Abstract
Lafutidine a newly developed histamine H2-receptor antagonist having biological half-life of 1.92 ± 0.94 h due to its selective absorption from upper part of gastrointestinal tract the development of mucoadhesive sustained release drug delivery system is recommended in order to enhance the bioavailability. A mucoadhesive tablets was developed using the natural polymer, sodium alginate, xanthan gum and karaya gum. Mucoadhesion is a complex phenomenon which involves wetting, adsorption and interpenetration of polymer chains. The prepared tablets of various formulations were evaluated for a total mucoadhesion time, buoyancy lag time and percentage drug released. The formulation with xanthan gum showed better results. Thus, it may be useful for prolonged drug release in stomach to improve the bioavailability and reduced dosing frequency. Non-fickians release transport was confirmed as the drug release mechanism from the optimized formulation by Korsmeyer–Peppas. The optimized formulation (B3) showed a mucoadhesive strength >35 g. In vivo study was performed using rabbits by X-ray imaging technique. Radiological evidences suggest that, a formulated tablet was well adhered for >10 h in rabbit’s stomach. Optimized lafutidine mucoadhesive tablets showed no significant change in physical appearance, drug content, mucoadhesive properties and in vitro dissolution pattern after storage at 40 °C temperature 75 ± 5% relative humidity for 3 months.
Introduction
Lafutidine, (±)-2-(furfuryl sulfinyl)-N-(4-[4-[piperidinomethyl]-2-pyridyl]oxy-(Z)-2-butenyl) acetamide is a newly developed second generation histamine H2-receptor antagonist (Ikawa et al., Citation2007). It is used in the treatment of gastric ulcers, duodenal ulcers and gastric mucosal lesions associated with acute gastritis and acute exacerbation of chronic gastritis (Onodera et al., Citation1995). It is slowly absorbed in upper duodenum. Due to higher solubility in intestinal pH, it reaches gastric cells via the systemic circulation and rapidly binds to histamine H2 receptors, resulting in immediate inhibition of gastric acid secretion (Suvarna et al., Citation2012). Lafutidine has been shown to increase the gastric mucosal blood flow, gastric mucus secretion and accelerates epithelial restitution in rats. Lafutidine has a receptor binding affinity which is 2–80 times higher than famotidine, ranitidine and cimetidine (Bhupesh & Raghuram, Citation2010). The Gastroretentive drug delivery system can improve oral bioavailability by controlled delivery of various drugs (Dollery, Citation1999; Caldwell et al., Citation1988) The formulation development of rate controlled oral drug delivery systems may overcome physiological adversities like short gastric residence and gastric emptying time (Caldwell et al., Citation1988; Chavanpatil et al., Citation2006) Depending upon the physiological state of the subject and the design of pharmaceutical formulation the emptying process can last from a few minutes to 12 h. This variability, in turn, may lead to unpredictable bioavailability and times to achieve peak plasma levels since the majority of drugs are preferentially absorbed in the upper part of the small intestine. Thus, control of placement of adhesion in a specific region of the gastrointestinal tract offers numerous advantages, especially for drugs exhibiting an absorption window in the GI tract or drugs with stability problem. Overall, the intimate contact of the mucoadhesive drug delivery system with the absorbing membrane maximizes drug absorption and may also influence the rate of drug absorption. These considerations have led to the development of oral controlled release (CR) dosage forms possessing the gastric retention capabilities. Mucoadhesive drug delivery is one of the approaches for gastro retention. While the system is mucoadhesion to the gastric contents, drug is released slowly at a predetermined rate (Singh & Kim, Citation2000; Arora et al., Citation2005). Xanthan gum is a polysaccharide secreted by the bacterium Xanthomonas Campestris. Sodium alginate is extracted from Macrocystis pyrifera and Karaya gum is natural polymers obtained as gum exudates of plant species Sterculia (Gershon et al., Citation1972). Gums of natural sources are biodegradable and non-toxic, which hydrates and swells on contact with aqueous media (Nokano & Ogata, Citation1984). Karaya gum is water soluble thickening agents which have not been much studied for their pharmaceutical applications.
