Abstract
The main objective of the study was to alter the dissolution profile of a practically insoluble Biopharmaceutics Classification System class II drug, aceclofenac, by formulating into lipid semisolid matrix (SSM) formulations using liquid filling technology in hard gelatin capsules, for both immediate and sustained release. SSM formulations of aceclofenac were prepared by melt fusion technique, using Gelucires (44/14, 50/13, 33/01 and 43/01), polyethylene glycols (4000 and 6000) and Poloxamer 188 at different levels. Role of additives like docusate sodium, Tween 80, Aerosil 200 and polyvinylpyrrolidone K-30 in enhancement of drug release was investigated. The optimized immediate and sustained SSM capsules were characterized in terms of assay, in vitro drug release, moisture uptake and differential scanning calorimetry. More than 80% of the drug was released within 15 min in various dissolution media studied, from Gelucire 44/14-based immediate release formulations. Incorporation of docusate sodium and Tween 80 provided further enhancement in drug dissolution when compared to plain drug and marketed tablet. SSM formulations based on Gelucire blends of 50/13 and 43/01 and 44/14 and 43/01 sustained the release of the drug for a period of 24 h following zero-order kinetics. The in vivo study of the optimized immediate release and sustained release formulations revealed significant enhancement in anti inflammatory activity (p < 0.01) in rats. From these findings, liquid fill technique in hard gelatin capsules using Gelucire and their blends might be an efficacious approach for designing immediate or sustained drug release profiles for poorly soluble drugs like aceclofenac.
Introduction
Active pharmaceutical ingredients are classified according to the Biopharmaceutics Classification System (BCS) (Amidon et al., Citation1995) where in more than 35% of the commonly prescribed drugs are poorly water-soluble (Wu & Benet, Citation2005). Although many novel techniques have emerged to increase solubility of these drugs, these techniques either use very expensive polymers or high amount of surfactants, which make them industrially unviable. Pharmaceutical industries are now exploring new ways to make use of conventional polymers and technologies to modulate the solubility of class II drugs.
One of the newer technologies for improving the bioavailability of poorly soluble drug includes use of novel excipient like Gelucire. Gelucires are solid waxy materials, which are amphiphilic in character and are identified by two values: their melting points and their hydrophilic–lipophilic balance (HLB). Gelucires are the saturated polyglycolized glycerides consisting of mono-, di- and tri-glycerides and of mono- and di-fatty acid esters of polyethylene glycol (PEG). The nature and proportion of each component are specific to a given grade of Gelucire. Gelucires have been widely studied as controlled release matrices (Dennis et al., Citation1990; Galal et al., Citation2004) as well as for modulating the solubility of poorly soluble drugs for enhancing their dissolution rate and subsequently their bioavailability (Galal et al., Citation2003; Yüksela et al., Citation2003). Gelucires on heating can give a molten/semisolid mass with drug in solubilized state, which can be easily filled in the hard gelatin capsule by liquid filling technique (Hauss, Citation2007), a technology that is more efficient and comparatively economic than powder capsule filling. It is a relatively simple process and is easily scaled up to make the technology commercially viable (Stegemann & Bornem, Citation1999).
Aceclofenac is a non-steroidal anti-inflammatory drug with potent analgesic and anti-inflammatory properties. It is widely used for rheumatoid arthritis, osteoarthritis and a variety of other acute and chronic musculoskeletal disorders (Gonzalez et al., Citation1994), which necessitates the requirement of immediate and sustained release formulations. Aceclofenac is a BCS class II drug as it is practically insoluble and its gastrointestinal absorption is limited by its dissolution rate (Soni et al., Citation2008).
This investigation was aimed at studying the role of lipid bases and their blends in modulating the dissolution rate of poorly water soluble drug to obtain release profiles for both immediate and sustained release formulations. A relatively new and less explored concept of semi solid matrix (SSM) formulations, using liquid fill technology in hard gelatin capsules, was explored for this purpose. Weakly acidic and poorly water soluble drug aceclofenac was selected as a model drug. Immediate and “once a day” sustained release semisolid matrix (SSM) were formulated using different materials like Gelucire, PEG and Poloxamer 188 by liquid filling technology in hard gelatin capsules. Role of additives like docusate sodium, Tween 80, Aerosil 200 and polyvinylpyrrolidone (PVP) K-30 in dissolution enhancement of aceclofenac was also studied. For sustained release formulations, Gelucire blends were used to tailor the drug release to follow zero order kinetics. The optimized SSM capsules were evaluated for drug content, in vitro dissolution, release kinetics, moisture pickup and differential scanning calorimetry (DSC) and were compared with marketed tablets. The optimized formulations were studied for their in vivo pharmacodynamic efficiency and compared with plain drug in rat model.
