Abstract
Inclusion complexes of salicylic acid (SA) and acetylsalicylic acid (aspirin, ASA) with polysaccharide arabinogalactan (AG) from larch wood Larix sibirica and Larix gmelinii were synthesized using mechanochemical technology. In the present study, we have investigated physicochemical properties of the synthesized complexes in solid state and in aqueous solutions as well as their anti-aggregation and ulcerogenic activity. The evidence of the complexes formation was obtained by nuclear magnetic resonance (NMR) relaxation technique. It was shown that in aqueous solution the molecules of SA and ASA are in fast exchange between the complex with AG macromolecules and solution. The stability constant of aspirin complex was calculated. It was shown that mechanochemically synthesized complexes are more stable when compared to the complex obtained by mixing solutions of the components. Complexes of ASA show two-fold increase of anti-platelet effect. It allows to reduce the dose of the antithrombotic drug and its ulcerogenic activity. These results substantiate the possibility to design new preparations on the basis of ASA with increased activity and safety.
Introduction
Many drugs demonstrate reduced bioavailability and efficacy due to low solubility in aqueous solutions. Therefore, to achieve the main therapeutic action it is necessary to use higher doses, which leads to an increase of unwanted side effects. In order to improve the solubility in aqueous or physiological media as well as toxicological and pharmacological properties of drugs, the formation of inclusion complexes with water-soluble natural or synthetic polymers or oligomers is used (Davis & Brewster, Citation2004; Marchessault et al., Citation2006; Krishnaiah, Citation2010; Bilensoy, Citation2011).
Earlier (Dushkin et al., Citation2008, Citation2010a, Citation2012), it was shown that the use of a number of drugs in the form of mechanochemically synthesized complexes with polysaccharides and glycyrrhizic acid (GA) allows significant reduction in their doses while maintaining the therapeutic activity and reducing ulcerogenic effect (in the case of NSAIDs (non-steroidal anti-inflammatory drugs)). In addition, it was demonstrated that supramolecular complexes with polysaccharides and GA show enhanced chemical stability of the components (Kornievskaya et al., Citation2007; Polyakov et al., Citation2009; Polyakov & Leshina, Citation2011). Mechanochemical way of preparation of such complexes (or solid dispersion systems that form these complexes when dissolved in water) has significant advantages over traditional “liquid-phase way”, providing increased stability and solubility of the complex (Dushkin et al., Citation2010b, Citation2012) as far as simplifying their production in a single step of processing the mixture of solid components in special mills.
At the present study our approach is used for drugs on the salicylate base. It is known that significant role in therapy of cardiovascular diseases belongs to antithrombotic drugs and among them the most popular are acetylsalicylic acid (ASA) agents (Awtry & Loscalzo, Citation2000). Considering that ASA therapy for primary and secondary prophylaxis of cardiovascular complications is life-long, the risk of undesirable side effects increases. The most frequent clinically significant side effect of aspirin is the irritation of gastrointestinal mucosa up to erosions and ulcers formation that is called “ulcerogenic effect” (Roderick et al., Citation1993; Lanas, Citation2001). Special studies show that this effect is dose dependent, and this is why in cardiology aspirin is used in relatively small doses (75–150 mg) (Campbell et al., Citation2007). Further dose reduction results in increase of antithrombotic effect. Another way to increase safety of ASA intake is the use of so-called enteric or “buffered” tablets but pharmacologist’s opinion about them is ambiguous (Rodriguez et al., Citation2001). So, it is highly urgent to develop novel effective antithrombotic agents with minimal side effects.