The present study deals with use of minimum amount of natural polymers for sustained drug release by utilizing the advantage of interactions of gums resulting in enhanced matrix strength. This research work is with new active pharmaceutical ingredient along with natural gum polymer. Prolonged gastric retention improves bioavailability and improves solubility of drugs that are less soluble in the high pH environment. Mucoadhesion can be defined as a state in which two components, of which one is of biological origin, are held together for extended periods of time by the help of interfacial forces (Gandhi & Robinson, Citation1998).
Mucoadhesive drug delivery systems is one of the most important novel drug delivery systems with its various advantages and it has a lot of potential in formulating dosage forms for various chronic diseases. Thus, aim was to develop mucoadhesive delivery systems for the treatment of ulcer which attempts to increase the gastric retention time thereby improving bioavailability of the lafutidine.
Materials and methods
Materials
Lafutidine was obtained as a gift sample from Alkem laboratories ltd. (Kachigam, India), Sodium alginate, MCC KG-100 from Lubrizol (Mumbai, India), Xanthan gum from Torrent Laboratory (Ahmadabad, India), purified talc, magnesium stearate, Karaya gum were gift samples from S.D. Fine Chemicals (Mumbai, India). All other chemicals were of analytical grades as required.
Methods
Drug excipients compatibility study
Fourier transformation-infrared spectroscopy (FTIR)
FTIR is used to identify the drug excipients interaction. FTIR studies were performed on drug, polymer and statistically optimized formulation at 40 °C/75% relative humidity for 4 weeks. Sample preparation involved mixing the samples with potassium bromide (KBr), triturating in glass mortar and placing in sample holder. Samples were analyzed by potassium bromide pellet method in an IR spectrophotometer (FTIR 8001, Shimadzu, Japan) in the region between 4200 and 400 cm−1 (Lachman et al., Citation1987).
Differential scanning calorimetry (DSC)
Differential scanning calorimetric analysis of drug, polymer and statistically optimized formulation were done using Differential Scanning Calorimeter (Perkin Elmer Cyris-DSC) at 40 °C/75% relative humidity for 4 weeks. In this process, ∼8–10 mg of the samples were weighed onto aluminum pan and then hermetically sealed with aluminum lids. In DSC analysis we obtain DSC curves at a scanning rate of 10 °C/min conducted over a temperature range of 25–250 °C. (Lachman et al., Citation1987). In addition, hermetically sealed pans were used and so no influence of the environment can be expected.
Preparation of mucoadhesive tablets of lafutidine
Each tablet containing ∼50 mg of lafutidine was prepared by direct compression method. The ingredients MCC KG 100 as diluents, sodium alginate, xanthan gum and karaya gum as CR polymer were weighed accurately and sifted through 30 meshes were then mixed thoroughly for 5 min in polybag, finally lubricated for 3 min with lubricant magnesium stearate and glident talc by passing through 60 meshes. Powder blend was then compressed into tablets by direct compression method using the single-punch tablet compression machine (Cadmach, Ahmadabad, India) using 7.0 mm standard concave punches. Tablet blend was evaluated for their bulk density, tapped density, flow properties and compressibility index.
In vitro buoyancy studies
The in vitro buoyancy was determined by buoyancy lag time as per the method described by Rosa et al. (Citation1994). The test was performed by placing each of the tablets in a 250 mL of beaker, containing 200 mL of 0.1 N HCl, pH 1.2. Physical state of tablet was observed for 12 h. The time between introduction of the tablets its buoyancy on the 0.1 N HCl (lag time) the time during which the tablets remains buoyant (total buoyancy time) were determined visually. Three replicates of each formula were performed.
Mucoadhesive strength and mucoadhesion time
These were measured by modified balance method, a balance left pan was replaced with a weight to the bottom of which a tablet was attached and both sides were balanced with weight. Porcine gastric mucosa having a thick layer of mucus was fixed to a rubber cork, which was previously attached to the bottom of the beaker containing related medium with a level slightly above the mucosa. The weight was attached to the tablet brought into contact with the porcine mucosa, kept undisturbed for 5 min and then the pan was raised. Weights were continuously added on the right side pan in small increments and the weight at which the tablet detached from the mucosa was recorded as the mucoadhesive strength (Chien, Citation1992). For measuring mucoadhesion time, 10 g of weight was put on right-side pan after raising it and the detachment time was noted. The time period throughout which the tablet remained attached to the mucosa is mucoadhesion time.
