., Citation2006; Huh et al., Citation2006) via the inhibition of the pro-angiogenic transcription factor hypoxia-inducible factor 1-α and the induced apoptosis of cancer cells (Chauhan et al., Citation2003). However, the poor solubility of 2-ME in water and the liver first pass effect have presented a serious obstacle for its practical use as a therapeutic agent (Ireson et al., Citation2004; Sweeney et al., Citation2005).
Recently, the formulations containing nanosized drug particles have been found to be promising candidates for enhancing the solubility of poorly water-soluble drugs. Nanosuspension could enhance the in vitro drug release and intestinal epithelium membrane permeability and improve the in vivo bioavailability (Wang et al., Citation2010). As a consequence, 2-ME nanosuspension was developed to overcome its defect in our previous studies (Du et al., Citation2010). The present research was designed to explore the tissue distribution, in vitro and in vivo antitumor activity of 2-ME nanosuspension in comparison with free 2-ME solution.
Materials and methods
Materials
2-ME (purity > 99%) was home made, phosphatidylcholine (PC, injection grade) was purchased from Siwei (Zhengzhou, China). Poloxamer188 (P188) and sodium carboxymethycellulose (CMC-Na) were obtained from Shenyang liqi Pharmaceutical Company (Shenyang, China). Sodium lauryl sulfate (SLS) was from Sigma (California, CA, USA). Dulbecco’s Modified Eagle Medium (DMEM) (high glucose) was purchased from Hyclone Laboratories (Logan, UT, USA). Fetal bovine serum (FBS) was supplied by Tissue Culture Biologicals (Long Beach, CA, USA). Penicillin and streptomycin were provided by Sigma (California, CA, USA). All the other chemicals and solvents utilized were of chromatographic and pharmaceutical grade.
Animals and cell line
Female Sprague–Dawley rats (200 ± 20 g) and female C57BL/6 mice (6–8 weeks of age) were supplied by Medical Animal Test Center of Zhengzhou University (Zhengzhou, China) and Experimental Animal Center of Academy of Military Medical Sciences (Beijing, China), respectively. The mice and rats used were maintained under specific pathogen-free conditions. Animals, housed in laminar-flow cages, were acclimatized to a temperature of 25 ± 2°C at a relative humidity of 75 ± 5% under natural light/dark conditions for one week before experiment. All experimental procedures comply with the principles of care and use of laboratory animals and were approved by the Institutional Animal Care and Use Committee of Zhengzhou University Health Science Center. Lewis lung carcinoma (LLC) cells were purchased from Shanghai Institute of Cell Resource Center of Life Sciences (Shanghai, China).
Preparation of 2-ME nanosuspension and 2-ME solution
2-ME nanosuspension was prepared by the nanoprecipitation-high-frequency ultrasonication technique (Hany et al., Citation2009; Müller and Peters, Citation1998). Briefly, required amount of 2-ME, phosphatidylcholine (3%, w/v) were dissolved in absolute alcohol. P188 (3%, w/v), CMC-Na (0.6%, w/v) and SLS (0.1%, w/v) were dispersed in phosphate-buffered saline (PBS, pH 7.4) to obtain the aqueous surfactant solution (Lu, Citation2005; Peters et al., Citation2000). The organic solution was injected into the aqueous solution under rapid stirring at 100 g for 30 min at 50°C. The primary slurry was dispersed by high speed stirrer (ultra-turrax T25, Germany) at 20,000 g for 5 min. The organic solvents were removed in the rotary evaporator at 30°C under vacuum pressure. The resultant nanosuspension was subjected to probe-ultrasonicator (400 w, 40 cycles/3s) (LTD JY92-II, Scientz Biotechnology Co., Ningbo, China) in an ice-bath. Homogeneous 2-ME nanosuspension, containing 2-ME of 3 mg per milliliter, was produced.
The mean particle size (z-average) and the polydispersity index (PDI) were measured by Zetasizer-nano-ZS90 (Malvern Instruments, Malvern, UK). Each of samples was diluted with PBS to get a suitable scattering intensity for collecting final experimental values.
Preparation of 2-ME solution: 2 mg/mL 2-ME solution was prepared by solving 2-ME in hydrogenated castoroil, alcohol and 0.9% saline solution mixture (1.5:0.5:8, v/v).
