Abstract
Although several studies have shown that chlorhexidine (Cx) has bactericidal activity and exerts toxic effects on periodontal tissues a few studies evaluated mechanisms to reduce its adverse effects maintaining the antimicrobial properties. Therefore, the aim of the present study was to investigate the in vitro antimicrobial activity and cellular cytotoxicity of Cx included on cyclodextrins (Cd), α, β or Hp-β-cyclodextrins (Hp-β-Cd). The influence of Cds was determined by increasing its molar rate 1:1 to 1:4 in relation with free Cx. The minimal inhibitory concentrations (MICs) for Candida albicans, Aggregatibacter actinomycetemcomitans actinomycemcomitans and Streptococcus mutans were determined. An ergosterol solubilization assay was carried out using the C. albicans model and osteoblasts, fibroblasts and tumoral Caco-2 cells for cytotoxicity assay. The antimicrobial activity results in a significant growth inhibition of C. albicans when it was treated with Cx:α-Cd complexes, whereas Cx:β-Cd was more effective for A. actinomycetemcomitans, and Cx:Hp-β-Cd complexes was for S. mutans when compared to the other complexes. The cytotoxicity for fibroblasts and osteoblasts decreased in relation with each kind of Cd been β-Cd ≤ Hp-β-Cd ≤ α-Cd. Although the Hp-β-Cd inclusion complexes had more severe effects on Caco-2 cells, all complexes exhibited less cytotoxicity than free Cx. The α-Cd, β-Cd and Hp-β-Cd increase the antimicrobial activity of Cx, but decrease its cytotoxic effects on mammalian cells. Taken together these findings suggest that cyclodextrins are a tool for modulation of effects of Cx. It could be useful to design Cx/Cd delivery systems with high efficacy and minimum cytotoxic effects.
Introduction
Currently, the management of periodontal disease begins with oral hygiene instructions and non-surgical mechanical root debridement to remove subgingival bacteria. This treatment is followed by a re-evaluation period to assess whether surgical treatment may be needed. However, when the mechanical treatment of periodontal pockets is not effective, antimicrobials may be locally applied to arrest the progression of the disease. In this case, the most frequently employed antimicrobial drug is chlorhexidine (Cx) (Jeffcoat et al., Citation1998; Drisko, Citation2001).
Cx is considered the gold standard among antimicrobial agents (Salim et al., Citation2013). It is a well-known inactivating agent of many non-sporulated bacteria, and exhibits some sporostatic and mycobacteriostatic action. It is also effective against yeasts and protozoa, with a minimum inhibitory concentration comparable to most antiseptics (Adriaanse et al., 1996; Best et al., Citation2007; Teixeira et al., Citation2012; Salim et al., Citation2013). The mechanism of action of Cx has been well described, and its main target has been identified as the bacterial membrane (Gianelli et al., Citation2008; Faria et al., Citation2009). However, the agent is also toxic to mammalian cells, as even diluted solutions of chlorhexidine can cause severe cytotoxic effects on fibroblasts, osteoblasts, (Hugo & Longworth, Citation1964; Best et al., Citation2007) and chondrocites (Denyer, Citation1995).
Cx possesses two biguanides that act as polar heads connected by a spacer chain of six methylenes and two chlorophenyl groups as the hydrophobic core. These cationic complexes initiate their interactions with cellular membranes by binding to surface phospholipids through electrostatic forces (Sheppard et al., Citation1997; McDonnell & Russel, Citation1999). In an effort to improve the effectiveness of Cx at lower concentrations, investigators have found that Cx inclusion complexes with β-cyclodextrin (β-Cd) have stronger antimicrobial activity than pure Cx (Cortés et al., Citation2001; Denadai et al., Citation2007; Teixeira et al., Citation2013), Cx gel formulations (Karpanen et al., Citation2008, Citation2011) or Cx formulations containing a silicate matrix or other vehicles (Loftsson & Brewster, Citation1996; Uekama et al., Citation1998; Raso et al., Citation2010). The enhanced antimicrobial activity of Cx was more evident at 1:3 and 1:4 molar ratios of drug-cyclodextrin complexes. In addition, sterol solubilization was also evident when 1:3 and 1:4 Cx:β-Cd complexes were analyzed using Candida albicans (C.a) as an experimental model (Teixeira et al., Citation2012).
Complexation of drugs in cyclodextrin (Cd) has been shown to represent an effective strategy for improving macromolecular drug therapy by stabilizing the guest molecule against aggregation, thermal denaturation or degradation (Uekama et al., Citation1998; Loftsson et al., Citation2004). Macromolecular drugs can be partially complexed by Cds via their hydrophobic side chains, and the ability of Cd to sequester hydrophobic moieties helps in improving drug stability. In forming the complex, the physicochemical and biological properties of the drug can be altered to affect an advantage (Uekama et al., Citation1998; Liu & Guo, Citation2002). Among all host potential molecules for drug formulations, Cds seem to be particularly important because of their capacity to associate with a variety of different molecules and potentially alter drug properties, specially the solubility (Loftsson et al., Citation2004).
Cd molecules have a hydrophilic outer surface and a hydrophobic inner core, which allows for the formation of inclusion complexes through binding of small amphiphilic molecules in the core (Loftsson & Brewster, Citation1996; Zheng et al., Citation2001; Liu & Guo, Citation2002; Abdelwahed et al., Citation2008). The most common cyclodextrins are α-cyclodextrin (α-Cd), β-cyclodextrin (β-Cd), and γ-cyclodextrin (γ-Cd), which contain six, seven and eight glucopyranose units, respectively. Cyclodextrins, such as (2-hydroxypropyl)-β-cyclodextrin (Hp-β-Cd), have been used specifically to solubilize drugs in aqueous solutions and as parenteral excipients for drugs (Loftsson & Brewster, Citation1996; Uekama et al., Citation1998; Loftsson et al., Citation2004).
The binding abilities of Cds exhibit substantial differences due to their differing chemical structures (Harada et al., Citation2009). For example, the chain length selectivity for each of the Cds is quite different from the others. The small cavity of α-cyclodextrin prohibits inclusion of some molecules that can be bound by the cavities of β-Cd or γ-Cd. However, α-Cd is more soluble in water than β-Cd and may display stronger inclusion properties for some drugs (Mura et al., Citation1992; Ahmed et al., Citation1994), in addition to better stabilizing capacity (Uekama et al., Citation1981) and absorption enhancing ability (Uekama et al., Citation1995; Haerbelin et al., Citation1996; Maggi et al., Citation1998).
β-Cd is very efficient as a sterol acceptor molecule, apparently because its inner hydrophobic cavity matches the size of the sterol molecule. Cholesterol can be removed from cells by extraction into small unilamellar vesicles (Ohvo-Rekila et al., Citation1997; Mesmin & Maxfield, Citation2009).