Swelling study
The lafutidine mucoadhesive tablets were weighed individually (W1) placed separately in a glass beaker containing 200 mL of 0.1 N HCl incubated at 37 ± 0.5 °C. At regular 1 h time intervals until 10 h, the tablets were removed from beaker and the excess surface liquid was removed carefully using the paper. The swollen lafutidine tablets were then re-weighed (W2) and the swelling index (SI) was calculated using the following formula (Dorozynski et al., Citation2004).
In vitro dissolution studies
In vitro drug release studies of various formulations (A1, A2, A3, B1, B2, B3 and C1, C2, C3) were performed using USP II (paddle with sinker) at 100 rpm in 900 mL of 0.1 N HCL medium which was maintained at 37 ± 0.5 °C. Then 10 mL of sample was withdrawn at predetermined time intervals until 12 h and replaced with fresh dissolution medium (Atyabi et al. Citation1996; Korsmeyer et al. Citation1983). The samples were analyzed by high performance liquid chromatography at 276 nm. A octadecylsilane column (250 mm × 4.6 mm, 5 μm) with mobile phase containing mixture of pH 6.5 buffer solution and acetonitrile in the ratio of 70:30 v/v. The flow rate was 1.0 mL/min. The retention times of was ∼7.5 min.
In vivo gastrointestinal retention time study
The protocol for in vivo study was approved by the Institutional Animal Ethics Committee of R.C.Patel Institute of Pharmaceutical Education and Research, Shirpur (RCPIPER/IPEC/2011-12/01) and is in accordance with guidance of Committee for the purpose of Control and Supervision of Experiments on Animals, Ministry of Social Justice and Empowerment, Government of India. All the in vivo experiments were performed under permission from animal ethical committee. In vivo study of the selected formulation B3 containing xanthan gum was performed using the albino rabbit by an X-ray imaging method (Whitehead et al., Citation1998; Desai & Bolton, Citation1993). Tablets containing 30% barium sulfate were selected for in vivo study given to rabbit followed by 30 mL of water. In each experiment, the animals were fasted overnight with free access of water and a radiograph was made just before the administration of the lafutidine mucoadhesive tablet to ensure the absence of any radio-opaque material in the stomach. The radiographic imaging was taken in a supine position and the distance between the sources of X-rays and the animal was kept constant for all imaging, thus the observation of the lafutidine mucoadhesive tablet movement could be easily noticed. Gastric radiography was carried out at 2 h time intervals for a period of 12 h using an X-ray machine.
Statistical analysis
Tests for significant differences between means were performed by one-way ANOVA using the software Sigma Stat version 10 was used to analyze swelling studies, mucoadhesive strength and the dissolution.
Kinetic modeling of drug release
The dissolution profile of all the batches were fit to zero order, first order (Gibaldi & Feldman, Citation1967), Higuchi (Citation1963), Hixon & Crowell (Citation1931), Korsmeyer–Peppas model (Korsmeyer et al., Citation1983; Peppas, Citation1985). The data obtained from dissolution kinetics studies were analyzed using PCP Disso v2.08 software.
Stability studies
Optimized batch B3 and A3 was kept in accelerated stability condition at 40 °C temperature 75 ± 5% relative humidity for a period 3 months as per International Conference on Harmonization guidelines (Mathews, Citation1999; International Conference on Harmonization Steering Committee, Citation1999). The samples were withdrawn at 1, 2 and 3-month intervals evaluation was carried out for appearance, thickness, hardness, friability, buoyancy lag time, drug content, mucoadhesive behavior and cumulative % drug released.