HPLC analysis
2-ME concentrations in solution were detected by Agilent 1200 series system (Agilent, USA). An octadecyl silane column (4.6 × 150 mm, 5 µm) (Agilent, USA) was used. The mobile phase was composed of 65% water and 35% methanol. The column was eluted at a flow rate of 1 mL/min at 30°C. The fluorescence detector was used and the excited wavelength and emission wavelength were 285 and 325 nm, respectively (Du et al., Citation2009).
Tissue distribution study
Two groups of 60 rats were used for the distribution studies of 2-ME in vivo. One group was administered with 2-ME nanosuspension and another group was administered with 2-ME solution as control. All animals received a single i.v. injection of 2-ME solution or nanosuspension at a dose of 10 mg/kg. The rats were anesthetized by inhalation of diethyl ether. Five rats in each group were taken out randomly at each time point (0.25, 0.5, 1, 2, 4, 8 h) after i.v. administration and sacrificed. The tissues, including heart, liver, spleen, lung, kidney, were harvested. Every organ sample was washed, weighed and homogenized. 0.5 mL aliquot of homogenized tissue was mixed with a 10 µL letrozole (internal standard), followed by the addition of 3 mL of ethyl acetate to fully extracted 2-ME and letrozole. The sample was then vigorously vortex-mixed for 3 min and centrifuged for 20 min at 2,000 g. The supernatant was collected to a clean microtube and evaporated under a gentle stream of nitrogen gas at 40°C. Finally, the residue was redissolved in 100 µL mobile phase, following centrifugation at 20,000 g for 15 min. 20 µL of the upper layer was injected into the HPLC system. The chromatographic analysis conditions were the same as mentioned above.
Overall targeting efficiency (TEQ) of 2-ME nanosuspension were calculated and compared with that of 2-ME solution to evaluate the tissue targeting property of 2-ME nanosuspension. TEQ can be calculated according to Eq. (1):
in which the denominator refers to the sum of drug exposure to all the tissues, including the target tissue.
In vitro cytotoxicity of 2-ME nanosuspension against LLC cells
LLC cells were maintained in DMEM medium (high glucose) supplemented with 10% FBS, 100 U/mL penicillin and 100 µg/mL streptomycin. Cells were cultured in a humidified atmosphere containing 5% CO2 at 37°C. The medium was exchanged every 2–3 days. In vitro cytotoxicity was determined using a standard MTT assay with protocol appropriate for the individual test system. In brief, exponentially growing LLC cells were plated into 96-well plates (5 × 103 cells/well in 100 µL of medium) for 18 h, and were incubated with 2-ME nanosuspension and the 2-ME solution (at concentrations of 0.1, 0.5, 1, 2, 5, 10 and 20 µM) for 24, 48 and 72 h, respectively. The control group was treated with the same volume of culture medium with 0.1% DMSO. Then, 20 µL MTT (5 mg/mL in PBS) was added to each cell and the plates were incubated for an additional 4 h. The medium was then replaced with 150 µL DMSO to solubilize the formazan produced by the viable cells. The absorbance of each well was recorded at 490 nm (Mosmann, Citation1983) by the microplate reader and the cell survival ratio was calculated according to Eq. (2).
All assays were done with six parallel samples. The cytotoxicity of 2-ME nanosuspension was expressed as IC50 that was defined as the drug concentration required to inhibit 50% of cell growth (Huang et al., Citation2010).
In vivo antitumor activity of 2-ME nanosuspension in C57BL/6 mice with LLC solid tumor
LLC solid tumor model and treatment regimen
LLC cells were kept in DMEM medium supplemented with 10% FBS, 100 U/mL penicillin and 100 µg/mL streptomycin. Female C57BL/6 mice (6–8 weeks old and weighing approximately 18–20 g) were used for the study. In the solid tumor model, viable cells (2 × 106 cells/mice) were injected subcutaneously into the right anterior limb of each C57BL/6 mouse (Zhu et al., Citation2011).