Materials and methods
Materials
Aceclofenac was obtained as a gift sample from Suraksha Pharma Pvt. Ltd., Hyderabad, India. Gelucire 44/14, Gelucire 50/13, Gelucire 33/01 and Gelucire 43/01 were kindly gifted by Colorcon Asia Pvt. Ltd., Goa, India. Transparent hard gelatin capsules “0”, “00” and “000” sizes and Aerosil 200 were gift samples by EEI Capsules Ltd, Mumbai, India, and K. Uttamlal & Company, Mumbai, India, respectively. PEG 4000, PEG 6000, PVP K-30 (Loba Chemie Pvt. Ltd., Mumbai, India), docusate sodium (SD Fine-Chem Ltd., Mumbai, India) and Tween 80 (Oxford Laboratories Pvt. Ltd., Mumbai, India) of analytical grade were purchased. All other chemicals used were of analytical grade. Marketed aceclofenac immediate release tablets (Hifenac 100 mg, Intas Pharmaceuticals Ltd., Gujrat, India) and sustained release tablets (Hifenac-SR, 200 mg, Intas Pharmaceuticals Ltd.) were purchased from local pharmacy.
Solubility studies
Semi-quantitative estimation of aceclofenac solubility in various SSM bases like Gelucire (44/14, 33/01, 50/13 and 43/01), PEGs (4000 and 6000) and Poloxamer 188 was carried out according to a method reported by S. Galal et al. (Citation2004). Accurately weighed 10 mg of aceclofenac was placed in different fusion tubes. Bases were accurately weighed and added to the tubes to obtain different mixtures with drug:bases ratios ranging from 1:1 to 1:20 for each matrix former base used. Drug:base mixtures were then kept in an oven at 60 °C for 6–8 h, and the mixtures were visually and microscopically observed for complete drug solubility.
Formulation of SSM and filling in hard gelatin capsule
Accurately weighed SSM bases were taken in glass beakers and heated to 10 °C above the melting point of the base on a constant temperature water bath. To this molten base, required quantity of aceclofenac was added and stirred continuously for 40 min to ensure homogeneity. The mass was cooled to 32–34 °C and then filled in the body of transparent hard gelatin capsules of size “0”, “00” or “000” using 1 ml of warm glass syringe, to contain either 100 mg (for immediate release) or 200 mg (for sustained release) of aceclofenac/capsule (). Size “000” capsules, although not recommended for oral use in humans, was, however, selected for few formulations to incorporate the higher dose, to maintain uniform dosage delivery system for all formulations under study and also to compare the formulation effect due to capsules. The capsules were left undisturbed for 20–30 min at 23 ± 1 °C until a solid plug was obtained. After solidification of SSM in capsules, the caps were fitted and the corresponding weights of filled capsules were noted. Sealing of the filled capsules was not required as any leakage or visible change in appearance was not apparent during the time of storage under ambient temperature for a period of six months. Batch size for all formulations was five capsules each and was performed in triplicate. The capsules were stored in air tight, glass bottles at room temperature till further use.
Table 1. Dissolution data of immediate release SSM aceclofenac capsule formulations in pH 6.8 buffer.
Table 2. Effect of excipients on Gelucire 44/14 based formulation (F18).
Table 3. Dissolution data of aceclofenac sustained release SSM capsules.
Immediate release SSM formulations
Immediate release liquid fill SSM formulations were prepared using Gelucire 33/01, Gelucire 44/14, PEG 4000, PEG 6000 and Poloxamer 188 (). Formulations F18 and F19 were filled in size “00” and size “000” capsules, respectively. Formulations F20 and F21 were not filled in capsules and were cooled and solidified in the beaker, powdered, sifted through 40# sieve and stored in air tight containers until further use. Other formulations were filled in size “0” capsules.
Effect of inclusion of additives on immediate release SSM formulations
The possibility of modification of drug release from Gelucire matrices by addition of hydrophilic additives like docusate sodium, Tween 80, Aerosil 200 and PVP K-30 was determined. Optimized formulation F18 was selected for the study. Accurately weighed additives were incorporated in the drug – Gelucire 44/14 – blended, stirred, cooled and filled in size “00” hard gelatin capsules. Each capsule contained 100 mg of aceclofenac with different percentages of hydrophilic additives (). The total weight of the formulation was adjusted to 600 mg/capsule with Gelucire 44/14.
Sustained release SSM formulation
Sustained release liquid fill SSM formulations for 24 h release were prepared using Gelucire 44/14, Gelucire 43/01 and Gelucire 50/13 blends and filled in different sized capsules ().
Assay
Accurately weighed SSM capsule contents equivalent to 100 mg of drug was taken in a 100 ml volumetric flask; 20 ml methanol was added and sonicated for 20 min to dissolve the drug. The volume was made to 100 ml with pH 6.8 buffer solution. The dispersion was filtered using 0.45 µm membrane filter. An aliquot of the above solution was diluted suitably with pH 6.8 buffer to obtain 10 mcg/ml of aceclofenac. The absorbance of sample solution was determined at 272 nm against buffer blank.
