Abstract
Context: This study presents novel self-nanoemulsifying drug delivery system potential of oral delivering which leads poorly aqueous soluble drug glimepiride.
Objective: The objective of this study was to prepare solid self-nanoemulsifying drug delivery system (S-SNEDDS) for the improved oral delivery of glimepiride and to evaluate its therapeutic efficacy in albino rabbits.
Results and discussion: The droplet size analyses revealed a droplet size of less than 200 nm. The solid state characterization of S-SNEDDS by scanning electron microscopy (SEM), X-ray powder diffraction and differential scanning calorimetry (DSC) revealed the absence of crystalline glimepiride in the S-SNEDDS. The in vitro dissolution studies revealed that the significant improvement in glimepiride release characteristics. The effect of S-SNEDDS on therapeutic efficacy of glimepride was assessed in albino rabbits by monitoring blood glucose levels and compared with free drug suspension, L-SNEDDS. The S-SNEDDS showed significant (p < 0.05) increase in in vitro drug release and therapeutic efficacy as compared with free drug.
Conclusion: This study demonstrated that S-SNEDDS is a promising novel drug delivery system of glimepride to enhance oral delivery.
Introduction
The oral delivery of many new drug molecules is recurrently associated with complications of low water solubility or high lipophilicity which leads to poor and highly variable oral bioavailability and the absence of dose proportionality (Kyatanwar et al., Citation2010). This class of drug compounds can be classified as low solubility and high permeability Bio Pharmaceutical Classification System (BCS) class II drugs. In these drugs, dissolution of the drug is the rate limiting step in the absorption process. To triumph over these obstacles, numbers of formulation approaches are reported including the use of surfactants (Allaboun et al., Citation2003; Balakrishnan et al., Citation2004; Chakraborty et al., Citation2009), lipids (Yeap et al., Citation2013), permeation enhancers (Burcin et al., Citation2010; Beg et al., Citation2011), formation of salt (Li et al., Citation2005; Serajuddin, Citation2007), co-crystallization (Shan & Zaworotko, Citation2008; Qiao et al., Citation2011; Chadha et al., Citation2012), solid dispersions (Serajuddin, Citation1999), inclusion complexes with cyclodextrins and modified cyclodextrins (Miyake et al., Citation2000; Veiga et al., Citation2000; Wang et al., Citation2000; Bannwart et al., Citation2001; Carrier et al., Citation2007; Gamsiz et al., Citation2010a,Citationb; Gamsiz et al., Citation2011; Badr-Eldin et al., Citation2008; Kumar et al., Citation2013), nanosuspensions (Patravale et al., Citation2004), and colloidal vesicles like liposomes (Nazzal et al., 2002a; Manconi et al., Citation2013; Yang et al., Citation2013), and niosomes (Khazaeli et al., Citation2007; Bayindir & Yuksel, Citation2010; Sezgin-Bayindir et al., Citation2013; Jin et al., Citation2013)
In modern years, self-nanoemulsifying drug delivery systems (SNEDDS) are the most popular and commercially feasible lipid-based formulation approach for improving oral bioavailability of poorly water soluble and lipophilic drugs (Pouton, Citation2006; Date, Citation2007; Shweta et al., Citation2011). SNEDDS are precisely defined as an isotropic multi-component drug delivery systems composed of a synthetic or natural oil, surfactant, and co-surfactant that have a unique ability of forming fine oil in water micro- or nano-emulsion upon mild agitation followed by dilution in aqueous media such as gastro-intestinal fluid. As SNEDDS self-emulsifies in the stomach and presents the drug in minute droplets of oil (<5 µm), it improves drug dissolution through presenting a large interfacial area for partitioning of the drug between the oil and the GIT fluid. The other advantages include increased stability of drug molecules and ease of administering the final formulation as gelatin capsules (soft gelatin capsules in the case of liquid self-nanoemulsifying drug delivery system (L-SNEDDS) and hard gelatin capsules in the case of solid self-nanoemulsifying drug delivery system (S-SNEDDS)).
Glimepiride,1-[[p-[2-(3-ethyl-4-methyl-2-oxo-3-pyrroline-1-carboxami-do) ethyl] phenyl] sulphonyl]-3-(trans-4-methylcyclohexyl) urea is the first third-generation sulphonyl urea. It is a very potent sulphonyl urea employed for concomitant use with insulin for the treatment of non-insulin-dependent (type II) diabetes mellitus. It produces hypoglycemia by stimulating release of insulin from pancreatic β cells and by increasing the sensitivity of peripheral tissue to insulin. It also supports the movement of sugar from the blood into the cells that need it. Glimepiride shows low, pH-dependent solubility. It exhibits very poor solubility at 37 °C (<0.004 mg/ml) in acidic and neutral aqueous media and it belongs to “BCS Class II” drugs (Lobenberg & Amidon, Citation2000). It is likely to show low and irregular bioavailability following oral administration due to the low water solubility (Amidon et al., Citation1995; Grunenberg et al., Citation1995). Hence administering glimepiride by oral appears as a tough challenge due to its poor absorption pattern and rapid and unpredictable hepatic first pass metabolism.