In the previous short communication, we had presented preliminary results on nuclear magnetic resonance (NMR) and anti-aggregation study of ASA inclusion complex with natural polysaccharide arabinogalactan (AG) isolated from the wood of larch Larix sibirica and Larix gmelinii (Dushkin et al., Citation2013). In this article, we have presented the detailed physicochemical study of the solid and liquid states of supramolecular complexes of ASA and its primary metabolite – salicylic acid (SA) with AG. For synthesis of these complexes the mechanochemical method was used, which has significant advantages over traditional liquid-phase techniques (Dushkin, Citation2010). The properties of these complexes in solid state were investigated by the methods of X-ray and thermal analysis, infrared (IR) spectroscopy and electron microscopy. Molecular dynamics of the complexes in solution was studied by 1H NMR. The composition of the complexes was monitored by HPLC and GPC. The changes of specific pharmacological activity were studied in vivo.
Materials and methods
Materials
SA (“Mosreactiv”, Moscow, Russia) and ASA (Shandong Xinhua Pharmaceutical Co. Ltd., Zibo city, China) of pharmaceutical grade were used without further purification (SA content in ASA was 0.6%). AG was provided by company “Wood Chemistry”, Russia. Purity of the AG > 99.5%, moisture – 0.01%, the content of phenolic impurities – 0.15%.
Preparation of inclusion complexes
The roll mill VM-1 was used to carry out mechanochemical synthesis. Processing mode: acceleration of grinding media – 1 g, weight of the material – 20 g, drum capacity – 300 ml, grinding media – steel balls (diameter 15 mm, 675 g load), processing time from 2 to 24 h.
We have also prepared supramolecular complexes by mixing the components in the same ratio in aqueous solution, without further isolation in solid form. This method was used for a comparative study of their properties in comparison with solutions of mechanochemically synthesized complexes.
Solubility determination
To determine the solubility of the drugs, 0.4 g of the complex (the ratio AG/drug = 10:1) or 0.8 g (20:1) was dissolved in 5 ml of distilled water with stirring at magnetic stirrer for 3 h at 25 °C. In all cases, the drugs in the solution were in equilibrium with the undissolved one. All AG was completely dissolved. pH of the obtained solutions was equal to 2.4. Drug concentration in the solution was determined by HPLC chromatograph Agilent 1200 with column Zorbax Eclipse XDB-C18, 4.6 × 50 mm at 30 °C and diode-array detector. Acetonitrile–acetate buffer (1:3) was used as eluent, pH = 3.4, flow rate = 0.8 ml/min, sample 5 µl, detection wavelength 240.8 nm.
Microscopy
Electronic images were obtained using Hitachi TM-1000 microscope (Japan).
X-ray powder diffraction analysis
X-ray diffraction analysis (XRD) of solid complexes was carried out on a DRON-4 equipment (“Byrevestnik”, St. Petersburg, Russia) using CuKα radiation, counter speed 2 deg/min, range of intensity measurement – 1000.
Differential scanning calorimetry
Thermal analysis of the samples was carried out by differential scanning calorimetry (DSC) with the DSC-550 instrument (Instrument Scientific Specialists Inc., Omaha, NE) in Ar atmosphere. Temperature program: 20–250 °C, the heating rate 10°/min.
Infrared spectroscopic analysis
IR spectra of the samples taken in tablets with KBr were measured using Fourier spectrometer “Infralum FT-801” (“Simeks”, Novosibirsk, Russia) in the range of 500–4000 cm−1.
Molecular weight distribution
The molecular weight distribution (MWD) of the samples was investigated by gel permeation chromatography (GPC) on the Agilent 1200 chromatograph with column PL aquel-OH 40, 300 × 7.5 mm column at 30 °C with refractometric detector. The solvent used is 0.1 M LiNO3 aqueous solution, flow rate = 1 ml/min. The calibration was performed by standard dextrans with molecular weights of 1, 5, 12, 25, 80, 150, 270 and 410 kDa. Agilent GPC date analysis program was used to process the results.