In vitro dissolution studies
Dissolution studies of all formulations were performed using 900 ml of dissolution medium maintained at 37 ± 0.5 °C and stirred at 100 rpm in a USP apparatus-I. The following media were used for dissolution studies of immediate release SSM capsules, immediate release marketed tablets and plain drug; 6.8 pH phosphate buffer, 7.4 pH buffer, 4.5 pH acetate buffer and 2% sodium lauryl sulfate (SLS) in 0.1 N HCl. In vitro dissolution studies for sustained release SSM capsules, and sustained release marketed tablets were conducted using 900 ml of 2% SLS in 0.1 N HCl dissolution medium for 2 h and replacing the whole buffer after 2 h with 6.8 pH phosphate buffer. Five milliliter samples were withdrawn at various intervals and filtered through 0.45 µm. An equal volume of fresh dissolution medium was immediately replaced. After suitable dilutions, the samples were analyzed spectrophotometrically at 272 nm.
Mechanism of drug release was obtained by applying the release rate data to zero order, first order, Higuchi and Korsmeyer–Peppas models (Costa & Sousa Lobo, Citation2001). The best fit model was determined based on smallest value of Akaike Information Criterion (AIC) and the mechanism of release was explained (Costa & Sousa Lobo, Citation2001). For immediate release formulations, cumulative percent of drug released at end of 5 (Q5 min), 15 min (Q15 min) and time taken for 100% of drug release (t100%) were obtained. Dissolution efficiency at end of 15 min (DE15) was also calculated (Khan, Citation1975). Cumulative drug release at end of 6 h (Q6 h), 12 h (Q12 h) 18 h (Q18 h) and 24 h (Q24 h) were obtained for sustained release formulations. Similarity factor f2 (SUPAC-MR FDA Guidance, Citation1997) was calculated using marketed product as reference.
Immediate release formulations with Q15 min > 85% drug release in all dissolution media and highest DE15 were selected for further studies. Sustained release formulations with highest correlation to zero order model, largest value of f2 and Q6 h, Q12 h, Q18 h and Q24 h in the range of 25–35%, 50–60%, 75–85% and >95%, respectively, were the acceptance criteria set to select and optimize different type of SSM formulation.
Moisture uptake studies
Moisture uptake studies were performed on optimized formulations and corresponding placebo formulations. The formulations were subjected to 75% relative humidity (RH) at ambient temperature. RH of 75% was obtained by placing a saturated salt solution of sodium chloride at 25 ± 2 °C in the bottom of the desiccators. The capsules were weighed after five days, and the percentage moisture uptake by different samples was determined gravimetrically.
Differential scanning calorimetry
DSC scans of aceclofenac, optimized formulations and placebo containing bases and additives in appropriate ratio were obtained. Scans were performed on Perkin-Elmer Thermal Analyzer equipped with a monitor and printer. The instrument was calibrated with indium standard. Sample weights of 6–28 mg were placed in an open, flat bottom, platinum sample pans. Thermograms were obtained by heating the sample from 20 °C to 250 °C at a constant rate 20 °C/min. A dry purge of nitrogen gas (20 ml/min) was used for all runs. The melting point, peak maxima, appearance of any new peak and peak shape was noted.
In vivo pharmacodynamic study
Preliminary studies for in vivo performance of optimized immediate release (A2) and sustained release formulations (S13) were assessed by carrageenan-induced rat paw edema model (Winter et al., Citation1962). The experimental procedure was in accordance with the Principles of Laboratory Animal Care and Committee for the Purpose of Control and Supervision of Experiments on Animals guidelines for laboratory animal facility. This study protocol (IICT/PHARM/SRK/ACE/10/2008) was approved by the local Institutional Ethical Committee for Animal Experimentation. Prior to oral drug administration, four groups of rats (n = 6) were fasted overnight (>12 h) and randomly divided into four treatment groups (n = 6). Group I received pure aceclofenac (10 mg/kg) p.o as 1% gum acacia suspension, Group II and Group III received fast release SSM formulation A3 and sustained release SSM formulation S13 containing equivalent amount of aceclofenac (10 mg/kg), respectively, and Group IV served as vehicle control group and received equivalent 1% gum acacia suspension without drug. Before the administration of carrageenan, a zero hour paw volume was measured for the rats using digital Plethysmometer (Ugo Basile, Comerio, VA, Italy). After 30 min of drug administration, rats in all the groups were challenged by subcutaneous injection of 0.1 ml of 1% carrageenan in saline into the sub plantar region of right hind paw by using 26 gauze needles. Paw volumes are again measured at different time intervals after the challenge with carrageenan. The percentage inhibition of paw volume for each rat in treated groups was calculated and expressed as mean ± SD percent inhibition of paw volume. Student’s paired t-test was employed to analyze the results (Data Analysis tool, MS Excel 2007). Difference below the probability level of 0.05 was considered as statistically significant.