The present study deals with formulation of an Aerosol® 200 based SNEDDS of a poorly water soluble drug (glimepride). The main objective of this study was to investigate solid self-nanoemulsifying drug delivery system, as a potential drug delivery system for glimepiride. S-SNEDDS (consisting of Tween® 80/PEG and 400/Mygliol® 812) was characterized with regard to morphological analysis, solid state characterization as well as its in vitro drug release and therapeutic efficacy in albino rabbits.
Materials
Glimepiride was kindly gifted by Dr Reddy’s Labs, Hyderabad, India. Miglyol® 812 (Capric Triglyceride), Cotton seed oil, Aerosol® 200 (Dioxosilane), and Cremophor® RH 40 (Macrogolglycerol hydroxystearate) were gift samples from Bari’s Pharmaceuticals, Hyderabad, India. Tween® 80 (Polyoxyethylene sorbitan monooleate), PEG 400 (polyethylene glycol 400), Span® 20 (Sorbitan monolaurate), oleic acid, soya bean oil, ethyl alcohol, and HPLC grade methanol were purchased from Merck Specialties Pvt. Ltd., Mumbai, India. Span® 80 (Sorbitan monooleate), propylene glycol, and potassium dihydrogen phosphate were obtained from SD-Fine Chemicals Ltd., Mumbai, India. All other chemicals and materials were of analytical grade.
Methods
Operating conditions of HPLC for glimepiride analysis
Glimepiride was determined by high-pressure liquid chromatography (HPLC) using HPLC system (UFLC prominence HPLC, Shimadzu, Kyoto, Japan) with a PDA detector (SPD20A) at a wavelength of 228 nm and a manual injector with injection volume setting at 20 µl. The mobile phase consists of Acetonitrile: 0.2 M phosphate buffer (pH 7.4) was pumped at a flow rate of 1 ml/min. The drug was separated by Gemini C18, 100 × 4.6 mm (ID), and 5 µm column at ambient temperature.
Solubility studies
The solubility of glimepiride was determined in oils (Miglyol® 812, castor oil, oleic acid, cotton seed oil, and soya bean oil), surfactants, and co-surfactants (Tween® 80, PEG 400, propylene glycol, Cremophor® RH 40, Span® 80, and Span® 20). An excess amount (500 mg) of drug was added to each 1.5 ml eppendorf tubes consisting of 1 ml of each vehicle and the contents were mixed using a vortex mixer (REMI CM 101DX, REMI Equipment, Mumbai, India) to facilitate uniform mixing of drug for 15 min. The Eppendorf tubes were kept at 25 ± 1.0 °C in an orbital shaker (CL 24, Remi Electrotech Ltd., Mumbai, India) for 48 h to reach equilibrium (Wang et al., Citation2009). The tubes were centrifuged at 5000 rpm using micro centrifuge (RM 12C, REMI Equipment, Mumbai, India) for 20 min. The supernatant was filtered through a 0.45-µm syringe filter and an aliquot of 0.1 ml from the filtered supernatant was collected and diluted with methanol. The quantification of glimepiride was performed by HPLC (Prominence HPLC, Shimadzu, Japan) at 228 nm using a PDA detector.
Selection of oil
The selection of oil was based on solubility of glimepiride in the oils. Higher the solubility of the drug, higher will be the drug loading potential (Pouton, Citation2000, Citation2006). In this study, the drug was significantly more soluble in Miglyol® 812. Thus, the same was chosen as the oil phase for formulating the SNEDDS system.
Selection of surfactant
The screening of surfactant was based on both the ability to solubilize the drug in it and its ability to emulsify the selected oil phase. The solubility of all the surfactants include Tween® 80, Span® 20, Span® 80, and Cremophor® RH 40 was determined from solubility studies. Then to examine their emulsification ability, 20 µl of surfactant and 20 µl of the above selected oil phase were mixed together thoroughly in an Eppendorf tube. From this, 25 µl of mixture was added to 25 ml of distilled water in a standard flask. The ease of emulsification was checked by the number of inversions of standard flask enough to produce to uniform emulsion. The formed emulsions were allowed to stand for 2 h. Then the transmittance was determined using a double beam UV-visible spectrophotometer (UV-3500, Labindia, Mumbai, India) at 638.2 nm against distilled water as the blank (Date & Nagarsenker, Citation2007; Shweta et al., Citation2011).
Selection of co-surfactant
The screening of co-surfactants was based on both the ability to solubilize the drug in it and their efficacy to improve the nanoemulsification ability of the selected surfactants. The solubility of the drug in the co-surfactants namely PEG 400, propylene glycol was determined from the solubility studies same as in the case of oils and surfactants. Then to screen the efficacy of co-surfactants, 40 µl of the selected surfactant and 20 µl of co-surfactant to be screened and 60 µl of selected oil phase were mixed together thoroughly in an Eppendorf tube. From this mixture, 25 µl was added to 25 ml of distilled water in a standard flask. The ease of emulsification was checked in a similar fashion as described above.