Results and discussion
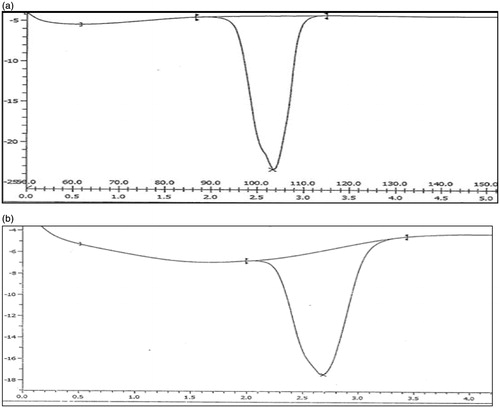
The drug excipients studies reveal that there were no physical changes in drug and excipients mixtures. Spectral observation indicated that the principal IR absorption peaks observed in spectra of drug were close to those in spectra of excipients indicated that there was no significance interaction between drug and excipients (). The major IR peaks observed lafutidine were 3328, 3205 (N–H stretching – aliphatic), 2933 (–C–H stretching – aromatic), 1658 (–C=N stretching), 1610 (N–H bending), 1350 (C–H bending) and 1278 (C–N stretching). IR spectrum indicated characteristics peaks belonging to measure functional group such as principle peaks at wave no. 3328, 3205 (N–H stretching – aliphatic), 2919 (–C–H stretching – aromatic), 1656 (–C=N stretching), 1612 (N–H bending), 1357 (C–H bending) and 1280 (C–N stretching).
Figure 1. (a) FTIR spectrum of lafutidine. (b) FTIR spectrum of lafutidine and excipients physical mixture.
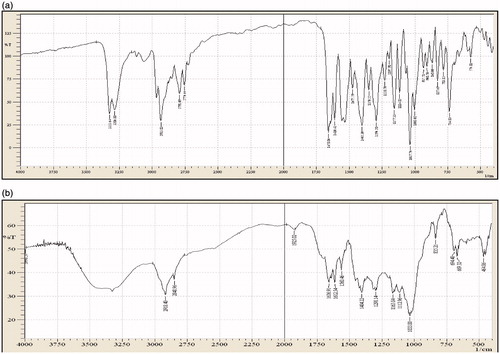
The drug, drug excipients physical mixture (microcrystalline cellulose, sodium alginate, xanthan gum, karaya gum, talc and magnesium stearate mixture) studies reveal that there were no significant change in position of peak in thermogram of drug, drug excipients was recorded. The diffential scanning calorimeter curves of lafutidine exhibited a single sharp endothermic peak at 102.99 °C related to its melting transition temperature showed in ) and drug excipients physical mixture shows melting endothermic peak of lafutidine at 102.13 °C shown in ). From drug excipients compatibility study, it was concluded that the given drug was compatible after 1-month treatment of 40 °C temperature 75 ± 5% relative humidity with all the excipients and it was confirmed by differential scanning colorimeter study. Therefore, this study revealed that there was no interaction between the drug, polymers and other excipients. Tests for significant differences between means were performed by one-way ANOVA using the software Sigma Stat version 10. Differences were considered significant at p < 0.05 level. The data clearly indicate that the values of mucoadhesive strength, % swelling and drug released, are strongly dependent on the independent variables.
Gastroretentive lafutidine mucoadhesive tablets were developed to increase the gastric residence time of the drug which retained in the stomach for a longer time, improving the buoyancy and drug release characteristics also help in CR of drug up to 12 h. The gastroretentive mucoadhesive tablets were made using gel-forming polymers such as sodium alginate, xanthan gum and karaya gum. The composition of lafutidine mucoadhesive formulations A1, A2, A3, B1, B2, B3, C1, C2 and C3 are shown in .
Table 1. Composition of lafutidine mucoadhesive tablets.
Evaluation of precompression parameters of powder blend
Prepared powder blend of all formulations of the lafutidine (A1, A2, A3, B1, B2, B3, C1, C2 and C3) were evaluated for their physical properties such as angle of repose, bulk density, tapped bulk density, Hausner’s ratio, Loss on drying Carr’s index, it can be clearly concluded that the powder blend with different formulations components were having good flow properties, good compressibility which allow these formulations to be directly compressed into tablets. Results of Precompression evaluations of formulations were shown in .
Table 2. Evaluation of precompression parameters of gastroretentive mucoadhesive tablets of lafutidine.