Results and discussion
Solubility
SSM formulations by liquid filling technique are usually prepared by dissolving/dispersing the drug and bases at higher temperatures and filling them in a molten state in hard gelatin capsules. SSM bases that can dissolve the drug usually improve the in vitro dissolution rate due to increased wettability and solubilization (Galal et al., Citation2004). Similarly, the ability of the base to prevent recrystallization or trap fine precipitates of the drug on cooling the molten mass, in capsules, may improve the stability of the product (El Massik et al., Citation2003).
This study was hence performed to check the solubilizing ability of various SSM bases and their ability to prevent recrystallization and precipitation on cooling. Solubility of aceclofenac in different SSM bases is shown in (). The solubility of aceclofenac in various hydrophilic excipients showed a following order: PEG 4000/PEG 6000 > Poloxamer 188 > Gelucire 44/14 > Gelucire 50/13. Among the amphiphilic Gelucires, grade 44/14 showed better solubility for the drug when compared to 50/13 probably due to lower melting point and higher HLB value, which in turn may improve drug dispersibility and miscibility in the carrier matrix. Lipophilic Gelucire 33/01 and 43/01 were poor solubilizers of aceclofenac due to their low HLB value. Based on this study, PEG 4000, PEG 6000, Poloxamer 188 and Gelucire grades: 44/14, 50/13 and 33/01 were selected for formulation of immediate release SSM formulations, while Gelucire 43/01 and blends with 44/14 and 50/13 were selected for sustained release formulations.
Table 4. Semiquantitative solubility of Aceclofenac in different bases.
The drug content was uniform for all batches of SSM formulations prepared and was found to be in the acceptable range of 99.16–104.35%.
In vitro dissolution rate of immediate release formulations
Attempts were made to design immediate release SSM formulations that could release more than 85% of drug within 15 min in various acidic and basic dissolution media encompassing the gastrointestinal tract (GIT) pH. This criterion was selected to avoid dissolution rate controlled bioavailability problems that is usually observed for a class II drug like aceclofenac (US FDA Guidance, Citation2000).
Dissolution of plain drug and marketed tablets (100 mg) in different media was performed before approaching for dissolution studies of SSM formulations. From the release studies, it was concluded that the weakly acidic aceclofenac shows poor dissolution in all pH except pH 6.8 and 7.4 (; ). Total amount of aceclofenac from pure untreated drug sample was released within 15 min in pH 6.8 and 7.4, against 5 and 12 h in 0.1 N HCl in 2% SLS and pH 4.5, respectively. The drug release from marketed product showed a similar pattern with rapid release of drug in basic media (pH 6.8 and 7.4) as compared to acidic media. This difference in the release was due to the pH-dependent solubility of the drug (Manjanna et al., Citation2009). A pH 6.8 buffer was hence selected as a dissolution media for preliminary screening and selection of immediate release SSM formulation, as recommended by Mutalik et al. (Citation2007) for aceclofenac.
Figure 1. Release profiles of aceclofenac and marketed tablets in different pH. D-plain drug, MT – immediate release marketed tablets. Each value represents mean ± SD (n = 6). Only positive SD bar is displayed for clarity.
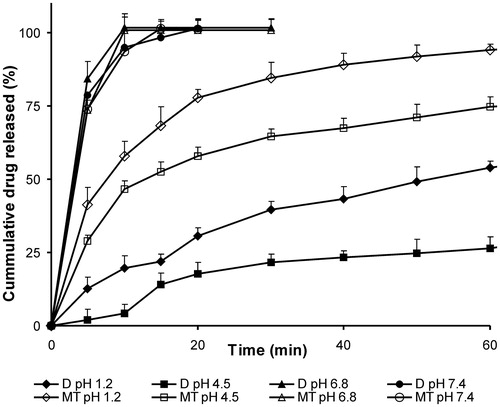
Table 5. Comparative studies of immediate release formulations in different media.