Construction of ternary phase diagram
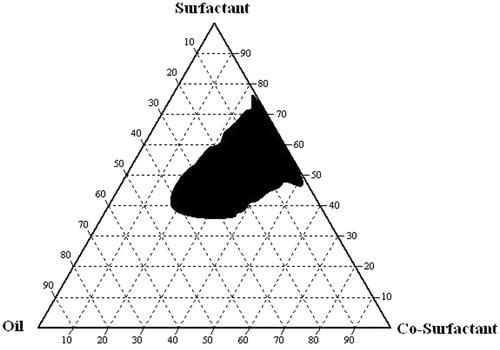
For the construction of ternary phase diagrams, the screened surfactant and co-surfactants were mixed (Smix) in three different weight ratios (1:1, 2:1, and 3:1). These Smix ratios were chosen to show increasing surfactant concentrations with respect to co-surfactants. The oil phase and each Smix ratios (a total of 51 ratios) were blended thoroughly in 17 different weight ratios from 1:9 to 9:1 (1:1, 1:2, 1:3, 1:4, 1:5, 1:6, 1:7, 1:8, 1:9, 9:1, 8:1, 7:1, 6:1, 5:1, 4:1, 3:1, and 2:1). From these each ratio, 0.1 ml of mixtures was transferred to separate glass beakers. To these contents, 100 ml distilled water was added gently agitated using a magnetic bar at 37 °C. The transmittance of the formed emulsions were determined using an UV–visible double beam spectrophotometer (UV-3500, Labindia, Mumbai, India) at 638.2 nm against distilled water as the blank. The resulted emulsions were examined for clarity, phase separation, and coalescence of oil droplets on standing for 2 h. When the oil droplets easily spread out in water and formed a clear, transparent emulsion, the emulsion was judged as “good” emulsion (% transmittance >85), and when there was poor or no emulsion formation with immediate coalescence of oil droplets, especially when stirring was stopped, the emulsion was judged as “bad” emulsion (% transmittance <85). The phase diagram was constructed using Chemix school® version 3.50 software (Arne Standnes, MN, USA) to identify the nanoemulsifying region, using oil and Smix ratios which form ‘good’ emulsions upon dilution with purified water. The nanoemulsion region in the ternary phase diagram was represented as shaded area ().
Preparation of L-SNEDDS
Twelve L-SNEDDS of glimepiride were formulated by using oil (5, 10, 20, and 30) and Smix in the ratios of 1:2.33, 1:4, 1:9, and 1:19 as well as surfactant and co-surfactants in the ratios of 1:1, 2:1, and 3:1. The drug loaded L-SNEDDS were prepared by adding the oil phase, containing accurately weighed quantity (20 mg) of glimepiride, drop wise into the Smix with constant stirring for 30 min. It was equilibrated at ambient temperature for 48 h and investigated for signs of turbidity or phase separation. The compositions of prepared L-SNEDDS were represented in .
Table 1. Droplet size and PDI of liquid SNEDDS of glimepiride.
Emulsion droplet size and PDI analysis
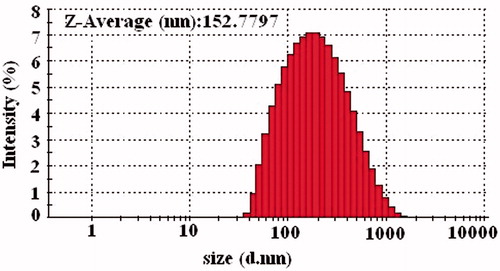
The globule size of the emulsion determines the rate and extent of drug release (Tarr & Yalkowsky, Citation1989). Aliquot (1 ml) of the optimized SNEDDS were diluted 100-fold with purified water to assess the globule size using Zetasizer (Zetasizer Nano ZS, Malvern Instruments, Worcestershire, UK) at a wavelength of 635 nm with dynamic light scattering angle of 90° at 25 °C. The Z-average diameter was derived from cumulated analysis by the auto-measure software.
Preparation of S-SNEDDS from L-SNEDDS
The simplest technique to convert L-SNEDDS to S-SNEDDS is by adsorption onto the surface of carriers. In the present study, Aerosol® 200 was used as an inert solid adsorption carrier. A fixed volume of formed L-SNEDDS equivalent to dose was transferred to a China dish, and to this, the Aerosol® 200 was added in increments with vigorous stirring until a free flow powder was obtained. Then the dose equivalent free flow powder was filled in hard gelatin capsules.
In vitro drug release studies
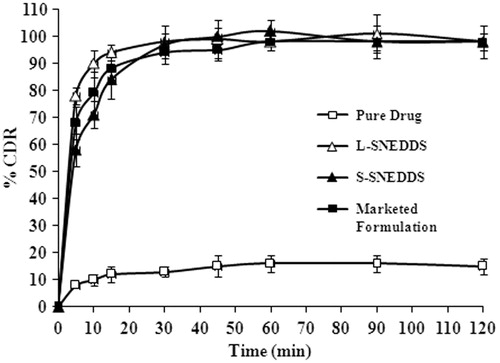
Pure glimepiride (2 mg), marketed tablet (2 mg dose), dose equivalent amount of glimepiride-loaded L-SNEDDS, and S-SNEDDS were placed in a USP-II dissolution test apparatus (DS 8000, LABINDIA, Mumbai, India). The dissolution test was performed using 900 ml of phosphate buffer pH 7.4 as a dissolution medium. Temperature and speed of the paddle were adjusted to 35 ± 0.5 °C and 100 rpm, respectively. At predetermined time intervals (0, 5, 10, 15, 30, 45, and 60 min) an aliquot (5 ml) of the samples was collected and filtered through a membrane filter (0.4 µm) at each time 5 ml of fresh medium was added to dissolution medium. The concentration of drug was determined by using HPLC (Prominence HPLC, Shimadzu, Kyoto, Japan) with a PDA detector (SPD-M20A) at 228 nm.