To improve some physicochemical properties of natural Cds, many types of derivatives have been developed: hydrophilic (methylated, hydroxyalkylated and branched), hydrophobic (ethylated), ionic (sulphated and phosphated) (Cal & Centkowska, 2008; Taneri et al, Citation2010). The presence of only primary hydroxyl functional groups increases the reactivity and the accessibility of both the 2- and 3-positions of this Cd in drug delivery (Badi & Guégan, Citation2007). The disruption of hydrogen bonding via molecular manipulation gives rise to an increase in water solubility. Hp-β-Cd is regarded as safe and non-irritating (Del Valle, 2004). Among Cd derivatives, only HP-β-Cd has a legal status and own pharmacopoeia monographs (Cal & Centkowska, 2008).
The toxicities associated with Cds are dependent upon their route of administration (Harada et al., Citation2009). Renal toxicity of α-Cd and β-Cd after parenteral administration, as well as problems with a number of modified Cds have been well documented (Frank et al., Citation1976; Irie et al., Citation1992; Davis & Brewster, Citation2004; Zhao et al., 2010). The toxicological effects of Cds are primarily due to to inclusion complexation with cholesterol and membrane lipids (Stella & He, Citation2008). These molecules may have catalytic effects on lipid transport, as coupling of cyclodextrins with lipid emulsions could enhance lipid transport. The high efficiency of the cyclodextrin is probably a function of its small relative size in comparison to cellular membranes, which allows large numbers of cyclodextrin molecules to directly associate with membranes (Atger et al., Citation1997). The location of cyclodextrin molecules near the surface of the cell allows solubilization of cholesterol from biological membranes by desorption directly into the hydrophobic core of the cyclodextrin without entering into the aqueous phase (Hidalgo & Dominguez, Citation2001; Besenicar et al., Citation2008).
We have analyzed the cytotoxic effects of Cx:Cd inclusion complexes on three different types of cells treated with: osteoblasts, fibroblasts and Caco-2 cells. We also compared the abilities of α-Cd, β-Cd and Hp-β-Cd to interact with ergosterol-containing cell membranes from C.a. Thus, we hypothesize that cholesterol solubilization ability and cytotoxicity can serve as factors predictive of the effects of Cd inclusion complexes that vary based on the structure of the derivative and the molar ratio of the components and the powerful pharmaceutical strategies of the use of different cyclodextrin based on biological properties.
Materials and methods
Chemicals and microorganism stocks
Chlorhexidine chlorhydrate (Cx) was obtained from Ecadil® (SP, Brazil), α-Cd from Wacker Biochem®, Co. (Eddyville, IA, USA), and β-Cd and Hp-β-Cd from Cerestar®, Co. (Milwaukee, WI, USA). Fluconazole was obtained from Pfizer® Pharmaceutics Group (New York, NY, USA). Sabouraud dextrose agar (SDA), broth (SDB), Blood Agar, Brain Heart Infusion (BHI) broth and agar were purchased from Biobras S.A® (MG, Brazil). Candida albicans (ATCC 18804), Streptococcus mutans (S.m; ATCC 25175), and Aggregatibacter actinomycetemcomitans (A.a; ATCC 29522) were obtained from the American Type Culture Collection (FioCruz, RJ, Brazil). All other materials and solvents were of analytical grade.
Preparation of supramolecular complexes
Complexes containing 1:1, 1:2, 1:3 and 1:4 molar ratios of α-Cd:Cx, β-Cd:Cx and Hp-β-Cd:Cx were prepared for chemical analysis and biological tests using the freeze-dry method as previously described (Cortés et al., Citation2001; Denadai et al., Citation2007; Teixeira et al., Citation2013). Briefly, Cx dichlorhydrate 0.59 g, 0.50 g and 0.44 g, respectively, for α-Cd (1 g), β-Cd (1 g) and Hp-β-Cd(1 g) were dissolved in 100 mL of milli-Q water. Solutions of Cx were mixed with α-Cd, β-Cd or Hp-β-Cd and stirred for 24 h at 50 °C. The resulting solutions were then frozen in liquid N2 and freeze dried over a period of 30 h using a FreeZone® 4.5 system under 50 mbar vacuum. The dried powders were stored in desiccators until further evaluation (Kasper & Friess, Citation2011).
Antimicrobials and minimal inhibitory concentration determinations
The activities of Cx:β-Cd inclusion complexes were analyzed by determining the minimal inhibitory concentration (MIC) against C.a, S.m and A.a microorganisms selected to model yeast and Gram-positive and Gram-negative pathogens, respectively. Microorganism cultures were cultured in appropriate media, specifically SDB for C.a and BHI supplemented with 1% hemin and 1% menadione for S.m and A.a. Prior to the assay, C.a cells were cultured for 24 h at 34 °C (Pfaller et al., 1988; CLSI, Citation2005a) under aerobic conditions in SDB. S.m and A.a (Slots & Ting, Citation1999; CLSI, Citation2005b) were cultured for 48 h at 37 °C under microaerophilic and anaerobic conditions, respectively. The cultures were then diluted to obtain a density of 1.5 × 108 colony forming units (CFU) for inoculums. MIC90, defined as the concentration required to inhibit the growth of 90% of the organisms compared to an untreated control, was determined using the macro dilution method CLSI (Clinical and Laboratory Standards Institute) (CLSI, Citation2005a,Citationb). Briefly, aliquots containing 500 μL of 1.0 µg/mL Cx or 1:1, 1:2, 1:3 or 1:4 Cx/CD complex solutions were serially diluted in 3.4 mL of SDB. Normalized Cx or Cx:β-Cd dilutions were then inoculated with 100 μL of C.a inoculums. Pure Cx and fluconazole (FLZ) were used as standard antimicrobial positive controls. Cyclodextrins alone served as negative controls. Sample absorbance was measured using a standard spectrophotometer (Thermo Scientific Multiskan® Spectrum, Vantaa, Finland) set to 580 nm, and sample data were analyzed and compared to controls (CLSI, Citation2005a,Citationb). The data was obtained from three independent experiments carried out in sixplicates. The MIC90 values were recorded and evaluated using the nonparametric Kruskal-Wallis.