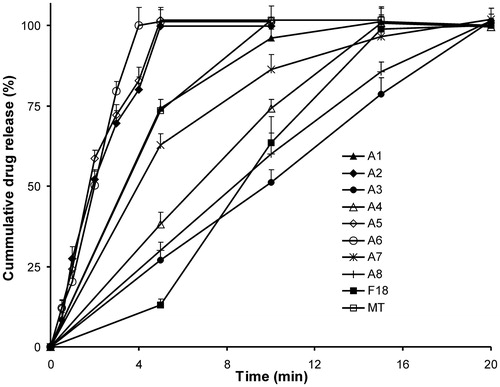
Dissolution data for various SSM bases studied are presented in and and . Among all the matrix excipient studied, Gelucire 44/14 showed higher dissolution rate at all ratios as evident from amount of drug released in 5 and 15 min and DE values. The following rank order with decreasing dissolution rate was obtained: Gelucire 44/14 > Gelucire 33/10 > PEG 4000 > PEG 6000 > Poloxamer 188 > Gelucire 50/13. Increasing the SSM base concentration increased the dissolution rate of aceclofenac in all bases. However, this increase in dissolution was not proportionate to the amount of base incorporated, especially at higher ratios. In case of Gelucire 44/14, the effect of increase in drug SSM base ratio 1:6 (F18) to 1:8 (F19) was not significant (p > 0.05) on Q15 min or DE15 values. The possible reason for this could be attributed to an increase in the diffusion pathway for the drug from the SSM matrix with increase in matrix former concentration. The formulations F20 and F21 were prepared without filling the SSM into hard gelatin capsules to study the impact of capsule shell on dissolution. A threefold increase in the initial release Q5 min was observed in F20 when compared to SSM filled in capsules F18, leading to 1.5 times improvement in DE. However, time taken for release of total amount of drug remained unchanged; indicating that once the capsule shell disintegrates, the release is rapid and comparable to the drug release from Gelucire matrix in absence of capsule.
Figure 2. Release profiles in pH 6.8 of drug:different SSM carriers (1:5). MT – immediate release marketed tablets, G – Gelucire. Each value represents mean ± SD (n = 6). Only positive SD bar is displayed for clarity.
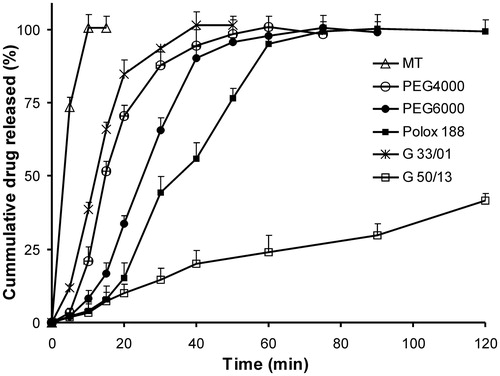
Figure 3. Release profile of Gelucire 44/14-based SSM at different ratios in pH 6.8. MT – immediate release marketed tablets. Each value represents mean ± SD (n = 6). Only positive SD bar is displayed for clarity.
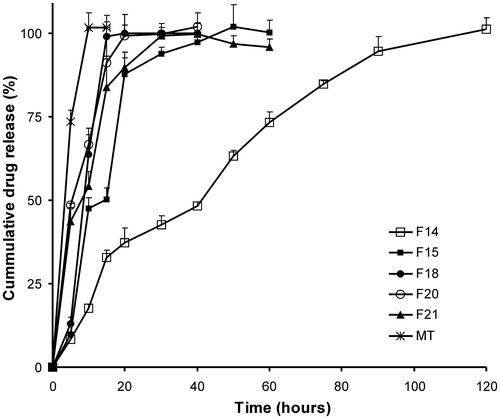
Based on the above findings, Gelucire SSM capsules containing Gelucire 44/14 at drug:base ratio 1:6 (F18) was selected for further studies. The drug release from F18 was determined in different media to encompass the range of likely pH values encountered in the GIT and compared with plain drug and marketed tablet (). More than 80% of the drug was released within 15 min, and total drug was released within 30 min in all pH range, which was acceptable (US FDA Guidance, Citation2000). The drug release rate was significantly more in acidic pH when compared to plain and marketed product as seen from Q15 min and t100% values indicative of improved wettability and emulsification of poorly soluble weakly acidic aceclofenac by an amphiphilic Gelucire 44/14. However, the drug release from immediate release SSM formulation F18 in basic media was significantly less than plain drug and marketed tablets. This was due to the significantly lesser amount of drug released at earlier time points, i.e. before disintegration of capsule shell of F18. However, the total amount of drug was released in 15 min as seen from Q15 min values, which were acceptable.
Role of additives
Incorporation of various additives in SSM formulations alters the dissolution rate (El Massik et al., Citation2003). Effect of inclusion of additives was investigated on formulation F18 with a view to reduce the disintegration time of the capsules and in turn improve the dissolution rate. Dissolution data of formulations A1-A8 in pH 6.8 is shown in . All the excipients studied had a significant impact on the initial release of the formulations as seen from Q5 min values (). SSM formulations prepared using 1% docusate sodium (A2) and 5% Tween 80 (A5) showed faster dissolution as compared to pure drug and marketed tablets with total drug released within 5 min. This enhanced drug release can be possibly attributed to the altered wettability and surface activity of drug by surface active agents like docusate sodium and Tween 80. The presence of these agents in the formulation prevents agglomeration of drug in the aqueous environment, which results in significant increase in surface area hence facilitating faster dissolution of a practically water insoluble drug like aceclofenac. The dissolution enhancement observed using docusate sodium was concentration dependent, the effect being maximal at 1%. A higher docusate sodium concentration (1.5%) formulation A3 resulted in a less dissolution-enhancing effect (). This may be attributed to an increased consistency of the SSM with increase in surfactant concentration posing a barrier to diffusion of drug into the dissolution media (El Massik et al., Citation2003). The optimized formulas (A2 and A5) showed enhanced dissolution rate when compared to marketed tablet and plain drug in 2% SLS in 0.1 N HCl, pH 4.5, pH 6.8 and 7.4, indicating efficiency of the SSM formulations for immediate release (; ).