Physicochemical characterization of lafutidine mucoadhesive tablets
Lafutidine mucoadhesive formulations A1, A2, A3, B1, B2, B3, C1, C2 and C3 were evaluated for average weight, thickness, hardness, friability and drug content the results were shown in . Physicochemical parameters of all the formulations were within the acceptance limit. The drug content was found to be within a narrow range as specified in pharmacopoeia (90–110%) in all the formulations.
Table 3. Physicochemical characterization of mucoadhesive tablets of lafutidine.
In vitro dissolution studies
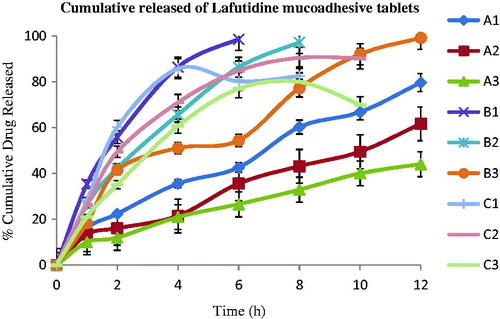
To evaluate different polymers such as sodium alginate (A1, A2, A3), xanthan gum (B1, B2, B3) and karaya gum (C1, C2, C3) used to prepare the gastroretentive mucoadhesive tablets of lafutidine and their individual % cumulative drug released profile was evaluated and showed in .
The lafutidine mucoadhesive tablets formulations A1, A2, A3, B1, B2, B3, C1, C2 and C3 exhibited cumulative % drug released were shown in .
Table 4. Determination of cumulative % drug released, buoyancy lag time and mucoadhesion time of lafutidine tablets.
Xanthan gum containing gastroretentive formulation B3 exhibited 99.59% cumulative drug released and good mucoadhesion time for 12 h. While B1, B2 could not maintain its matrix integrity for >8 h. sodium alginate containing formulation A1, A2, A3 forms a thick gel structure that delayed drug released, i.e. 78.95%, 62.65% and 46.20%, respectively, over a period of 12 h. Also, karaya gum containing formulation C1, C2, C3 showed drug released up to 10 h. A significantly higher rate extended release of drug was found to be near required theoretical profile value (which was calculated using the equations for an immediate release dose maintenance dose) from batch B3 and A3 compaired with other batches. The lafutidine mucoadhesive tablets containing sodium alginate (A3) showed a higher rate extended released up to 12 h as compared to the formulation with karaya gum (C3) which was upto 10 h. The data obtained from in vitro dissolution studies were fitted to zero-order; first-order, Higuchi and Korsmeyer–Peppas equations. The data were analyzed, and the n value of optimized formulation (B3) was found to be 0.652, which is in range of 0.5 < n < 1 and k value was found to be 11.57. The diffusion exponent value indicates that the drug release follows Non-Fickian transport.
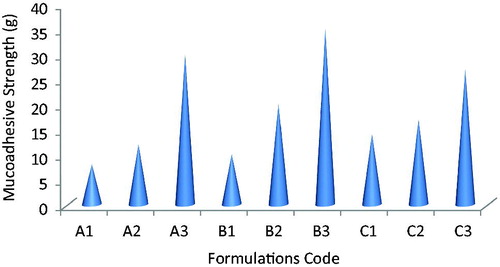
All formulation shows good mucoadhesive performance with mucoadhesive resistance time range from 6 h for A1, B1, C1 and 10 h for A2, B2, C2, C3 >12 h for formulations B3, A3. The bioadhesive strength of lafutidine mucoadhesive tablets were shown in . It was revealed that increasing polymer amount increases bioadhesive strength due to providing more adhesive sites and polymer chains for construal with mucin. Formulation with xanthan gum showed stronger mucoadhesion than formulation containing sodium alginate and karaya gum this may be due to capability of sodium alginate and karaya gum to form mucoadhesion bond with mucin and construal of polymer chain in the interfacial region.