Sterol quantization method
Total sterols were extracted from C.a using the method described by Arthington-Skaggs et al. (Citation1999) and Vieira et al. (Citation2008) with slight modifications. Briefly, a single C.a colony from an SDA plate that had been incubated overnight was used to inoculate SDB broth containing 1 mg/L of Cx or Cx:α-Cd, Cx:β-Cd, or Cx:Hp-β-Cd inclusion complexes. The cultures were incubated for 24 h at 34.0 °C. The cells were harvested by centrifugation at 2700 rpm in a centrifuge (Herolab Instruments®, Piracicaba, SP, Brazil) for 5 min and washed once with sterile distilled water. The weight of the cell pellet was determined. To each pellet was added 3 mL of a 25% ethanol KOH solution, followed by vortexing for 1 min. The resulting cell suspensions were then incubated at 85 °C in a water bath for 1 h. Following this incubation, the tubes were allowed to cool to room temperature. Sterols were then extracted by the addition of a mixture of 1 mL sterile distilled water and 3.0 mL n-heptane, followed by vigorous vortexing for 3 min. The n-heptane layer was transferred to a clean tube and stored at 20.0 °C for 24 h. Prior to analysis, a 20 µL aliquot of sterol extract was diluted five times in 100% ethanol and spectroscopically analyzed over a range of wavelengths from 240 to 300 nm using a Gilford Response Spectrophotometer (Gilford Systems, Oberlin, Ohio, USA). The presence of ergosterol and the late sterol intermediate 24(28) DHE (di-hydroergosterol) in the extracted sample resulted in a characteristic four-peak curve. Absence of detectable ergosterol in extracts was indicated by a flat line. A dose-dependent decrease in the height of the absorbance peaks was evident, corresponding to decreased ergosterol concentration. The data was obtained from three independent experiments carried out in triplicates. Ergosterol content was calculated as a percentage relative to a control C.a sample using the following formula:
Mammalian cell culture
The mouse fibroblast cell line L929 was cultured in Modified Eagle Medium (MEM) containing 4.5 g/L glucose, 2.0 mM l-glutamine, 2.2 M sodium pyruvate, 10.0 mM N-2-hydroxyethylpiperazine N-2-ethanesulfonic acid (HEPES) buffer, 2.0 g/L sodium bicarbonate, 100 U/mL amphotericin–gentamicin and 10% fetal bovine serum (Sigma, St. Louis, MO, USA) (Rhoads et al., Citation2010).The fibroblasts was tested on the 7th passage in 80% confluent monolayer.
Primary osteoblasts were isolated from neonate Wistar rats as described by Wong & Cohn (Citation1975). Briefly, calvarias were subjected to four sequential 15-min digestions in 3 mL of enzyme mixture containing 0.05% trypsin-EDTA and 0.1% collagenase at 37.0 °C. Cell fractions were pooled and resuspended in 10 mL of Dulbecco’s Modified Eagle Medium (DMEM) containing 10% FBS, 4.5 g/L glucose, 2.0 mM l-glutamine, 2.2 M sodium pyruvate, 10.0 mM HEPES, 2.0 mM non-essential amino acids, 2.0 g/L sodium bicarbonate, 100 U/mL penicillin and 100 mg/mL streptomycin. Cells were plated at a density of 1 × 104 cells/cm2, and the medium was changed 24 h later. All animal-related experiments were performed in accordance with the guidelines proposed by the Center for Laboratory Animal Medicine and Care at the University of Texas Health Science Center at Houston (UTHSCH) (Kong et al., Citation2009; Rhoads et al., Citation2010; Zhang et al., 2010). The osteoblasts were tested on the 4th passage in 80% confluent monolayer.
The human colorectal carcinoma cell line Caco-2 (ATCC, Rockville, MD, USA) was cultured in 10 mL of DMEM containing 4.5 g/L glucose, 2.0 mM l-glutamine, 2.2 M sodium pyruvate, 10.0 mM HEPES, 2.0 mM non-essential amino acids, 2.0 g/L sodium bicarbonate, 100 U/mL amphotericin–gentamicin and 10% fetal bovine serum.
Cells were subcultured after reaching confluence as observed under a phase-contrast microscope. A single cell suspension was obtained after trypsinization, and the cells were counted in a hemocytometer (Reichert, Buffalo, NY, USA). Cells were then allowed to attach to the plates for 48 h in a humidified atmosphere containing 5% CO2 at 37.0 °C (Rhoads et al., Citation2010). The Caco-2 cells were tested on the 30th passage in 80% confluent monolayer.
Cell viability assay
The neutral red (NR) assay, a colorimetric method for determining the number of viable cells in cytotoxicity assays, was conducted. To begin the assay, the cells were plated in 96-well plates (1.5 × 105 cells/well). After 48 h, the cells were treated with the experimental suspensions in serum-free DMEM at 37 °C. To prepare the suspensions, the stock suspension was vortexed to distribute the particles and then dilutions were made in serum-free DMEM to achieve final test concentrations. The test concentrations were vortexed before addition to the cell monolayers. Untreated controls were exposed to 100 µL of serum-free DMEM alone and were processed in a manner identical to the experimental groups by incubation in phenol red-free medium for 24 h. After exposure to the test suspensions, the cells were rinsed with phosphate buffered saline (PBS) and then incubated with NR solution (100 μg/mL in serum-free DMEM) at 37.0 °C for 2 h. Cells were then incubated in 2.5% glutaraldehyde fixing solution for 2 min; the cells were extracted by adding 200 µL of basic methanol (49% v/v methanol in water and 2% acetic glacial acid) to each well. After 15 min, the plate was analyzed (Thermo Scientific Multiskan® Spectrum, Vantaa, Finland) using an excitation wavelength of 540 nm and emission wavelength of 630 nm. The data was obtained from three independent experiments carried out in sixplicates. Data are reported as the percentage of untreated controls and was calculated as follows:
Cytotoxicity was assessed as described by Kong et al. (Citation2009) based on cell viability relative to controls using the following criteria: non-cytotoxic, >90% cell viability; slightly cytotoxic, 60–90% cell viability; moderately cytotoxic, 30–59% cell viability; severely cytotoxic, ≤30% cell viability.
Statistical analysis
Data were statistically analyzed using non-parametric one-way analyses of variance (ANOVA). Differences between the coefficients of variation (CVs) for the different test groups were analyzed with Bonferroni post-test. p Values less than 0.05 were considered significant.
Results
Antimicrobials and MIC determination
MIC data are summarized in . Pure Cx exhibited antifungal and antimicrobial activity against C.a, S.m and A.a. Significant efficacy was observed for 1:4 Cx inclusion complexes that are significantly more effective than Cx alone (). The 1:1 and 1:2 Cx:α-Cd complexes exhibited good antifungal activity against C.a, while the 1:3 and 1:4 Cx:β-Cd were more effective against Gram-negative bacteria (A.a), and Cx:Hp-β-Cd complexes were more effective against Gram-positive bacteria (S.m) (1:4).
Table 1. Minimal Inhibition concentration in µg/mL of chlorhexidine (Cx) and Cx-cyclodextrin inclusion complexes at 1:1; 1:2; 1:3; 1:4 molar rate against Candida albicans (C.a), Streptococcus mutans (S.m) and Aggregatibacter actinomycetemcomitans (A.a).