In vitro dissolution rate of sustained release
SSM formulations using lipophilic Gelucire grades were prepared to obtain sustained release formulations of aceclofenac that could retard the drug release for a period of 24 h. An acceptance criterion of a zero order release profile was set. Initially, SSM formulations (S1–S4) using poor solubilizers like Gelucire 50/13 and Gelucire 43/01 alone at different levels were screened for their sustained release effect. Complete drug release within 10–13 h occurred in Gelucire 50/13 formulations, whereas only 11–14% of drug was released within 24 h for formulations of Gelucire 43/01 (; ). This difference is probably due to the lower HLB value and greater lipophilic nature of Gelucire 43/01, which inhibits the drug release into the aqueous dissolution media. To obtain the desired release profile, subsequent trials were carried out by blending highly retardant Gelucire 43/01 with Gelucire 50/13 (S5–S9) or Gelucire 44/14 (S10–S15) at different ratios. From all these formulations, S7, S12 and S13 gave sustained release profile till 24 h ( and ; ). The release profile of S7 (f2 = 56.3%) and S12 (f2 = 72.1%) was similar to marketed tablets (). The release profile of formulation S13 followed zero order kinetics as seen from the least AIC values reported in . Similarly, the n value of Peppas model of 0.9988 also substantiated a concentration independent release profile for S13. Based on the above findings, S7 and S13 were selected for further studies. SSM formulation S12 was, however, not selected for further studies as >95% of drug was released within 20 h.
Figure 5. Dissolution profiles of sustained release Gelucires 50/13- and 43/01-based SSM formulations. MT – sustained release marketed tablets. Each value represents mean ± SD (n = 6). Only positive SD bar is displayed for clarity.
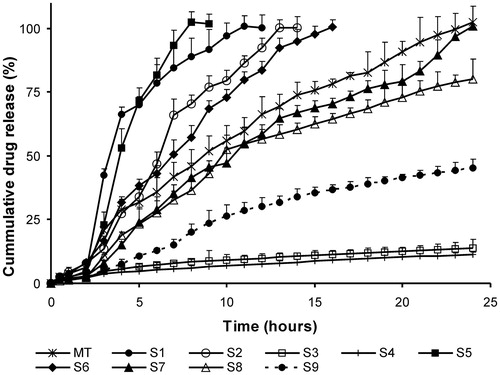
Figure 6. Dissolution profiles of sustained release Gelucires 43/01- and 44/14-based SSM formulations. MT – sustained release marketed tablets. Each value represents mean ± SD (n = 6). Only positive SD bar is displayed for clarity.
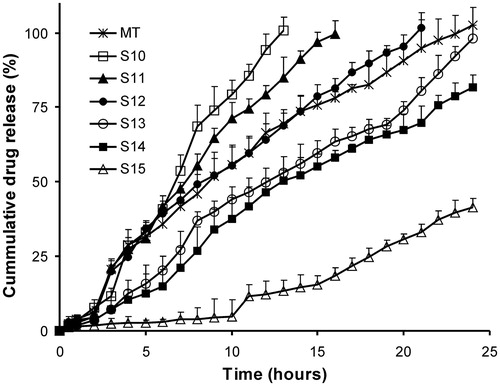
Table 6. Kinetic data of sustained release SSM capsules.
The treated kinetic data suggest that the sustained release SSM matrix formulations (S2, S5, S6, S10, S11, S13 and S14) predominately follow erosion controlled systems as Peppas n value ≈ 1, which is also indicative of zero order release profile as seen from the least AIC values. Formulations S3 and S4 exhibited drug release via Higuchi diffusion mechanism, while anomalous behavior with drug diffusion and matrix erosion occurring simultaneously was observed with remaining formulations ().
Moisture uptake studies
SSM formulations should be stable at every environmental zone as described by International Conference on Harmonisation (ICH) guidelines. Moisture uptake study at 75% RH was conducted to have an insight into the stability of the formulation. The empty gelatin capsules picked up 0.2% moisture, while the immediate release SSM formulations, F18, A2 and A5, picked up 0.7%, 0.8% and 0.6% moisture, respectively. The moisture pickup by the sustained release SSM formulations S7 and S13 were 0.2% and 0.4%, respectively. The SSM formulations were found be stable and acceptable at 75% RH as the percent of moisture uptake was less than 2% (Barakat, Citation2006; Stegemann & Bornem, Citation1999).