When the tumor grew to the volume of 100 mm3 (about a week after the inoculation), the LLC-bearing mice were randomly divided into following six groups (six per group) and 0.2 mL of a respective treatment was continuously administrated via tail veins, once daily for 7 days. The first group of mice was negative control and treated with free nanosuspension, the second group of mice was positive control and received cyclophosphamide at a dose of 30 mg/kg/day, the third group of mice was treated with 2-ME solution at a dose of 30 mg/kg/day, the fourth, fifth and sixth group of mice were injected with 2-ME nanosuspension at the dose of 15, 30 and 60 mg/kg/day, respectively. The tumors were measured daily with a vernier caliper, and the volume of the solid tumor was calculated using the following formula: tumor volume (mm3) = LW2/2, where L and W represent the largest diameter and the smallest diameter, respectively.
Antitumor effect of 2-ME nanosuspension on tumor growth in mice
The in vivo antitumor activity of 2-ME nanosuspension in the solid tumor model was determined by the reduction of the solid tumor volume, compared with that of non-treated control mice. On day 8 (the first day mice received treatment was set as day 0), the mice were sacrificed and the tumors were excised and weighted. The tumor growth inhibiting ratio was calculated according to Eq. (3):
where C is average tumor weight of the negative control group, and T is average tumor weight of the tested group.
Effects of 2-ME nanosuspension on the main immune organs indices
Relative organs were excised and weighed immediately after the mice were sacrificed as an index of systemic toxicity. Organ index was expressed as the organ weight relative to body weight.
Statistical analysis of the data
Results were presented as the mean values ± standard deviation. The statistical analysis was performed by applying the Student’s unpaired t-test. For p < 0.05, the difference was considered statistically significant.
Results
The result of particle size and zeta potential measurement
The size distribution of the nanosuspension was 168.4 ± 3.2 nm with PDI 0.25 ± 0.02, the negative zeta potential was −29.79 ± 1.89 mV. Both induced degree of supersaturation and acoustic cavitation in solution created by ultrasound waves played an important role for producing nanosized crystal (Zhang et al., Citation2011). Long-chain polymeric surfactants and a negative surface charge would enhance the physical stability of nanosuspension.
Tissue distribution of 2-ME in rats
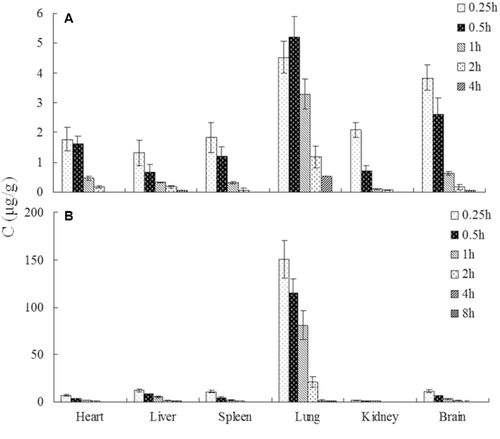
Distribution of 2-ME in the heart, liver, spleen, lung and kidney was examined after the treatment of 2-ME solution and 2-ME nanosuspension at the dose of 10 mg/kg. The LOD was found to be 10 ng/mL and the linearity of the method was demonstrated over the concentration range 0.025–6.4 µg/mL by analyzing tissue standards in triplicate (r > 0.9994). The absolute recoveries of 2-ME in tissues were from 73.42 to 85.70%, and the relative recovery was from 92.64 to 109.16%. Intra- and inter-day precision were <9%, which were within the acceptance range.
As shown in , the 2-ME accumulation in the lung was significantly higher than that of other organs and this phenomenon was shown both in rats treated with 2-ME solution and 2-ME nanosuspension. In addition, the 2-ME nanosuspension showed a better lung targeting property, with the lung TEQ of 2-ME nanosuspension increased from 28.71 to 51.95% compared with 2-ME solution, as shown in .
Table 1. Overall targeting efficiency of 2-ME in tissues after i.v. administration of 2-ME nanosuspension and 2-ME solution to rats (mean ± SD, n = 5).