Solid state characterization of optimized S-SNEDDS
Morphological analysis of S-SNEDDS (SEM)
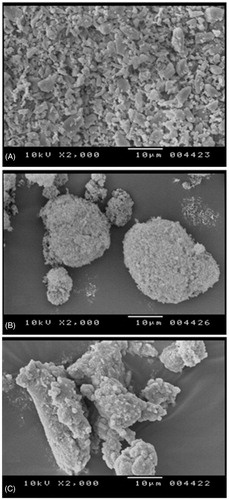
The morphological features of solid glimepiride-loaded S-SNEDDS, pure glimepiride, and aerosol were observed by SEM (S-4100, Hitachi, Shiga, Japan). The analysis was performed by placing the samples on a brass stub using a double-sided adhesive tape and was made electrically conductive by coating in vacuum (6 pas) with platinum using an ion sputter (E-1030) at 15 mA. The micro-photographs were taken at an excitation voltage of 10 kV.
Differential scanning calorimetry (DSC)
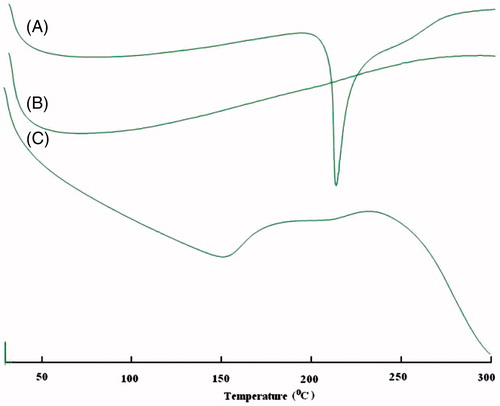
The thermal investigations of glimepiride S-SNEDDS, pure glimepiride, and pure Aerosol® 200 were performed by using a DSC (SIIO, 6300, Tokyo, Japan) by placing about 3 mg of each separated sample in sealed standard aluminum pans before heating under a nitrogen flow (50 ml/min) and setting the heat flow was from 0 °C to 300 °C.
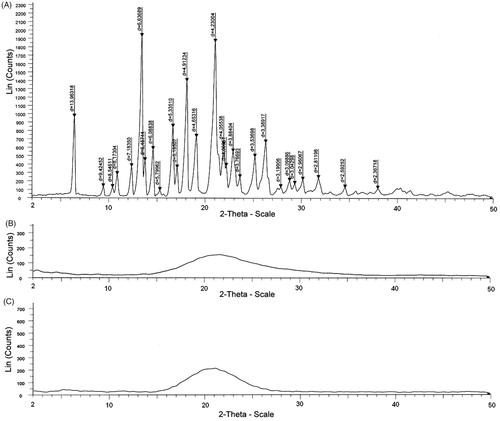
X-ray powder diffraction (XRPD)
The powder crystallinity of the S-SNEDDS formulation, pure glimepiride, and Aerosol® 200 were assessed with an X’Pert PRO diffractometer (PW 1729, Philips, Amsterdam, The Netherlands) at room temperature using monochromatic Cu-Kα radiation, over a range of 2θ angles from 2° to 50°, with an angular increment of 0.02° per second.
Stability studies
The stability studies were conducted to determine the changes in in vitro drug release studies, drug content, emulsion droplet size, and PDI, on storage, according to ICH guidelines at 40 °C/75 ± 5% RH (Jain et al., Citation2005; Girish et al., Citation2007; Srinivas et al., Citation2011) by storing the optimized S-SNEDDS for 3 months. After 3 months, the samples were collected and analyzed for drug content, emulsion droplet size, PDI, and in vitro release studies.
Assessment of therapeutic efficacy in albino rabbits
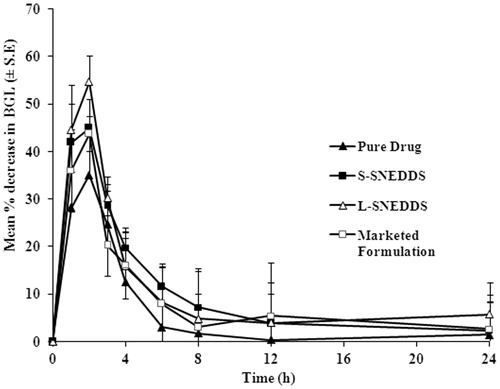
This study was with a single dose design using male albino rabbits weighing 1–1.5 kg. The study was conducted prior approval from institution animal ethical committee, School of Pharmacy, Anurag Group of institutions, Hyderabad, India. The animals were kept on a standard diet and fasted overnight. Rabbits were divided into four groups, each of six animals. Each animal of these first group received pure glimepiride (1 mg/kg body weight), second group received marketed formulation, third group received S-SNEDDS, and forth group received L-SNEDDS in an amount equivalent to calculated dose (1 mg/kg body weight). Blood samples were withdrawn from the marginal ear vein of the animal. Fasting blood glucose level was assessed using Semi-Auto analyzer (Optimas, Labindia, Mumbai, India). Blood glucose level (BGL) was measured at different time intervals (0, 1, 2, 3, 4, 5, 6, 8, 12, 18, and 24 h) up to 24 h. Each animal served as its own control and hence, the hypoglycemic response was evaluated as percentage decrease in blood glucose level. The pharmacodynamic parameter of the area under percentage decrease in BGL versus time curve (AUC0–24h) was calculated by adopting the trapezoidal rule (Wagner, Citation1975; Ammar et al., Citation2006; Sandya et al., Citation2012).