Swelling study
The mucoadhesive tablets containing xanthan gum showed constant increased in swelling because of more water sorption capacity than sodium alginate and karaya gum; this can be shown by released pattern of the batches A3 and C3. This erosion of polymers dominates over water sorption after 7 h. Hence the reduction in tablet weight occurs after 8 h because of erosion of matrix. In case of increasing concentrations of sodium alginate and karaya gum showed an increase in swelling but not to the extent of xanthan gum (B3). The formulation A3 containing sodium alginate showed less swelling index at the beginning but was found higher at the end of 8 h also maintains their matrix integrity up to 8 h. The swelling index of lafutidine mucoadhesive tablets of formulations B3 was 125 ± 2.0% and A3 was 105 ± 1.5% at the end of 10 h shown in .
Table 5. Swelling index of lafutidine mucoadhesive tablets.
In vivo study
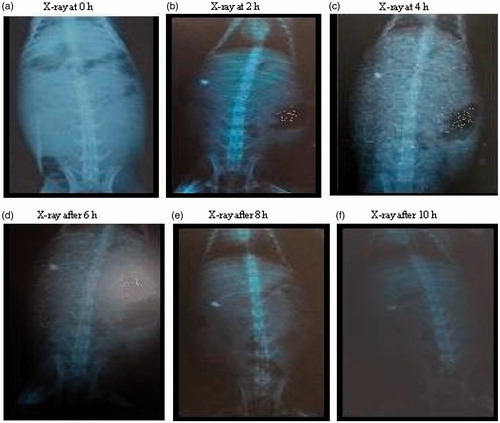
The prepared gastroretentive mucoadhesive tablets of lafutidine containing xanthan gum were selected for evaluation of gastric retention using X-ray imaging. In vivo study of final formulation (B3) was performed using new zeal albino rabbits by X-ray imaging technique. Prepared tablets of various concentration of barium sulfate were evaluated for in vitro mucoadhesive study. It was observed that tablets containing xanthan gum formulation showed good mucoadhesion property and sufficient integrity of lafutidine mucoadhesive tablets. Radiological evidences suggest that the formulated gastroretentive mucoadhesive tablet of lafutidine (B3) was well floated >10 h in rabbit’s stomach; hence we can conclude that the gastroretentive mucoadhesive tablets was satisfactory floated to the rabbit stomach were shown in .
Stability studies
The lafutidine mucoadhesive tablets containing xanthan gum were selected for stability study on the basis of mucoadhesive properties and in vitro drug dissolution studies. Lafutidine mucoadhesive tablets were stored at 40 °C/75% relative humidity in closed high-density polyethylene bottles for 3 months. Mucoadhesive tablets did not showed any significant change in physicochemical parameters and drug contents as shown in . Thus, it was found that the B3 formulation of lafutidine tablets were stable under 40 °C/75% relative humidity storage conditions for a period of 3 months. Formulation B3 results was found satisfactory compared to formulation A3 were shown in .
Table 6. Evaluation of lafutidine mucoadhesive tablets kept in stability at 40 °C/75% relative humidity.
Conclusion
Lafutidine mucoadhesive gastroretentive tablet is developed using naturally occurring plant based polymers showed desirable high-drug content, mucoadhesive strength, swelling index and adequate release characteristics. The use of natural gum in pharmaceutical dosage forms is of increasing interest due of their low production cost, lesser toxic effects and regulatory acceptance. The gastroretentive mucoadhesive tablets of lafutidine were formulated by using gelling polymer xanthan gum showed pleasing results with short buoyancy lag time, total mucoadhesion time >10 h, controlled drug released up to 12 h comparing with karaya gum. Thus, it was conclude that the mucoadhesive formulation with xanthan gum of lafutidine were stable at 40 °C/75% relative humidity for a period of 3 months results were found satisfactory.