In vitro cytotoxicity of 2-ME nanosuspension against LLC cells
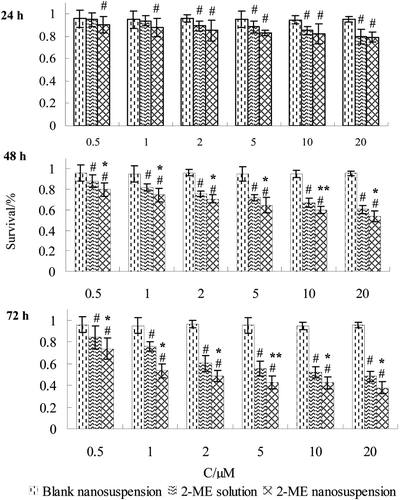
The in vitro cytotoxicity of 2-ME nanosuspension and 2-ME solution against LLC cells was expressed as survival percentage. indicated that 2-ME nanosuspension and 2-ME solution inhibited the proliferation of LLC cells in a dose-dependent manner at the range of 0.5–20 µM and the activity of 2-ME nanosuspension increased with prolonged time of treatment. Meanwhile, blank nanosuspension was not able to significantly decrease the number of viable LLC cells. Based on the optical density values determined, the IC50 values of 2-ME nanosuspension and 2-ME solution in LLC cells were observed to be 3.56 and 6.35 µM after 72h, respectively, and the difference was significant (p < 0.05) between the nanosuspension and the solution.
In vivo antitumor effects of 2-ME nanosuspension in LLC bearing C57BL/6 mice
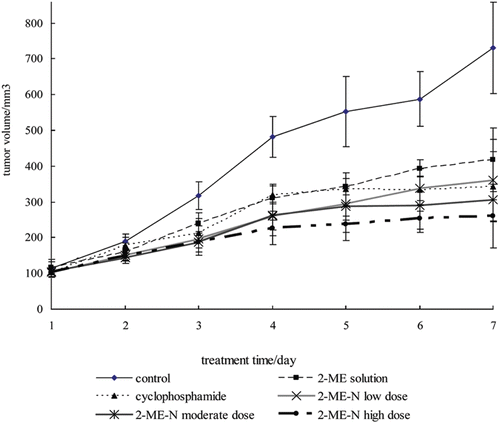
The in vivo antitumor activity of 2-ME nanosuspension was evaluated against C57BL/6 mice bearing LLC xenograft model. None of the mice died during the assay, and it could be seen that mice in the negative control group exhibited piloerection and diminished vigor, but mice treated with the 2-ME nanosuspension remained vigorous and had a healthy appearance. and listed the tumor weight, tumor inhibition rate and tumor volume changes of all the tested groups, respectively. Both 2-ME nanosuspension and 2-ME solution yielded tumor inhibition with a marked reduction of the tumor volume and weight compared with the negative control group. In relation to the negative control group, the weight inhibition rates were 43 and 56% for the 2-ME solution and 2-ME nanosuspension at the same dose of 30 mg/kg, respectively, which were statistically different (p < 0.05). 2-ME solution has an antitumor activity nearly the same as cyclophosphamide at the same dose (p > 0.05).
Table 2. Inhibition effect of 2-ME nanosuspension on tumors weight (mean ± SD, n = 6).
Effects of 2-ME nanosuspension on the main immune organs indices
Relative organ index of mice were calculated for investigating the toxicity. As shown in , relative organ index of liver was increased in the 2-ME nanosuspension group, which means 2-ME nanosuspension has less liver toxicity. A great organ index loss of spleen in cyclophosphamide group could be seen (p < 0.05), which account for the immunosuppressive side effect by cyclophosphamide during the therapy.
Table 3. Effects of 2-ME nanosuspensions on organ index of lewis tumour bearing mice (mean ± SD, n = 6).
Discussions
2-ME is an endogenous metabolite of estrogen, and it had been proved that lung was a target organ for estrogens (Benttarari et al., Citation1983). As such, we speculated that specific affinity site or receptor for 2-ME would be in the lungs. Moreover, 2-ME solution is unimolecular, while 2-ME nanosuspension encapsulate multiple molecules of drug to bind the receptor, and nanosuspension composed of phospholipid weaken the tensile force of alveolus surface, speeding the spread and absorbance of drug. The higher concentrations of 2-ME nanosuspension in the lungs indicated that 2-ME nanosuspension might have superiority for treatment of lung cancer. This surmise would be verified in the subsequent experiments.