1H NMR spectroscopy
1H NMR spectra in solution were recorded by “Bruker” NMR spectrometer DPX-200. The compositions SA–AG and ASA–AG in a weight ratio of 1:10 and 1:20, prepared mechanochemically and by mixing of components in solution were investigated in D2O as a solvent (Aldrich, 99.8%). Measurement of the spin–spin relaxation time T2 was carried out using the standard Carr-Purcell-Meiboom-Gill (CPMG) sequence from Avance version of Bruker pulse sequence library: P1(90°) – (τ − P2(180°) − τ)n – registration, where τ = 0.6 ms – fixed time delay, and n varied from 0 to 2048. It is known that the spin-lattice T1 and spin–spin T2 relaxation times are very sensitive to the intermolecular interaction and the diffusion mobility of molecules (Emsley et al., Citation1965; Popova et al., Citation2004). This is due to the change of the rotational correlation time τC in the complex, which is estimated by the Stokes–Einstein–Debye τC = 4πa3η/3kT. After moving the “guest” molecule inside the complex, the relaxation times of its protons significantly reduced by slowing the diffusion and rotational mobility. In general, biexponential decay kinetic of the echo signal might be observed in NMR experiment.
(1)
Here, T21 and T22 correspond to the different relaxation times of the guest molecules in the bound and “free” states. The fast component A1 corresponds to the fraction of the guest molecules are in the complex, while the slow A2 – to the fraction of these molecules in solution. Typical T2 value for the molecules in solution is 0.5–5 s, and in AG complex is 50–150 ms. Such a pattern usually was observed for complexes of hydrophobic molecules. In a situation where the molecules in the complex and in solution are in rapid exchange (compared with the relaxation time), the change of the NMR signal by varying the delay between the pulses is described by monoexponential function. In these conditions, the observed relaxation time is a superposition of values T21 and T22 (2).
(2)
Using these data, one can estimate the stability constant of the complex by Equation (Equation3(3) ) for 1:1 complex.
(3)
Such approximation can also be used if we assume that the AG macromolecule may involve several “guest” molecules.
Pharmacological study
Specific pharmacological action was studied in vivo. The experiments involved male rats of the Wistar line (180–230 g). The animals were obtained from the laboratory of experimental animals of the Institute of Cytology and Genetics (SB RAS, Novosibirsk) and divided into groups of six animals in each. ASA–AG 1:10 or 1:20 and SA–AG 1:10 complexes were injected intragastrically. Blood samples were collected through heart puncture after 3 h, after intragastric dose in anaesthetized animals (Sodium thiopental, 30 mg/kg i.p.) into vacuum tubes with CTAD system (Vacuette, Greiner Bio-One GmbH, Austria). All manipulations were performed in full accordance with the rules and principles of human–animal treatment (Commission of the European Communities, Citation1991).
To obtain platelet-rich plasma, blood was centrifuged at 100 g/15 min. After plasma collection, remains were again centrifuged at 2000g/15 min to collect platelet-poor plasma. As an aggregation inducer, the ADP (Tekhnologija-Standart, Barnaul, Russia) was used at a concentration of 1 mg/ml. Platelet aggregation was evaluated by turbidimetric method on aggregometer Solar 2110 (Solar, Minsk, Belarus). The method is based on change in platelets optical density after addition of an inducer into platelet-rich plasma-containing tube and measured against platelet-poor plasma. The optical density change is evaluated in % and its velocity in %/min, that is, aggregation rate and velocity, respectively. Statistical analysis was made using “Statistica 7.0” software (Statsoft Inc., Tulsa, OK) and Student's t test. Data are shown as mean ± standard error of the mean (SE).
Results and discussion
Composition of mechanochemically synthesized solid dispersions
HPLC data on drug content and molecular-mass characteristics of AG in preparing solid dispersions ASA–AG are shown in .
Table 1. The content of the active substance (ASA and SA) and the molecular-mass characteristics of AG in the obtained solid dispersions (VM-1, 24 h)a.
HPLC and GPC analyses indicate that under conditions used in the mechanical activation, within the experimental error, there are no significant chemical transformation of the studied drugs, as well as a significant destruction of AG macromolecules.