Acknowledgements
Authors would like to thank Vice President, Alkem Research Centre for useful comments and advices during the development of the formulation. The authors are thankful to Dr. S. J. Surana, principal, R. C. Patel Institute of Pharmaceutical Education & Research, Shirpur, India, for providing necessary facilities to carry out this research work. The authors are thankful to Alkem laboratories ltd. (Kachigam, India) for providing a gift sample of drug, S.D fine chemicals (Mumbai, India), Torrent Laboratory, (Ahmadabad, India) and Lubrizol (Mumbai, India) for providing gift samples of excipients.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
References
- Arora S, Ali J, Ahuja A, et al. (2005). Floating drug delivery systems: a review. AAPS PharmSciTech 6:E372–90
- Atyabi F, Sharma H, Mohammad H, Fell J. (1996). In vitro evaluation of a novel gastro retentive formulation based on ion exchange resins. J Control Release 42:105–13
- Bhupesh D, Raghuram C. (2010). An open-label randomized cross-over bioequivalence study of lafutidine 10 mg under fasting condition. World J Gastrointest Pharmacol Ther 1:112–18
- Caldwell L, Gardner R, Cargill R, et al. (1988). Drug delivery device which can be retained in the stomach for a controlled period of times. US patents, 30th August 1988; 4,767,627
- Chavanpatil MD, Jain P, Chaudhari S, et al. (2006). Novel sustained release, swellable bioadhesive gastroretentive drug delivery system for ofloxacin. Int J Pharm 316:89–92
- Chien Y. (1992). Novel drug delivery systems. 2nd ed. New York: Marcel Dekker Inc., 171–6
- Desai S, Bolton S. (1993). A floating controlled-release drug delivery system: in vitro-in vivo evaluation. Pharm Res 10:1321–5
- Dollery C. (1999). Therapeutic drugs. Edinburgh, Scotl: Churchil Livingstone, C113YC117
- Dorozynski P, Jachowicz R, Kulinowski P, et al. (2004). The macromolecular polymers for the preparation of hydrodynamically balanced systems – methods of evaluation. Drug Dev Ind Pharm 30:947–57
- Gandhi RB, Robinson JR. (1998). Bioadhesion in drug delivery. Ind J Pharm Sci 50:145–52
- Gershon S, Pader M, Balsam M, et al. (1972). Cosmetics science and technology. New York: Wiley-Interstice, 423
- Gibaldi M, Feldman S. (1967). Establishment of sink conditions in dissolution rate determinations. Theoretical considerations and application to nondisintegrating dosage forms. J Pharm Sci 56:1238–42
- Higuchi T. (1963). Mechanism of sustained-action medication. Theoretical analysis of rate of release of solid drugs dispersed in solid matrices. J Pharm Sci 52:1145–9
- Hixson AW, Crowell JH. (1931). Dependence of reaction velocity upon surface agitation. Ind Eng Chem 23:923–93
- Ikawa K, Shimatani T, Hayato S, et al. (2007). Pharmacokinetic and pharmacodynamic properties of lafutidine after postprandial oral administration in healthy subjects: comparison with famotidine. Biol Pharm Bull 30:1003–6
- International Conference on Harmonization Steering Committee (1999). Q1A – Stability testing of new drug substances and products
- Korsmeyer R, Gurny R, Peppas N. (1983). Mechanisms of solute release from porous hydrophilic polymers. Int J Pharm 15:25–35
- Lachman L, Hebert AL, Joseph LK. (1987). Theory and practice of pharmacy. 3rd ed. Varghese Publication House, 430–4
- Mathews BR. (1999). Regulatory aspects of stability testing in Europe. Drug Dev Ind Pharm 25:831–56
- Nokano M, Ogata A. (1984). Examination of natural gums as matrices for sustained release of Theophylline. Chem Pharm Bul 32:782–5
- Onodera S, Shibata M, Tanaka M, et al. (1995). Gastroprotective activity of FRG-8813, a novel histamine H2-receptor antagonist, in rats. Jpn J Pharmacol 68:161–73
- Peppas NA. (1985). Analysis of Fickian and non-Fickian drug release from polymers. Pharm Acta Helv 60:110–11
- Rosa M, Zia H, Rhodes T. (1994). Dosing testing in vitro bioadhesive floating drug delivery system for oral application. Int J Pharm 105:65–70
- Singh BN, Kim KH. (2000). Floating drug delivery systems: an approach to oral controlled drug delivery via gastric retention. J Control Release 63:235–59
- Suvarna K, Manisha S, Sugandha V. (2012). Development and validation of an UV spectrophotometric method for estimation of Lafutidine in bulk and tablet. In: contemporary investigation and observation in pharmacy, 1:5–8
- Whitehead L, Fell JT, Collett JH, et al. (1998). Floating dosage forms: an in vivo study demonstrating prolonged gastric retention. J Control Release 55:3–12