Differential scanning calorimetry
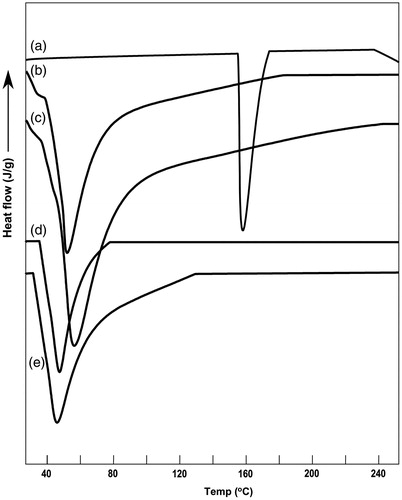
DSC of the pure drug () showed a sharp peak at 155.8 °C corresponding to the melting point of aceclofenac. The enthalpy change (ΔH) was calculated as 82.3 J/gm. The dummy formulations of A2 (Gelucire 44/14 + docusate sodium) and A5 (Gelucire 44/14 + Tween 80) showed a broad melting point endotherm at 48.9 °C and 51.1 °C, respectively, corresponding to the melting point range of Gelucire 44/14 (). The DSC of optimized formulations A2 and A5 () showed a single melting endotherm of Gelucire 44/14, but no endotherm corresponding to the melting point of the drug was observed. Similar observations were noted for DSC scans of S7 and S13 formulations (scans not shown). This loss in drug melting peak may be due to the complete solubilization of the drug in the matrix base or its conversion to amorphous form. However, few authors have claimed that DSC was not a suitable technique for characterization of SSM formulations as the crystalline drug may dissolve in the lower melting point matrix below its own melting temperature when subjected to higher temperature during DSC scans, thus making it difficult to detect whether the drug in formulation originally exists in solubilized state or gets dissolved in liquid matrix during DSC scan (Fini et al., Citation2005).
In vivo pharmacodynamic study
The anti-inflammatory activity of optimized immediate release SSM (A2) and sustained release SSM (S13) was evaluated based on its ability to inhibit the edema produced in the hind paw of rats when challenged with carrageenan. SSM formulations A2 and S13 showed significant anti-inflammatory activity (p < 0.05) when compared to plain drug (). A2 showed maximum edema inhibition of about 80 ± 6% when compared to 68 ± 5% for plain drug. Although A2 and plain drug gave maximum edema inhibition at 3 h, significant reduction in inflammation (p < 0.01) was observed with A2 from two hours onwards indicating rapid onset of action () when compared with plain drug. The possible reason for this could be due to the solubilization of the drug in Gelucire SSM matrix, which presented the drug in very fine form in the GIT, thus enhancing the dissolution and subsequently the absorption and bioavailability.
Figure 8. Percent inhibition by aceclofenac SSM formulations on carrageenan-induced rat paw edema. Each value represents mean ± SD (n = 6). Only positive SD bar is displayed for clarity.
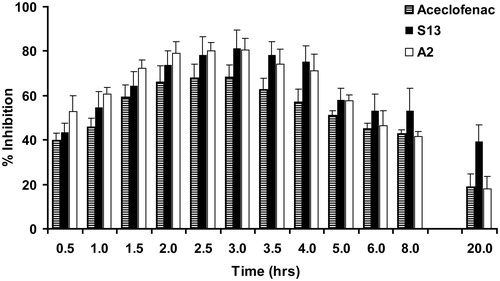
In order to obtain preliminary data for justifying sustained release claim, in vivo studies were also performed on S13 formulation, using carrageenan-induced rat paw edema model. Many authors have successfully used this acute model of inflammation for evaluating sustained release formulations (Arora & Mukherjee, Citation2002; Panusa et al., Citation2011). Sustained release SSM formulation, S13, also gave maximum edema inhibition of 81 ± 8% at 3 h, which was statistically significant at p < 0.01 when compared to plain drug. However, the anti-inflammatory activity of S13 persisted till a period of 20 h, while the activity of plain drug and A3 starting gradually decreasing from 5 h onwards. A significant (p < 0.05) edema inhibition of 39 ± 8% at end of 20 h was observed for S13 formulation when compared to plain drug and immediate release A3 formulation, which showed negligible edema inhibition in range of 10–23% at 20 h. The edema inhibition for a longer duration clearly indicates that the drug release from S13 was sustained; as a result, the circulating drug levels were maintained within the therapeutic range for a period of 20 h when compared to plain drug and A3. This outcome clearly justifies the sustained release claim for S13 formulation.
Conclusion
This study results clearly demonstrate the capabilities of Gelucire as a SSM base for preparation of SSM of aceclofenac by liquid filling technology in hard gelatin capsules. Immediate release SSM formulations with enhanced dissolution rate were successfully formulated using Gelucire 44/14. Incorporation of docusate sodium or Tween 80 further improved the dissolution rate, which was significantly better than plain drug or marketed tablets. In addition, these formulations also showed pH-independent release profiles. Sustained release SSM formulations with tailor-made dissolution profile following zero order kinetics were obtained with Gelucire grades and their blends. This commercially feasible process based on the ease of operations will be useful in lending a “one-technology for many solutions” for a variety of BCS class II drugs. Future work needs to be directed toward exploring the potential of these SSM bases on various drugs with different kind of biopharmaceutical profiles.