Sterol quantitation method
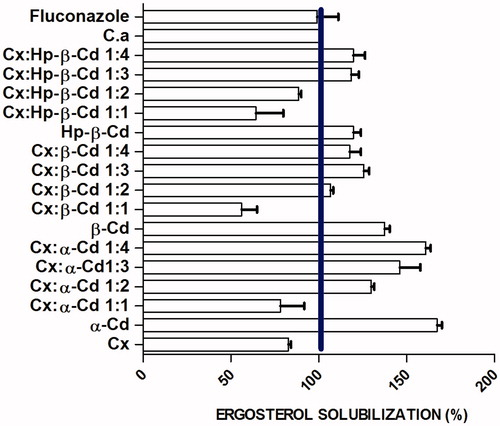
In order to characterize the interactions between the inclusion complexes and cellular membranes, we analyzed the effects of these complexes on ergosterol solubilization. Yeast was treated for 24 h with concentrations of the complexes equivalent to the MIC90 values. Treatment with Cx and 1:1 Cx:α-Cd, 1:1 Cx:β-Cd, and 1:1 and 1:2 Cx:Hp-β-Cd inclusion complexes resulted in a significant reduction in cellular ergosterol levels (). The mean of the in ergosterol solubilization increments to α-CD groups ranged from 30% to 60% after exposure to Cx-α-Cd complexes ranging from 1:1 to 1:4 molar ratios (). No significant difference in ergosterol solubilization was observed in cells treated with 1:3 and 1:2 Cx:α-Cd. The 1:1 molar ratio complexes exhibited a cyclodextrin dose-dependent decrease in total solubilization of ergosterol. The greatest levels of ergosterol solubilization were observed in cells treated with 1:3 and 1:4 Cx-Cd complexes and with pure α-Cd, β-Cd and Hp-β-Cd.
Cell viability assay
To explore the viability of osteoblasts in primary culture, fibroblasts and Caco-2 cells, we used a colorimetric method based on the properties of neutral red (NR). NR is a weak cationic dye that readily penetrates the cell membrane and accumulates in intracellular lysosomes, where it binds to anionic sites in the lysosome matrix. The NR assay revealed that treatment with Cx and Cx-Cd inclusion complexes affected cell viability in a dose-dependent manner (). Osteoblast cells appeared highly sensitive to the treatment (), as their viability was significantly reduced after exposure to 100 µg/mL Cx for 24 h (moderate cytotoxicity). Progressive decreases in the concentration of the antiseptic agent (from 100 to 1 µg/mL) were correlated with parallel increases in osteoblast cell survival (up to 80%). Treatment with α-Cd and β-Cd had moderately toxic effects on osteoblasts. Treatment with pure Hp-β-Cd at 100, 10 and 1 µg/mL stimulated proliferation of fibroblasts and osteoblasts. As shown in , treatment with 100 µg/mL α-Cd was not cytotoxic for fibroblasts and was moderately toxic for osteoblasts (), while 100 µg/mL α-Cd stimulated fibroblast proliferation. The β-Cd groups did not show cytotoxic effects. Treatment with 100 µg/mL and 10 µg/mL Cx:α-Cd and 100 µg/mL Cx:β-Cd had slight cytotoxic effects on fibroblasts; however, no cytotoxicity was observed for complexes containing Hp-β-Cd at any concentration. Among complexes containing α-Cd, the 1:2, 1:3 and 1:4 mixtures were statistically similar. At a 1 µg/mL concentration, all of the complexes proved to be biocompatible, while Cx had a moderate cytotoxic effect. Moderate cytotoxicity was observed with 1:4 Cx:α-Cd. In general, fibroblasts appeared to be more resistant to the treatment (), exhibiting substantial cell viability (moderate to no cytotoxicity) even in the presence of higher Cx concentrations. The Cx:α-Cd and Cx:β-Cd complexes were moderately cytotoxic to osteoblasts and fibroblasts, while the Cx:Hp-β-Cd inclusion complexes were the most compatible with these types of cells.
Figure 2. (A) Cell viability/proliferation of osteoblastic cells cultured for 24 h by NR Assay. Control cultures (Chlorhexidine and Osteoblasts); Cx-treated cultures at: 100 µg/mL. The values are expressed as mean ± SD obtained from three independent experiments carried out in sixplicate. (B) Cell viability/proliferation of osteoblastic cells cultured for 24 h by NR assay. Control cultures (chlorhexidine and osteoblasts); Cx-treated cultures at: 10 µg/mL. The line is at 90% level of viability. The values are expressed as mean ± SD obtained from three independent experiments carried out in sixplicate. (C) Cell viability/proliferation of osteoblastic cells cultured for 24 h by NR assay. Control cultures (chlorhexidine and osteoblasts); Cx-treated cultures at: 1 µg/mL. The line is at 90% level of viability. The values are expressed as mean ± SD obtained from three independent experiments carried out in sixplicate.
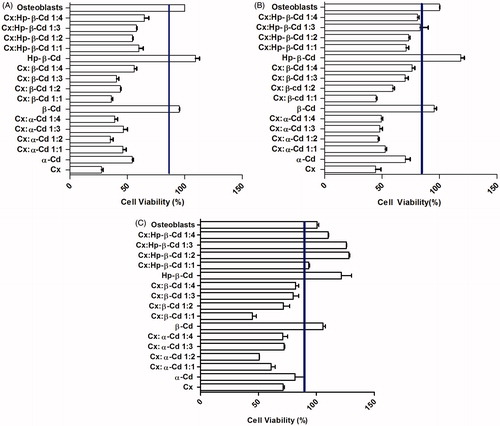
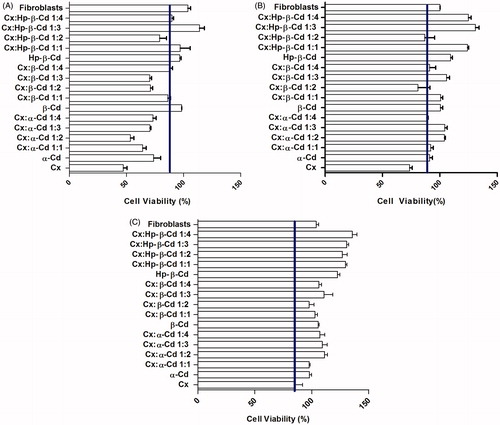
Figure 3. (A) Cell viability/proliferation of fibroblasts cultured for 24 h by NR assay. Control cultures (chlorhexidine and fibroblasts); Cx-treated cultures at: 100 µg/mL. The line is at 90% level of viability. The values are expressed as mean ± SD obtained from three independent experiments carried out in sixplicate. (B) Cell viability/proliferation of fibroblasts cultured for 24 h by NR assay. Control cultures (chlorhexidine and fibroblasts); Cx-treated cultures at: 10 µg/mL (B). The line is at 90% level of viability. The values are expressed as mean ± SD obtained from three independent experiments carried out in sixplicate. (C) Cell viability/proliferation of fibroblasts cultured for 24 h by NR assay. Control cultures (chlorhexidine and fibroblasts); Cx-treated cultures at: 1 µg/mL. The line is at 90% level of viability. The values are expressed as mean ± SD obtained from three independent experiments carried out in sixplicate.