Results and discussion
Selection of oil
The determination of solubility of glimepiride in oils, surfactants, and co-surfactants is the most important principle for the selection of components for SNEDDS formulation. The equilibrium solubility of glimepiride in different oils is shown in . Among the all selected oils (Miglyol® 812, Oleic acid, Sunflower oil, Soya bean oil, Isopropyl Myristate) Miglyol® 812 and Oleic acid exhibited highest and lowest solubilization capacity for glimepiride, respectively. As Miglyol® 812 had good solubility for glimepiride compared to all other oils, it was selected as oil phase for SNEDDS formulation.
Table 2. Solubility of glimepiride in various oils.
Selection of surfactant
Non-ionic surfactants are usually accepted for oral ingestion and are considered safer than the ionic surfactants. The non-ionic surfactants used were Tween® 80, Cremophor® RH 40, Span® 20, and Span® 80. These surfactants were screened on the basis of its ability to emulsify the selected oil as well as its capacity to solubilize glimepiride. Tween® 80 showed highest solubilization capacity for glimepiride () and had ability to emulsify the selected oil, i.e., Miglyol® 812. The emulsifying abilities were determined by measuring the % transmittance of the resultant nanoemulsion. Since the use of Tween® 80 and Cremophor® RH 40 was found to give transmittance above 90% and as glimepiride was found to have considerable solubility in these surfactants, they were chosen for further investigations.
Table 3. Solubility of glimepiride in various non-ionic surfactants and their emulsification efficiency.
Selection of co-surfactant
The % transmittance obtained using PEG 400 and propylene glycol as co-surfactants in combination with the above-selected surfactants (namely Tween® 80 and Cremophor® RH 40) and Miglyol® 812 as the oil are given in . Among the two co-surfactants examined, PEG-400 was found to display highest % transmittance when used in combination with Tween® 80 as a surfactant while propylene glycol was found to show low % transmittance when combined with Cremophor® RH 40 as the surfactant compared to PEG 400. The co-surfactant was finally selected based on its ability to solubilize the glimepiride and its ability to improve the nanoemulsification efficiency of the selected surfactant. Accordingly, PEG-400, which afforded high solubility of glimepiride and which showed the highest transmittance (98.55%), was selected as the co-surfactant for further investigations.
Table 4. Emulsification efficiency with different co-surfactants and selected surfactants.
Ternary phase diagram
The ternary phase diagram was constructed to identify the nano-emulsifying region and to optimize the concentration of the selected oil, surfactant, and co-surfactants (namely Miglyol® 812, Tween® 80, and PEG 400, respectively). For the development of a SNEDDS formulation, optimum ratios of excipient concentrations established by means of phase diagram studies provided the area of the monophasic region. It is important to determine this area in order to confirm successful aqueous dilution without breaking the nanoemulsion (Setthacheewakul et al., Citation2010). shows the ternary phase diagram of the system containing selected oil, surfactant, and co-surfactant. It was noted that incorporation of the co-surfactant, PEG 400, within the self-emulsifying region increased the spontaneity of the self-emulsification process. The efficiency of emulsification was good when the surfactant/co-surfactant concentration was more than 75% v/v of the SNEDDS formulation. It was observed that spontaneous emulsion formation was not effective with less than 50% v/v of the surfactant in the SNEDDS. In the present system, the formulations surrounding the good self-emulsifying region in the phase diagram exhibited a poor emulsion forming ability. It has been reported that the drug incorporated in the SNEDDS may have some effect on the self-emulsifying performance (Oh et al., Citation2011). However, in our study, no significant differences were found in the self-emulsifying performance.
Optimization of glimepiride L-SNEDDS
The amount of oil, surfactant, and co-surfactant for the final formulation of glimepiride SNEDDS was optimized on the basis of Z-average size and PDI. The results are shown in . Less than 200 nm Z-average size was observed with two SNEDDS compositions namely 5:47.5:47.5 and 5:71.25:23.75 (oil:surfactant:co-surfactant, respectively). But if the PDI value higher than 0.8, the systems were considered as polydisperse. Hence the results obtained for SNEDDS with oil:surfactant:co-surfactant of 5:71.25:23.75 was the most consistent, it was considered as optimized L-SNEDDS (F9) and composition was represented in .
Table 5. Composition of S-SNEDDS.
Emulsion droplet size analysis
The particle size distribution is one of the important parameters affecting the in vivo fate of emulsions. The droplet size of the emulsion also defines the rate and extent of drug release (Krutika et al., Citation2011). The smaller the emulsion droplet size, larger the surface area provided for the drug absorption. The Z-average size of the resulted nanoemulsion at 100 times dilution was determined to be 152 nm and the PDI value was low (0.211), representing that the system had narrow size distribution ().
Characterization of optimized S-SNEDDS of glimepiride
Morphological analysis of S-SNEDDS (SEM)
The scanning electron microscopic pictures of glimepiride powder, Aerosol® 200, and S-SNEDDS final formulation are presented in . The pure glimepiride powder () appeared as smooth surfaced, irregular shaped crystals. Aerosol® 200 () appeared as a rough surface with porous particles. However, the S-SNEDDS () appeared as smooth surfaced particles without any crystalline shape which indicates complete adsorption of liquid NEDDS containing glimepiride inside the pores of Aerosol® 200.