Declaration of interest
The authors of this article declare no conflict of interests and have no financial and personal relationships with other people or organizations that could influence their work.
References
- Amidon GL, Lennernas H, Shah VP, Crison JR. (1995). A theoretical basis for a biopharmaceutic drug classification: the correlation of in vitro drug product dissolution and in vivo bioavailability. Pharm Res 12:413–20
- Arora P, Mukherjee B. (2002). Design, development, physicochemical, and in vitro and in vivo evaluation of transdermal patches containing diclofenac diethylammonium salt. J Pharm Sci 91:2076–89
- Barakat NS. (2006). Etodolac-liquid-filled dispersion into hard gelatin capsules: an approach to improve dissolution and stability of etodolac formulation. Drug Dev Ind Pharm 32:865–76
- Costa P, Sousa Lobo JM. (2001). Modeling and comparison of dissolution profiles. Eur J Pharm Sci 13:123–33
- Dennis AB, Farr SJ, Kellaway W, et al. (1990). In vivo evaluation of rapid release and sustained release Gelucire capsule formulations. Int J Pharm 65:85–100
- El Massik MA, Abdallah OY, Galal S, Daabis NA. (2003). Semisolid matrix filled capsules: an approach to improve dissolution stability of phenytoin sodium formulation. Drug Dev Ind Pharm 29:531–43
- FDA Guidance for Industry. (Sept 1997). SUPAC-MR – modified release solid oral dosage forms: scale-up and post approval changes. Rockville, MD: Center for Drug Evaluation and Research (CDER)
- Fini A, Moyano JR, Ginés JM, et al. (2005). Diclofenac salts, II. Solid dispersions in PEG6000 and Gelucire 50/13. Eur J Pharm Biopharm 60:99–111
- Galal S, El Massik M, Abdallah O, Daabis N. (2003). Formulation of fast release glibenclamide liquid and semi solid matrix filled capsules. Acta Pharm 53:57–64
- Galal S, El Massik MA, Abdallah OY, Daabis NA. (2004). Study of in-vitro release characteristics of carbamazepine extended release semi solid matrix filled capsules based on gelucire. Drug Dev Ind Pharm 30:817–29
- Gonzalez E, De La Cruz C, De Nicolás R, et al. (1994). Long-term effect of nonsteroidal anti-inflammatory drugs on the production of cytokines and other inflammatory mediators by blood cells of patients with osteoarthritis. Agents Actions 41:171–8
- Hauss DJ. (2007). Oral lipid-based formulations. Adv Drug Deliv Rev 59:667–76
- Khan KA. (1975). The concept of dissolution efficiency. J Pharm Pharmacol 27:48–9
- Manjanna KM, Pramod Kumar TM, Shivakumar B. (2009). Effect of manufacturing conditions on physico-chemical characteristics and drug release profiles of aceclofenac sodium microbeads. Drug Invention Today 1:98–107
- Mutalik S, Naha A, Usha AN, et al. (2007). Preparation, in vitro, preclinical and clinical evaluations of once daily sustained release tablets of aceclofenac. Arch Pharm Res 30:222–34
- Panusa A, Selmin F, Rossoni G, et al. (2011). Methylprednisolone-loaded PLGA microspheres: a new formulation for sustained release via intra-articular administration. A comparison study with methylprednisolone acetate in rats. J Pharm Sci 100:4580–6
- Soni T, Nagda C, Gandhi T, Chotai NP. (2008). Development of discriminating method for dissolution of aceclofenac marketed formulations. Dissolution Technologies 15:31–5
- Stegemann S, Bornem C. (1999). Hard gelatin capsules today – and tomorrow. Capsugel Library 1:1–23
- US FDA Guidance for Industry. (August 2000). Waiver of in vivo bioavailability and bioequivalence studies for immediate-release solid oral dosage forms based on a biopharmaceutics classification system. Rockville, MD: Center for Drug Evaluation and Research (CDER)
- Winter CA, Risley EA, Nuss GW. (1962). Carrageenin-induced edema in hind paw of the rat as an assay for antiinflammatory drugs. Proc Soc Exp Biol Med 111:544–7
- Wu CY, Benet LZ. (2005). Predicting drug disposition via application of BCS: transport/absorption/elimination interplay and development of a biopharmaceutics drug disposition classification system. Pharm Res 22:11–23
- Yüksela N, Karataşa A, Özkanb Y, et al. (2003). Enhanced bioavailability of piroxicam using Gelucire 44/14 and Labrasol: in vitro and in vivo evaluation. Eur J Pharm Biopharm 56:453–9