The lower IC50 value of 2-ME nanosuspension exhibited that 2-ME nanosuspension was more potent against LLC cells. This phenomenon could be explained by a few reasons. First, after longer incubation periods, cells entered the G2 and M phases of the cell cycle during which 2-ME was known to be more effective (Hughes et al., Citation2002; Gui and Zheng, Citation2006). Second, it is considered that the nanosuspension could be transported via endocytosis (Gratton et al., Citation2008). Third, possessing a significantly increased solubility and dissolution rate, 2-ME nanosuspension induced sufficient molecular concentration around the cells, and adhered to the mucus layer prolonging their in vivo residence on mucosa surface compared with the 2-ME solution. All of these can improve the interaction between the drug and the cells, and enhance the suppression of 2-ME nanosuspension against cells. In addition, as the decrease of particle size of nanosuspension, the inhibition rate increased (Zheng et al., Citation2011).
It has been acknowledged that the nanoparticles could escape from the vasculature through the leaky endothelial tissue that surrounds the tumor and then accumulate in certain solid tumors by the enhanced permeability and retention (EPR) effect (Brannon-Peppas and Blanchette, Citation2004). Besides, our previous studies demonstrated that pharmacokinetic parameters of 2-ME nanosuspension, such as T1/2α, T1/2β and V(c), were longer than that of 2-ME solution, and these results indicated that 2-ME nanosuspension prolonged its in vivo residence and maintained a longer circulation time, which might produce a prolonged exposure of the tumor cells to the drug. As such, for the 2-ME nanosuspension, drug molecule could arrive at the tumor site via EPR effect and sustain the treatment concentration over time, and the antitumor effects are better than that of 2-ME solution.
Conclusions
In this study, 2-ME nanosuspension was prepared for applying to intravenous administration. Compared with 2-ME solution, 2-ME nanosuspension improved the overall targeting efficiency to the lungs of rats, which suggest that 2-ME nanosuspension may serve as passive targeting agents for lung cancer therapy. Cytotoxicity and antitumor effect studies demonstrated that 2-ME nanosuspension had stronger activity compared to the 2-ME solution both in the inhibition of cell growth and in treating tumor bearing mice. Based on these results, it can be concluded that 2-ME nanosuspension may be an effective drug delivery strategy, especially for relatively insoluble anticancer drugs.
Acknowledgements
We thank Chongwei Zhang providing the materials of 2-ME. We also thank Dr. Zhengquan Zhang for helpful proposition.
Declaration of interest
This work was financed supported by the Technology Innovation Talents Program Foundation of Henan Province, No. 094200510002. All authors explicitly state that they all have no conflicts of interest to report.
References
- Benttarari RR, Amit T, Youdim M. Binding of oestradiol, progesterone and prolactin in rat lung. J Endocrinol 1983;97:301–311.
- Brannon-Peppas L, Blanchette JO. Nanoparticle and targeted systems for cancer therapy. Adv Drug Deliv Rev 2004;56:1649–1659.
- Dobos J, Tímár J, Bocsi J, Burián Z, Nagy K, Barna G, Peták I, Ladányi A. In vitro and in vivo antitumor effect of 2-methoxyestradiol on human melanoma. Int J Cancer 2004;112:771–776.
- Du B, Li Y, Li XT, Youmei A, Zhang ZZ. LC with fluorescence detection for the tissue pharmacokinetics of 2-methoxyestradiol solution and liposome after intravenous administrations to rats. Chromatographia 2009;70:1287–1290.
- Du B, Li XT, Zhao Y, A YM, Zhang ZZ. Preparation and characterization of freeze-dried 2-methoxyestradiol nanoparticle powders. Pharmazie 2010;65:471–476.
- Garcia GE, Wisniewski HG, Lucia MS, Arevalo N, Slaga TJ, Kraft SL, Strange R, Kumar AP. 2-Methoxyestradiol inhibits prostate tumor development in transgenic adenocarcinoma of mouse prostate: role of tumor necrosis factor-α-stimulated gene 6. Clin Cancer Res 2006;12:980–988.
- Gratton SE, Ropp PA, Pohlhaus PD, Luft JC, Madden VJ, Napier ME, DeSimone JM. The effect of particle design on cellular internalization pathways. Proc Natl Acad Sci USA 2008;105:11613–11618.
- Gui Y, Zheng XL. 2-methoxyestradiol induces cell cycle arrest and mitotic cell apoptosis in human vascular smooth muscle cells. Hypertension 2006;47:271–280.