Physicochemical study of solid state compositions
Physicochemical changes in the solid phases of individual substances and drug compositions with AG during mechanical activation were examined by electron microscopy, IR, DSC and X-ray analysis.
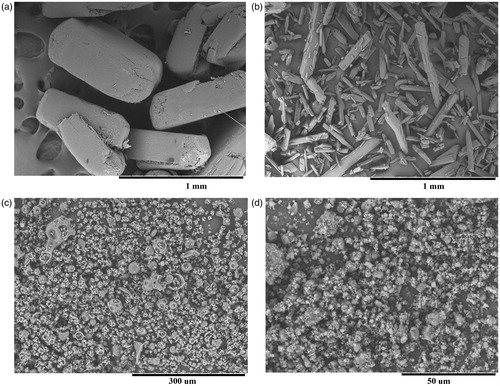
Under mechanochemical processing, a destruction of the crystalline particles of drugs and AG occurs followed by the formation of polydisperse powder, mainly consisting of particles of irregular shape and size of 5–20 µm and their aggregates ().
Figure 1. Electron micrographs of: A – Salicylic acid; B – Aspirin; C – Arabinogalactan; D – treated mixture of arabinogalactan/salicylic acid 10:1 in the VM-1 for 24 h.
Investigation of solid dispersions by IR spectroscopy revealed no significant changes due to mechanochemical treatment in the spectrum at 2500–3500 cm−1. On the other hand, in the stretching vibration of hydroxyl group at 3000–3500 cm−1 it is not possible to isolate specific bands of SA and ASA due to large excess of hydroxyl groups of polysaccharide. Consequently, one cannot conclude that there is a breakdown of the acids dimers in solid dispersions which are characteristic for their crystalline phase (Lieserowitz, Citation1976).
The results of DSC experiments are shown in . Mechanical processing of the raw substances does not lead to significant changes in their X-ray and DSC curves. At the same time, such changes occur during processing of AG mixtures with the drugs. As a result of mechanochemical treatment, XRD intensity of the reflections and the fusion heat of all compounds are significantly reduced. From these data, it follows that there is the loss of crystalline phase in mechanically processed mixture. Apparently, the amorphization or molecular dispersion of solid drugs in excess of AG occurs during mechanochemical treatment. X-ray diffraction reflections intensity of acids in their mixtures with AG is too low due to the mass excess of AG in the mixture. Hence, the DSC method is more suitable for quantitative analysis because of its high sensitivity even in mixtures with low mass fraction of acids.
Figure 2. DSC thermograms: A – (1) acetylsalicylic acid initial; (2) ASA treated in the VM-1 mill for 30 min. B – (1) salicylic acid initial; (2) SA treated for 30 min. C – (1) AG–ASA 10:1 not treated; (2) AG–ASA 10:1 processed in a mill for 2 h; (3) 4 h; (4) 8 h; (5) 16 h; (6) 24 h; (7) initial arabinogalactan. D – (1) AG–SA 10:1 not treated; (2) processed in a mill for 2 h; (3) 4 h; (4) 8 h; (5) 16 h; (6) 24 h; (7) initial arabinogalactan.