Differential scanning calorimetry (DSC)
The DSC thermograms of pure glimepiride, Aerosol® 200, and S-SNEDDS are shown in . Pure glimepiride showed one sharp endothermic peak at 212.2 °C (), corresponding to its melting point indicating its crystalline nature. S-SNEDDS showed one broad endothermic peak with reduced intensity at 153 °C (). It might be indicating that the drug is present in molecularly dissolved and amorphous state in S-SNEDDS (Nazzal et al., 2002b; Balakrishnan et al., Citation2009; Wang et al., Citation2009; Shanmugam et al., Citation2011).
X-ray powder diffraction (XRPD)
The XRPD patterns of pure glimepiride powder, Aerosol® 200, and S-SNEDDS are shown in . XRPD was used for further verification of the internal physical state of glimepiride in the S-SNEDDS. Pure glimepiride powder showed sharp peaks at the diffraction angles (2θ), such as 10.9°, 12°, 16.5°, 17°, 18.8°, 21°, 23°, 28°, and 30° () indicating a typical crystalline pattern. Aerosol® 200 showed no peaks at diffraction angles, indicating an amorphous pattern (). No obvious peaks representing crystals of glimepiride were observed for the S-SNEDDS final formulation (), indicating the absence of crystalline structure of glimepiride in the final formulation.
In vitro drug release studies
The dissolution profiles of L-SNEDDS, S-SNEDDS, and marketed formulation were compared with that of the pure drug (), using phosphate buffer pH 7 as a dissolution medium. Both liquid and S-SNEDDS and marketed formulation of glimepiride showed more than 85% drug within 15 min, whereas the pure drug showed less than 15% release of the total amount of drug. Within 30 min, the drug is completely released from the both solid and L-SNEDDS as a result of the spontaneous emulsification and the resulted nano-level droplet size. At the same time % drug release from pure drug was found to be only 16% of overall drug release. The solid carrier used for S-SNEDDS did not interfere with the drug release. The in vitro dissolution studies of S-SNEDDS revealed that initial glimepiride release from porous carriers (Aerosol® 200) was slower compared with L-SNEDDS, This could be because of additional steps involved during dissolution such as disintegration of solid structure of S-SNEDDS and desorption of L-SNEDDS from the voids of porous carriers. The S-SNEDDS when exposed to dissolution medium leads to desorption of the L-SNEDDS from the Aerosol® 200 surface due to stronger interaction between Aerosol® 200 and dissolution medium than those between Aerosol® 200 and L-SNEDDS.
Stability studies
To determine the temperature sensitivity on dissolution release profile, emulsion droplet size, PDI, and drug content of optimized S-SNEDDS, the stability study was performed at 40 °C and 75% ± 5% RH for 3 months. The stability samples were evaluated for dissolution release profile, emulsion droplet size, PDI, and drug content. Samples withdrawn after 3 months showed no significant changes in in vitro drug release studies, drug content, emulsion droplet size, and PDI. The similarity factor (f2) for the optimized S-SNEDDS was found to be 94.51 (), which indicates good similarity of dissolution release profile, prior and after stability studies. No significant difference (p > 0.05) was observed in drug content, emulsion droplet size, and PDI data, before and after storage.
Table 6. Results for stability studies of S-SNEDDS.
Assessment of therapeutic efficacy in albino rabbits
Blood glucose level (BGL) was measured at different time intervals up to 24 h. Each animal served as its own control and hence, the hypoglycemic response was evaluated as percentage decrease in blood glucose level. The pharmacodynamic parameter of the area under percentage decrease in BGL versus time curve (AUC0–24h) was calculated adopting the trapezoidal rule
shows the mean percentage decrease in blood glucose level (BGL) in normal rabbits after administration of pure glimepiride, glimepiride S-SNEDDS, marketed formulation, and L-SNEDDS. It is evident that the AUC-values for S-SNEDDS, L-SNEDDS, and marketed formulation were higher than the corresponding values of pure glimepiride (). The results revealed that there was a significant increase in the biological performance of S-SNEDDS and L-SNEDDS than pure glimepiride, and there was no significant difference among the S-SNEDDS, L-SNEDDS, and marketed formulation.
Table 7. Therapeutic efficacy of glimepiride pure and L-SNEDDS, S-SNEDDS, and marketed formulation.