- Chauhan D, Li G, Hideshima T, Podar K, Mitsiades C, Mitsiades N, Munshi N, Kharbanda S, Anderson KC. JNK-dependent release of mitochondrial protein, Smac, during apoptosis in multiple myeloma (MM) cells. J Biol Chem 2003;278:17593–17596.
- Hany SM, Peters Y, Nicholas B. Preparation of hydrocortisone nanosuspension through a bottom-up nanoprecipitation technique using microfluidic reactors. Int J Pharm 2009;375:107–113.
- Huang XH, Xiong PC, Xiong CM, Cai YL, Wei AH, Wang JP, Liang XF, Ruan JL. In vitro and in vivo antitumor activity of Macrothelypteris torresiana and its acute/subacute oral toxicity. Phytomedicine 2010;17:930–934.
- Hughes RA, Harris T, Altmann E, McAllister D, Vlahos R, Robertson A, Cushman M, Wang Z, Stewart AG. 2-Methoxyestradiol and analogs as novel antiproliferative agents: analysis of three-dimensional quantitative structure–activity relationships for DNA synthesis inhibition and estrogen receptor binding. Mol Pharmacol 2002;61:1053–1069.
- Huh JI, Calvo A, Charles R, Green JE. Distinct tumor stage-specific inhibitory effects of 2-methoxyestradiol in a breast cancer mouse model associated with Id-1 expression. Cancer Res 2006;66:3495–3503.
- Ireson CR, Chander SK, Purohit A, Perera S, Newman SP, Parish D, Leese MP, Smith AC, Potter BV, Reed MJ. Pharmacokinetics and efficacy of 2-methoxyoestradiol and 2-methoxyoestradiol-bis-sulphamate in vivo in rodents. Br J Cancer 2004;90:932–937.
- Lu B. New Techniques and New Dosage Forms of Drugs, 2nd ed. Bei Jing: People's Medical Publishing House, 2005:158–167.
- Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods 1983;65:55–63.
- Müller RH, Peters K. Nanosuspensions for the formulation of poorly soluble drugs: preparation by a size reduced technique. Int J Pharm 1998;160:229–237.
- Peters K, Leitzke S, Diederichs JE, Borner K, Hahn H, Müller RH, Ehlers S. Preparation of a clofazimine nanosuspension for intravenous use and evaluation of its therapeutic efficacy in murine Mycobacterium avium infection. J Antimicrob Chemother 2000;45:77–83.
- Sève P, Reiman T, Dumontet C. The role of betaIII tubulin in predicting chemoresistance in non-small cell lung cancer. Lung Cancer 2010;67:136–143.
- Spivey KA, Banyard J, Solis LM, Wistuba II, Barletta JA, Gandhi L, Feldman HA, Rodig SJ, Chirieac LR, Zetter BR. Collagen XXIII: a potential biomarker for the detection of primary and recurrent non-small cell lung cancer. Cancer Epidemiol Biomarkers Prev 2010;19:1362–1372.
- Sweeney C, Liu G, Yiannoutsos C, Kolesar J, Horvath D, Staab MJ, Fife K, Armstrong V, Treston A, Sidor C, Wilding G. A phase II multicenter, randomized, double-blind, safety trial assessing the pharmacokinetics, pharmacodynamics, and efficacy of oral 2-methoxyestradiol capsules in hormone-refractory prostate cancer. Clin Cancer Res 2005;11:6625–6633.
- Wang Y, Zhang D, Liu Z, Liu G, Duan C, Jia L, Feng F, Zhang X, Shi Y, Zhang Q. In vitro and in vivo evaluation of silybin nanosuspensions for oral and intravenous delivery. Nanotechnology 2010;21:155104–155110.
- Zhang H, Hollis CP, Zhang Q, Li T. Preparation and antitumor study of camptothecin nanocrystals. Int J Pharm 2011;415:293–300.
- Zheng D, Wang Y, Zhang D, Liu Z, Duan C, Jia L, Wang F, Liu Y, Liu G, Hao L, Zhang Q. In vitro antitumor activity of silybin nanosuspension in PC-3 cells. Cancer Lett 2011;307:158–164.
- Zhu WJ, Han B, Sun Y, Wang ZY, Yang XH. Immunoregulatory effects of a glucogalactan from the root of Panax quinquefolium L. Carbohyd Polym 2011;87:2725–2729.