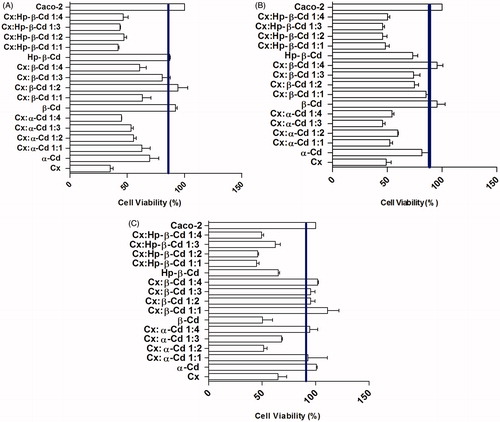
No matter which cyclodextrin used, the inclusion complexes tested were not able to stimulate the proliferation of Caco-2 cells (). None of the concentrations tested were observed to have severe cytotoxic effects, and the highest levels of cytotoxicity observed were moderate in cells treated with 100 µg/mL doses.
Figure 4. (A) Cell viability/proliferation of Caco-2 cells cultured for 24 h by NR assay. Control cultures (chlorhexidine and Caco-2 cells); Cx-treated cultures at: 100 µg/mL. The line indicated de 90% level, which correspond to the no cytotoxic results. The values are expressed as mean ± SD obtained from three independent experiments carried out in sixplicate. (B) Cell viability/proliferation of Caco-2 cells cultured for 24 h by NR assay. Control cultures (chlorhexidine and Caco-2 cells); Cx-treated cultures at: 10 µg/mL. The line indicated de 90% level, which correspond to the no cytotoxic results. The values are expressed as mean ± SD obtained from three independent experiments carried out in sixplicate. (C) Cell viability/proliferation of Caco-2 cells cultured for 24 h by NR assay. Control cultures (chlorhexidine and Caco-2 cells); Cx-treated cultures at: 1 µg/mL. The line indicated de 90% level, which correspond to the no cytotoxic results. The values are expressed as mean ± SD obtained from three independent experiments carried out in sixplicate.
Together, our results indicate that Cx exhibits specific cytotoxicity when associated with CD. Viability of osteoblasts and fibroblasts was more affected by complexes containing α-Cd compared to the other Cds, and this effect was dose-dependent. However, absence of cytotoxicity for osteoblasts and fibroblasts was observed after treatment with Hp-β-Cd complexes. These results are in agreement with those from a previous study of Cx cytotoxicity in fibroblasts and osteoblasts in vitro (Gianelli et al., Citation2008; Faria et al., Citation2009).
Discussion
Current drug treatments are largely effective in control of gingival disease; however, resistant strains and side effects associated with the use of high concentrations of Cx are rapidly emerging. In this study, the effects of a variety of Cx-cyclodextrin inclusion complexes on cell viability were tested and compared in order to develop new potential therapies that may minimize these problems. The effectiveness of these complexes was verified, as previously reported (Teixeira et al., Citation2012, Citation2013). In all groups, 1:3 and 1:4 Cx:Cd molar ratios improved the antimicrobial activity of the inclusion complexes, irrespective of the identity of the Cd.
The different physical and chemical properties of cyclodextrins can change the properties of guest molecules. Based on this concept, we hypothesized that the activity of a drug/Cd complex can be tailored by varying the identity and concentration of the Cd. This study corroborated data from a preliminary study of Cx-β-Cd inclusion complexes (Teixeira et al., Citation2012) and compared the effectiveness of these complexes to that of α-Cd- and Hp-β-Cd-containing complexes. Hp-β-Cd and Cx-β-Cd complexes exhibited a lower ability to extract ergosterol when compared with α-Cd complexes.
High concentrations of Cds have been shown to have limited specificity for cholesterol and to cause significant membrane disorganization in previous studies, consistent with our results (Irie et al., Citation1992; Ohvo-Rekila et al., Citation2000; Kiss et al., Citation2010). However, we observed saturation of ergosterol solubilization for 1:3 and 1:4 Cx:Cd complexes (). These observations are consistent with results from Ohvo-Rekila et al. (Citation2000), who found that cyclodextrin concentrations reach a saturating level (2.5 mM) at which desorption of sterols from the bilayer membrane was rate-limiting (Clejan & Bittman, Citation1984; Haynes et al., Citation2000). Atger et al. (Citation1997) observed that the location of cyclodextrin molecules near the surface of the cell allows membrane cholesterol to desorb directly into the hydrophobic core of the cyclodextrin (Besenicar et al., Citation2008).
In recent years, Cds have been used as tools to manipulate the lipid composition of biological and model membranes (Clejan & Bitman, Citation1984). Sterols can be removed from cells by extraction, forming small unilamellar vesicles. Similarly, chlorhexidine induces changes in the fluidity of the yeast membrane (Teixeira et al., Citation2012). Antibiotic molecules must compete with phospholipids in order to interact with cholesterol and form pores (Pankov et al., Citation2006; Shaw et al., Citation2008). Potentially, variations in membrane lipid composition could be a factor that modulates membrane fusogenic capacity and affects membrane and cellular pathology (Pankov et al., Citation2006). Recent evidence suggests that lipid rafts can serve as docking sites for a broad range of pathogens, including viruses and bacteria (Yunomae et al., Citation2003; Pucadyil & Chattopadhyay, Citation2007; Yang et al., Citation2007). Removal of cholesterol from cell membranes is reported to disrupt the integrity of these domains, and, therefore, represents a widely used strategy to disrupt putative functions mediated by these domains. This approach forms the basis of a number of reports implicating such domains in important cellular functions, such as signal transduction and entry of pathogens (Yunomae et al., Citation2003; Pucadyil & Chattopadhyay, Citation2007).
The cellular cytotoxicity of these complexes confirms the results from ergosterol solubilization experiments, as complexes with higher cytotoxicity also exhibited the greatest ergosterol solubilization. The cytotoxicity of these inclusion complexes may be associated with increased cell permeability and membrane disorganization caused by solubilization of membrane sterols. Changes in the cell surface or the sensitive lysosomal membrane lead to lysosome fragility and other changes that gradually become irreversible (Haynes et al., Citation2000; Kiss et al., Citation2010).