Conclusion
In the present study, the optimal S-SNEDDS formulation that showed significantly improved in vitro release of glimepiride when compared with pure glimepiride was successfully developed. The S-SNEDDS readily released the lipid phase to form a fine oil-in-water nanoemulsion, with a narrow distribution size. The solid state characterization of S-SNEDDS by SEM, DSC, and X-ray diffraction analysis suggested that glimepiride in the S-SNEDDS was in the amorphous or molecular dispersion state. The in vitro dissolution test showed that the S-SNEDDS had a faster in vitro release rate than the pure glimepiride. The AUC of glimepiride in S-SNEDDS was increased compared with the pure glimepiride. Thus, the prepared S-SNEDDS for the delivery of glimepiride would be a promising dosage form in the maintaining blood glucose level.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
References
- Allaboun H, Alkhamis KA, AlMomani WY. (2003). The application of the convective diffusion model and the film equilibrium model to surfactant-facilitated dissolution of gliclazide. Eur J Pharm Sci 19:231–6
- Amidon GL, Lennernäs H, Shah VP, Crison JR. (1995). A theoretical basis for a biopharmaceutic drug classification: the correlation of in vitro drug product dissolution and in vivo bioavailability. Pharm Res 12:413–20
- Ammar HO, Salama HA, Ghorab M, Mahmouda AA. (2006). Implication of inclusion complexation of glimepiride in cyclodextrin–polymer systems on its dissolution, stability and therapeutic efficacy. Int J Pharm 320:53–7
- Badr-Eldin SM, Elkheshen SA, Ghorab MM. (2008). Inclusion complexes of tadalafil with natural and chemically modified beta-cyclodextrins. I: preparation and in-vitro evaluation. Eur J Pharm Biopharm 70:819–27
- Balakrishnan A, Rege BD, Amidon GL, Polli JE. (2004). Surfactant-mediated dissolution: contributions of solubility enhancement and relatively low micelle diffusivity. J Pharm Sci 93:2064–75
- Balakrishnan P, Lee BJ, Oh DH, et al. (2009). Enhanced oral bioavailability of dexibuprofen by a novel solid Self-emulsifying drug delivery system (SEDDS). Eur J Pharm Biopharm 72:539–45
- Bannwart B, Bertin P, Péhourcq F, et al. (2001). Piroxicam concentrations in plasma and synovial fluid after a single dose of piroxicam-beta-cyclodextrin. Int J Clin Pharmacol Ther 39:33–6
- Bayindir ZS, Yuksel N. (2010). Characterization of niosomes prepared with various nonionic surfactants for paclitaxel oral delivery. J Pharm Sci 99:2049–60
- Beg S, Swain S, Rizwan Md, et al. (2011). Bioavailability enhancement strategies: basics, formulation approaches and regulatory considerations. Curr Drug Deliv 8:1–12
- Burcin Y, Erem B, Imran V, Murat S. (2010). Alternative oral exemestane formulation: improved dissolution and permeation. Int J Pharm 398:137–45
- Carrier RL, Miller LA, Ahmed I. (2007). The utility of cyclodextrins for enhancing oral bioavailability. J Control Release 123:78–99
- Chadha R, Saini A, Arora P, Bhandari S. (2012). Pharmaceutical cocrystals: a novel approach for oral bioavailability enhancement of drugs. Crit Rev Ther Drug Carrier Syst 29:183–218
- Chakraborty S, Shukla D, Jain A, et al. (2009). Assessment of solubilization characteristics of different surfactants for carvedilol phosphate as a function of pH. J Colloid Interface Sci 335:242–449
- Date AA, Nagarsenker MS. (2007). Design and evaluation of self-nanoemulsifying drug delivery systems (SNEDDS) for cefpodoxime proxetil. Int J Pharm 329:166–72
- Gamsiz ED, Miller L, Thombre AG, et al. (2010a). Modeling the influence of cyclodextrins on oral absorption of low solubility drugs: II. Experimental validation. Biotechnol Bioeng 105:421–30
- Gamsiz ED, Miller L, Thombre AG, et al. (2010b). Modeling the influence of cyclodextrins on oral absorption of low-solubility drugs: I. Model development. Biotechnol Bioeng 105:409–20
- Gamsiz ED, Thombre AG, Ahmed I, Carrier RL. (2011). Drug salts and solubilization: modeling the influence of cyclodextrins on oral absorption. Ann Biomed Eng 39:455–68
- Girish SS, Devendra KJ, Dhananjay MM. (2007). Preparation and in vitro evaluation of bilayer and floating bioadhesive tablets of rosiglitazone maleate. Asian J Pharm Sci 2:161–9
- Grunenberg A, Keil B, Henck JO. (1995). Polymorphism in binary mixtures, as exemplified by nimodipine. Int J Pharm 118:11–21
- Jain SK, Awasthi AM, Jain NK, Agrawal GP. (2005). Calcium silicate based microspheres of repaglinide for gastro retentive floating drug delivery: preparation and in vitro characterization. J Control Release 107:300–9
- Jin Y, Wen J, Garg S, et al. (2013). Development of a novel niosomal system for oral delivery of Ginkgo biloba extract. Int J Nanomed 8:421–30
- Khazaeli P, Pardakhty A, Shoorabi H. (2007). Caffeine-loaded niosomes: characterization and in vitro release studies. Drug Deliv 14:447–52
- Krutika K. Sawant, Shweta Guptha, Sandip Chavhan. (2011). Self-nanoemulsifying drug delivery system for Adefovir dipivoxil: design, characterization, in vitro and ex vivo evaluation. Colloids Surf A 392:145–55