Several studies have reported that Cx was able to cause a large variety of cytotoxic effects on eukaryotic cells like. This antimicrobial drug has toxic effects on cultured of alveolar bone (Cabral & Fernandes, Citation2007) and gingival epithelial cells (Babich et al., Citation1995) and was able to induce a dose-dependent reduction of human gingival fibroblast proliferation and reduces both collagen and non-collagen protein production (Mariotti & Rumpf, Citation1999; Flemingson et al., Citation2008). Cx prevents fibroblast attachment to root surfaces and interferes with periodontal regeneration (Alleyn et al., Citation1991). Our results of cytotoxicity assays are supported by those of Gianelli et al. (Citation2008), who characterized chlorhexidine cytotoxicity by colorimetric assay and microscopy. Based on previous about Cx-cyclodextrin we had been demonstrating the long term effect of this system (Yue et al., Citation2004). In this study we are given the higher focus in chlorhexidine-cyclodextrin effect on biological properties for the best understanding.
The external component of Cd, which contains many hydrophilic hydroxide groups, may improve the interaction between Cd and cells (Yunomae et al., Citation2003; Loftsson et al., Citation2007). Yang et al. (Citation2007) reported that the introduction of α-Cd decreased the cytotoxicity of polyether-imide (PEI). It is thought that the high density of amino groups and the high molecular weight were the reasons underlying the high cytotoxicity of PEI.
Due to the possibility of anti-tumor effects, inclusion complex-induced cytotoxicity of Caco-2 cells was analyzed. In previous studies dimethyl-β-Cd and methyl-β-Cd modified cyclodextrins were shown to markedly reduce the viability of Caco-2 cells at concentrations greater than 50 mM. In our study, we found that Hp-β-Cd inclusion complexes were more cytotoxic against Caco-2 cells, consistent with previous results (Loftsson et al., Citation2007).
As expected, Cx treatment caused massive cell death in all cell types at any concentration; however, addition of cyclodextrin decreased this effect. The 1:3 Cx:Hp-β-Cd complexes were the most biocompatible. The cytotoxic properties of the best system for cholesterol solubilization were associated with the most toxic derivatives (α-Cd). α-Cd demonstrated the best activity against C.a. Additionally, β-Cd and Hp-β-Cd had significantly lower cytotoxic effects than the other complexes. These findings suggest abilities of different cyclodextrins to interact with membranes in different manners and to specifically modulate the activity of Cx on different cell types.
The differential activity of 1:4 Cx-Cd complexes may be explained by changes in adhesion of cyclodextrin to the membrane, as opposed to free Cx. (Loftsson et al., Citation2004, Citation2007). The hydrophilic outer surface of Cd molecules forms a weak interaction with biological membranes (Yue et al., Citation2004; Abdelwahed et al., Citation2008). Complexes derived from α-Cd would be more likely to bind cells with high ergosterol content. Hp-β-Cd would be more suitable for formation of complexes for which the cytotoxic effects should be minimal or absent, such as anti-inflammatories, analgesics, anesthesics, or anti-depressive drugs. The presence of β-Cd exerts a positive influence on the antimicrobial activity of Cx with mild cytotoxic effects on eukaryotic cells, as treatment with 1:2 and 1:3 Cx:β-Cd complexes yielded the best results.
These results could be explained as a function of variations in the properties of biological membranes. The major membrane components of prokaryotic cells are phospholipids, and their membranes contain higher amounts of anionic lipids than eukaryotic cell membranes, but no cholesterol (Barman et al., Citation2006; Gianelli et al., Citation2008). This composition seems to be of fundamental importance for membrane interaction, activity, and selectivity of antimicrobial drugs (Ohvo-Rekila et al., Citation2000). Hydrogen bonding is responsible for the tight packing of lipid rafts but still ensures a high degree of lipid mobility (Ohvo-Rekila et al., Citation1997). The acyl chains between the phospholipids are held together by van der Waals interactions, which are considerably weaker than hydrogen bonds and, therefore, are responsible for looser packing of prokaryotic membranes and non-lipid raft portions of eukaryotic cell membranes (Irie et al., Citation1992; Barman et al., Citation2006).
The approach analyzed in this study is well-suited to the characterization of cyclodextrin-cell interactions. This fact may critically contribute to decreasing toxicological effects of drugs on cells being that the appropriate cyclodextrin could be used for specific interaction with the membrane or cell wall.
Conclusion
Results from this study suggest that α-Cd, β-Cd and Hp-β-Cd inclusion complexes could be promising tools that could be used as an alternative to treatment with chlorhexidine alone. Future research regarding the in vitro/in vivo correlation efficacy of these complexes in animal models may clarify their clinical utility. The hypothesis that α-Cd, β-Cd and Hp-β-Cd modulate the cellular effects of chlorhexidine is supported by the following experimental observations: (i) the addition of cyclodextrin to Cx decreases cytotoxicity in eukaryotic cells; (ii) Cx:Cd complexes inhibit microorganism growth and increase ergosterol solubilization in a dose-dependent manner, with saturation at a 1:3 drug/cyclodextrin molar ratio; (iii) treatment with pure cyclodextrin stimulates osteoblast and fibroblast proliferation; and (iv) all complexes are more cytotoxic to osteoblasts than fibroblasts, and α-Cd inclusion complexes increase cytotoxic effects on Caco-2 cells. Based on the observations above, we propose that α-Cd, β-Cd and Hp-β-Cd were able to modulate the cellular effects of Cx. We also believe that the cholesterol-solubilizing properties of these complexes depend on Cd structure and may serve as predictive factors of cellular cytotoxicity. Cyclodextrins provide a unique tool to modulate the activity of Cx and could be used for selective functions.
Declaration of interest
This study was carried out as part of our routine work. The National Institute of Science and Technology (15/2008-Nanobiofarmaceutics) and Fundação de Amparo à Pesquisa de Minas Gerais (APQ-02348-10) provided the financial support. We would like to thank to the Coordenação de Aperfeiçoamento Pessoal de Nível Superior (CAPES) for the PhD scholarship (063.08/2012).
References
- Abdelwahed W, Degobert G, Dubes A, et al. (2008). Sulfated and non-sulfated amphiphilic-β-cyclodextrins: impact of their structural properties on the physicochemical properties of nanoparticles. Int J Pharm 351:289–95
- Adriaanse AH, Muytjens HL, Heessen FWA, et al. (1996). In vitro activity of chlorhexidine, hexetidine and bacitracin against perinatal pathogens. J Antimicrob Chemother 37:192–4
- Ahmed SM, Casu B, Cedro A, et al. (1994). Disruption of micellar aggregates of glangioside GM-1 by complexation with α-cyclodextrin. Int J Pharm 109:99–106
- Alleyn CD, O’Neal RB, Strong SL, et al. (1991). The effect of chlorhexidine treatment of root surfaces on the attachment of human gingival fibroblast in vitro. J Periodontol 62:434–8
- Arthington-Skaggs BA, Jradi H, Desai T, et al. (1999). Quantitation of ergosterol content: novel method for determination of fluconazole susceptibility of Candida albicans. J Clin Microbiol 37:3332–7
- Atger VM, Moya ML, Stoudt GW, et al. (1997). Cyclodextrins as catalysts for the removal of cholesterol from macrophage foam cells. J Clin Invest 99:773–80
- Babich H, Wurzburger BJ, Rubin YL, et al. (1995). An in vitro study on the cytotoxicity of chlorhexidine digluconate to human gingival cells. Cell Biol Toxicol 11:79–88
- Badi N, Guégan P. (2007). Per-O-(3-hydroxy)propyl-β-cyclodextrin: a cyclodextrin derivative bearing only primary hydroxyl groups. Carbohydrate Res 342:1989–91
- Barman H, Walch ML, Latinovic-Golic S, et al. (2006). Cholesterol in negatively charged lipid bilayers modulates the effect of the antimicrobial protein granulysin. J Membrane Biol 212:29–39
- Beseničar MP, Bavdek A, Kladnik A, et al. (2008). Kinetics of cholesterol extraction lipid membranes by methyl-β-cyclodextrin – A surface plasmon resonance approach. Biochem Biophys Acta 1778:175–84
- Best AJ, Nixon MF, Taylor GJS. (2007). Brief exposure of 0.05% chlorhexidine does not impair non-osteoarthritic human cartilage metabolism. J Hospital Infect 67:67–71
- Cabral CT, Fernandes MH. (2007). In vitro comparison of chlorhexidine and povidone–iodine on the long-term proliferation and functional activity of human alveolar bone cells. Clin Oral Invest 11:155–64
- Cal K, Centkowska K. (2008). Use of cyclodextrins in topical formulations: practical aspects. Eur J Pharm Biopharm 68:467–78
- Clejan S, Bittman R. (1984). Kinetics of cholesterol and phospholipid exchange between Mycoplasma gallisepticum cells and lipid vesicles. Alterations in membrane cholesterol and protein content. J Biol Chem 259:441–8
- CLSI (Clinical and Laboratory Standards Institute/NCCLS). (2005a). Performance Standards for Antimicrobial Susceptibility Testing; Fifteenth Informational Supplement. CLSI/NCCLS document M100-S15 [ISBN 1-56238-556-9]. Wayne, Pennsylvania USA, 19087-98
- CLSI (Clinical and Laboratory Standard Institute). (2005b). Reference method for broth dilution Antifungal susceptibility testing of yeast: approved standards. Document CLSI M27-A2, CLSI, Wayne, Pennsylvania USA
- Cortés ME, Sinisterra RD, Ávila-Campos MJ, et al. (2001). The chlorhexidine:beta-ciclodextrin inclusion complex: preparation, characterization and microbiological evaluation. J Inclusion Phen Macrocyclic Chem 40:297–302
- Davis ME, Brewster ME. (2004). Cyclodextrin-based pharmaceutics: past, present and future. Nature Rev 3:1023–35
- Del Valle EMM. (2004). Cyclodextrins and their uses: a review. Process Biochem 39:1033–46
- Denadai AML, Teixeira KIR, Santoro MM, et al. (2007). Supramolecular self-assembly of β-cyclodextrin: an effective Carrier of the antimicrobial agent chlorhexidine. Carbohydr Res 342:2286–96
- Denyer SP. (1995). Mechanisms of action of antibacterial biocides. Int Biodeterior Biodegrad 36:227–45
- Drisko CH. (2001). Non-surgical periodontal therapy. Periodontology 2000 25:77–88
- Faria G, Cardoso CRB, Larson RE, et al. (2009). Chlorhexidine-induced apoptosis or necrosis in L929 fibroblasts: a role for endoplasmic reticulum stress. Toxicol Appl Pharm 234:256–65
- Flemingson EP, Ambalavanan N, Ramakrishnan T, Vijayalakshmi R. (2008). Effect of three commercial mouth rinses on cultured human gingival fibroblast:Na in vitro study. Indian J Dent Res 19:29–35
- Frank DW, Gray JE, Weaver RN. (1976). Cyclodextrin nephrosis in the rat. Am J Pathol 83:367–82
- Gianelli M, Chellini F, Margheri M, et al. (2008). Effect of chlorhexidine digluconate on different cell types: a molecular and ultra structural investigation. Toxicol in vitro 22:308–17
- Harada A, Takashima Y, Yamaguchi H. (2009). Cyclodextrin-based supramolecular polymers. Chem Soc Rev 38:875–82
- Haeberlin B, Gengenbacher T, Meinzer A. (1996). Cyclodextrin: useful excipients for oral peptide administration? Int J Pharm 137:103–10
- Haynes MP, Phillips MC, Rothblat GH. (2000). Efflux of cholesterol from different cellular pools. Biochem 39:4508–17
- Hidalgo E, Dominguez C. (2001). Mechanism’s underlying chlorhexidine-induced cytotoxicity. Toxicol in vitro 15:271–6
- Hugo W, Longworth A. (1964). Some aspects of the mode of action of chlorhexidine. J Pharm Pharmacol 16:655–62
- Irie T, Fukunaga K, Pitha J. (1992). Hydroxypropylcyclodextrins in parenteral use. I: lipid dissolution and effects on lipid transfers in vivo. J Pharm Sci 81:521–3
- Jeffcoat MK, Bray KS, Ciancio SG, et al. (1998). Adjunctive use of a subgingival controlled-release chlorhexidine chip reduces probing depth and improves attachment level compared with scaling and root planing alone. J Periodontol 69:989–97
- Karpanen TJ, Casey AL, Conway BR, et al. (2011). Antimicrobial activity of a chlorhexidine intravascular catheter site gel dressing. J Antimicrob Chemother 66:1777–84
- Karpanen TJ, Worthington T, Hendry ER, et al. (2008). Antimicrobial efficacy of chlorhexidine digluconate alone and in combination with eucalyptus oil, tea tree oil and thymol against planktonic and biofilm cultures of Staphylococcus epidermidis. J Antimicrob Chemother 62:1031–6
- Kasper JC, Friess W. (2011). The freezing step in lyophilization: physico-chemical fundamentals, freezing methods and consequences on process performance and quality attributes of biopharmaceuticals. Eur J Pharm Biopharm 78:248–63
- Kiss T, Fenyvesia F, Bácskaya I, et al. (2010). Evaluation of the cytotoxicity of β-cyclodextrin derivatives: evidence for the role of cholesterol extraction. Eur J Pharm Sci 40:376–80