- Kumar N, Shishu, Bansal G, et al. (2013). Preparation and cyclodextrin assisted dissolution rate enhancement of itraconazolium dinitrate salt. Drug Dev Ind Pharm 39:342–51
- Kyatanwar AU, Gajbhiye ND, Jadhav KR, Kadam VJ. (2010). Solid self emulsifying drug delivery system: a review. J Pharm Res 3:877–82
- Li S, Wong S, Sethia S, et al. (2005). Investigation of solubility and dissolution of a free base and two different salt forms as a function of pH. Pharm Res:628–35
- Lobenberg R, Amidon GL. (2000). Modern bioavailability, bioequivalence and biopharmaceutics classification system. New scientific approaches to international regulatory standards. Eur J Pharm Biopharm 50:3–12
- Manconi M, Nácher A, Merino V, et al. (2013). Improving oral bioavailability and pharmacokinetics of liposomal metformin by glycerolphosphate-chitosan microcomplexation. AAPS Pharm Sci Tech 14:485–96
- Miyake K, Arima H, Hirayama F, et al. (2000). Improvement of solubility and oral bioavailability of rutin by complexation with 2-hydroxypropyl-beta-cyclodextrin. Pharm Dev Technol 5:399–407
- Nazzal S, Guven N, Reddy IK, Khan MA. (2002a). Preparation and characterization of coenzyme QlO-Eudragit solid dispersion. Drug Dev Ind Pharm 28:49–57
- Nazzal S, Smalyukh II, Lavrentovich OD, Khan MA. (2002b). Preparation and in vitro characterization of a eutectic based semisolid self nanoemulsified drug delivery system (SNEDDS) of ubiquinone: mechanism and progress of emulsion formation. Int J Pharm 235:247–65
- Oh DH, Kang JH, Kim DW, et al. (2011). Comparison of solid self-microemulsifying drug delivery system (solid SMEDDS) prepared with hydrophilic and hydrophobic solid carrier. Int J Pharm 420:412–8
- Patravale VB, Date AA, Kulkarni RM. (2004). Nanosuspensions: a promising drug delivery strategy. J Pharm Pharmacol 56:827–40
- Pouton CW. (2000). Lipid formulations for oral administration of drugs: non-emulsifying, self-emulsifying and ‘self-microemulsifying' drug delivery systems. Eur J Pharm Sci 11:S93–8
- Pouton CW. (2006). Formulation of poorly water-soluble drugs for oral administration: physicochemical and physiological issues and the lipid formulation classification system. Eur J Pharm Sci 29:278–87
- Qiao N, Li M, Schlindwein W, et al. (2011). Pharmaceutical cocrystals: an overview. Int J Pharm 419:1–11
- Sandya RT, Sujatha S, Veeresham C. (2012). Pharmacokinetic and pharmacodynamic interaction of curcumin with glimepiride in normal and diabetic rats. Phcog Commn 2:14–21
- Serajuddin AT. (2007). Salt formation to improve drug solubility. Adv Drug Deliv Rev 59:603–16
- Serajuddin TM. (1999). Solid dispersion of poorly water-soluble drugs: early promises, subsequent problems, and recent breakthroughs. J Pharm Sci 88:1058–66
- Setthacheewakul S, Mahattanadul S, Phadoongsombut N, et al. (2010). Development and evaluation of self-microemulsifying liquid and pellet formulations of curcumin, and absorption studies in rats. Eur J Pharm Biopharm 76:475–85
- Sezgin-Bayindir Z, Onay-Besikci A, Vural N, Yuksel N. (2013). Niosomes encapsulating paclitaxel for oral bioavailability enhancement: preparation, characterization, pharmacokinetics and biodistribution. J Microencapsul, [Epub ahead of print]. Pages 1–9
- Shan N, Zaworotko MJ. (2008). The role of cocrystals in pharmaceutical science. Drug Discov Today 13:440–6
- Shanmugam S, Baskaran R, Balakrishnan P, et al. (2011). Solid self-nanoemulsifying drug delivery system (S-SNEDDS) containing phosphatidylcholine for enhanced bioavailability of highly lipophilic bioactive carotenoid lutein. Eur J Pharm Biopharm 79:250–7
- Shweta G, Sandip C, Krutika KS. (2011). Self-nanoemulsifying drug delivery system for adefovir dipivoxil: design, characterization, in vitro and ex vivo evaluation. Colloid Surf A 392:145–55
- Srinivas M, Krishna S, Prabhakar RV. (2011). Design and evaluation of hydrochlorthiazide gastroretentive floating drug delivery system. Asian J Pharm Sci 6:208–17
- Tarr BD, Yalkowsky SH. (1989). Enhanced intestinal absorption of cyclosporine in rats through the reduction of emulsion droplet size. Pharm Res 6:40–3
- Veiga F, Fernandes C, Teixeira F. (2000). Oral bioavailability and hypoglycaemic activity of tolbutamide/cyclodextrin inclusion complexes. Int J Pharm 202:165–71
- Wagner SG. (1975). Fundamentals of clinical pharmacokinetics. Hamilton, IL: Drug Intelligence Publications Inc
- Wang D, Miller R, Zheng J, Hu C. (2000). Comparative population pharmacokinetic-pharmacodynamic analysis for piroxicam-beta-cyclodextrin and piroxicam. J Clin Pharmacol 40:1257–66
- Wang L, Dong J, Chen J, et al. (2009). Design and optimization of a new self-nanoemulsifying drug delivery system. J Colloid Interface Sci 330:443–8
- Yang Z, Lu A, Wong BC, et al. (2013). Effect of liposomes on the absorption of water-soluble active pharmaceutical ingredients via oral administration. Curr Pharm Des 19:6647–54
- Yeap YY, Trevaskis NL, Quach T, et al. (2013). Intestinal bile secretion promotes drug absorption from lipid colloidal phases via induction of supersaturation. Mol Pharm 10:1874–89