- Kong N, Jiang T, Zhou Z, et al. (2009). Cytotoxicity of polymerized resin cements on human dental pulp cells in vitro. Dental Mater 25:1371–5
- Liu L, Guo QX. (2002). The driving forces in the inclusion complexation of cyclodextrins. J Incl Phenom Macro 42:1–14
- Loftsson T, Vogensen SB, Brewster ME, et al. (2007). Effects of cyclodextrins on drug delivery through biological membranes. J Pharm Sci 96:2532–46
- Loftsson T, Masson M, Brewster ME. (2004). Self-association of cyclodextrins and cyclodextrin complexes. J Pharm Sci 93:1091–9
- Loftsson T, Brewster ME. (1996). Pharmaceutical applications of cyclodextrins. 1. Drug solubilization and stabilization. J Pharm Sci 85:1017–25
- Maggi L, Conte U, Bettinetti GP. (1998). Technological properties of crystalline and amorphous α-cyclodextrin hydrates. Int J Pharm 172:211–17
- Mariotti AJ, Rumpf D. (1999). Chlorhexidine-induced changes to human gingival fibroblasts collagen and non-collagen protein production. J Periodontol 70:1443–8
- McDonnell G, Russell AD. (1999). Antiseptics and disinfectants: activity, action and resistance. Clin Microbiol Rev 12:147–79
- Mesmin B, Maxfield FR. (2009). Intracellular sterol dynamics. Biochim Biophys Acta 1791:636–45
- Mura P, Liguori A, Bramanti G, et al. (1992). Improvement of dissolution properties and microbiological activity of miconazole and econazole by cyclodextrin complexation. Eur J Pharm Biopharm 38:119–23
- Ohvo-Rekila H, Bjorn A, Kerlund J, et al. (2000). Cyclodextrin-catalyzed extraction of fluorescent sterols from monolayer membranes and small unilamellar vesicles. Chem Phys Lipids 105:167–78
- Ohvo-Rekila H, Olsio C, Slotte JP. (1997). Effects of sphingomyelin and phosphatidylcholine degradation on cyclodextrin-mediated cholesterol efflux from cultured fibroblasts. Biochim Biophys Acta 1349:131–41
- Pankov R, Markovska T, Antonov P, et al. (2006). The plasma membrane lipid composition affects fusion between cells and model membranes. Chem Biol Interact 164:167–73
- Pfaller MA, Burmeister L, Bartlett MS, et al. (1988). Multicenter evaluation of four methods of yeast inoculum preparation. J Clin Microbiol 26:1437–41
- Pucadyil TJ, Chattopadhyay A. (2007). Cholesterol depletion induces dynamic confinement of the G-protein coupled serotonin1A receptor in the plasma membrane of living cells. Biochim Biophys Acta 1768:655–68
- Raso EMG, Cortes ME, Teixeira KIR, et al. (2010). A new controlled release system of chlorhexidine and chlorhexidine:β-cd inclusion complexes based on porous silica. J Inclusion Phen Macrocyclic Chem 67:159–68
- Rhoads LS, Silkworth WT, Roppolo ML, et al. (2010). Cytotoxicity of nanostructured vanadium oxide on human cells in vitro. Toxicol in vitro 24:292–6
- Salim N, Moore C, Silikas N, et al. (2013). Candidacidal effect of fluconazole and chlorhexidine released from acrylic polymer. J Antimicrob Chemother 68:587–92
- Shaw JE, Epand RF, Hsu JCY, et al. (2008). Cationic peptide-induced remodeling of model membranes: direct visualization by in situ atomic force microscopy. J Struct Biol 162:121–38
- Sheppard FC, Mason DJ, Bloomfield SF, et al. (1997). Flow cytometric analysis of chlorhexidine action. FEMS Microbiol Lett 154:283–8
- Slots J, Ting M. (1999). Actinobacillus actinomycetemcomitas and Porphyromonas gingivalis in human periodontal disease: occurrence and treatment. Periodontol 2000 20:82–121
- Stella VJ, He Q. (2008). Cyclodextrins. Toxicol Pathol 36:30–42
- Taneri F, Ozcan I, Guneri T. (2010). In vitro and in vivo evaluation of oral tablet formulations prepared with ketoconazole and hydroxypropyl-β-cyclodextrin. Drug Delivery 17:152–7
- Teixeira KIR, Araújo PV, Neves BRA, et al. (2013). Ultrastructural changes in bacterial membranes induced by nano-assemblies beta-cyclodextrin chlorhexidine: SEM, AFM, and TEM evaluation. Pharm Develop Tech 18:600–8
- Teixeira KIR, Araújo PV, Sinisterra RD, et al. (2012). Chlorhexidine:beta-cyclodextrin blocks yeast growth by extraction of ergosterol. Braz J Microbiol 43:810–18
- Uekama K, Hirayama F, Irie T. (1998). Cyclodextrin drug carrier systems. Chem Rev 98:2045–76
- Uekama K, Kondo K, Nakamura K, et al. (1995). Modification of rectal absorption of morphine from hollow-type suppositories with a combination of α-cyclodextrin and viscosity-enhancing polysaccharide. J Pharm Sci 84:15–20
- Uekama K, Hirayama F, Wakuda T, et al. (1981). Effect of cyclodextrins on the hydrolysis of prostacyclin and its methyl ester in aqueous solution. Chem Pharm Bull 29:213–19
- Vieira FT, Menezes DC, De Lima GM, et al. (2008). Effect of diorganotin(IV) carboxylate complexes, [N-(2-carboxyphenyl)salicylideneiminato]dimethyltin(IV), bis(µ3-oxo)bis(µ-O-aminobenzoato-O,O′)bis(O-minobenzoato)tetrakis[dimethyltin(IV)] and bis(O-aminobenzoato-O,O′) di-n-butyltin(IV), on the membrane of Candida albicans cells – a mechanistic investigation of the antifungal activity of organotin complexes. App Organometal Chem 22:433–9
- Zhang W, Swearingen EB, Jun J, et al. (2010). Porphyromonas gingivalis invades osteoblasts and inhibits bone formation. Microbes Infect 12:838–45
- Zhao M, Wang H, Yang B, et al. (2010). Identification of cyclodextrin inclusion complex of chlorogenic acid and its antimicrobial activity. Food Chem 120:1138–142
- Zheng XM, Lu WM, Sun DZ. (2001). Enthalpy and entropy criterion for the molecular recognize of some organic complexes with beta cyclodextrin. Acta Phys Chim Sin 17:343–7
- Wong GL, Cohn DV. (1975). Target cells in bone for parathormone and calcitonin are different: enrichment for each cell type by sequential digestion of mouse calvaria and selective adhesion to polymeric surfaces. Proc Natl Acad Sci USA 72:3167–71
- Yang C, Li H, Goh SH, et al. (2007). Cationic star polymers consisting of alphacyclodextrin core and oligoethylenimine arms as non-viral gene delivery vectors. Biomaterials 28:3245–54
- Yue IC, Poff J, Cortés ME, et al. (2004). A novel polymeric chlorhexidine delivery device for the treatment of periodontal disease. Biomaterials 25:3743–50
- Yunomae K, Arima H, Hirayama F, et al. (2003). Involvement of cholesterol in the inhibitory effect of dimethyl-β-cyclodextrin on P-glycoprotein and MRP2 function in Caco-2 cells. FEBS Lett 536:3225–31