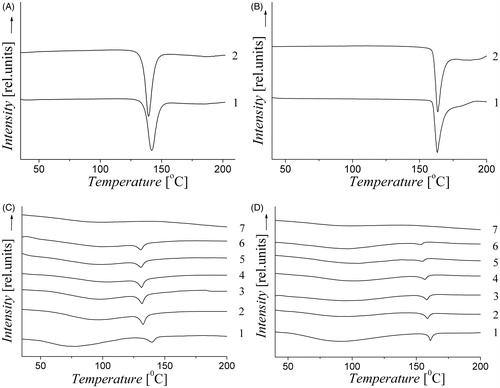
The dependences of the fusion heats on the treatment time in the mill are shown in . In our opinion, the loss of crystallinity of the drugs can be accompanied by penetration of their molecules into the solid phase of polysaccharide, with the formation of intermolecular complexes. Under this hypothesis, it is possible to estimate the fraction of the drug in the complex from the data shown in . To estimate the content of the crystalline drug phase in the complex, each heat of fusion value was related to the heat of fusion of the corresponding acid. The result represented in % is the fraction of drug which is not in the complex. So we can calculate the fraction of drug in the complex as 100% minus the fraction of the crystalline drug. After the 24-h treatment, the yields of the complex are 58 and 92% for ASA–AG mixture 1:10 and 1:20, respectively, and 86 and 93% for SA formulations 1:10 and 1:20. These estimates imply that the excess of AG promotes the formation of the complexes, and that SA–AG complexes are formed easily than ASA–AG complexes.
Table 2. The change of specific heat of fusion, normalized to the amount of acid in the sample, depending on the processing time in the mill. Analysis error is ± 10%.
Solubility of solid dispersions
Solubility of SA, ASA and their solid dispersions with AG measured in the present study are shown in .
Table 3. The solubility of pure SA, ASA and their solid dispersions with AG in water.
We suppose that the observed increase in solubility of these drugs from their solid dispersions with AG is due to the formation of water-soluble intermolecular complexes. However, the absolute value of change in solubility is small, probably because the investigated acids are sufficiently hydrophilic molecules. Earlier it was shown that the highest increase in solubility as a result of complexation with AG is achieved for low-soluble, hydrophobic drugs (Duskin et al., Citation2008, Citation2010b, Citation2012). In the case of SA and ASA, such a small increase in solubility, in our opinion, is insufficiently reliable for the conclusion about complex formation. Therefore, to prove the formation of inclusion complexes and to obtain quantitative characteristics of the complexes, the method of NMR relaxation was applied.
NMR spectroscopy in solution
It is known that relaxation rates of the molecules in solution are very sensitive to the intermolecular interaction and the diffusion mobility of molecules (Emsley et al., Citation1965; Popova et al., Citation2004). It allows one to use this technique to prove the intermolecular complex formation when the changes of other physical characteristics (solubility, spectral parameters) are very small. In the previous short communication, we had presented the evidence of ASA–AG inclusion complex formation obtained by NMR relaxation technique (Dushkin et al., Citation2013). In the present article, we have extended our studies by investigation of SA conjugation with AG, and by comparing the complexes prepared by liquid-phase and solid-phase synthesis. For this purposes relaxation times, T2 of ortho-protons of SA and ASA were measured in the aqueous solution in pure form as well as in the complexes with AG. Mechanochemically obtained complexes were compared with similar compositions obtained by mixing same compounds in water solution. Note that all kinetics show monoexponential time dependence (see, e.g. ). This means rapid exchange of “guest” molecules between the complex and solution (“Experimental” section).
Figure 3. Time dependence intensity of the echo signal (in logarithmic scale) of aromatic protons of SA in an aqueous solution; the solid dispersion (SA–AG 1:10) and mixture (SA + AG 1:10 without treatment) in aqueous solution (▪ – experiment, solid lines – calculation). Approximation error is 2–4%.
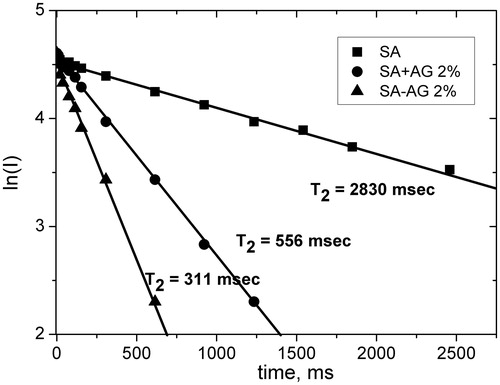
As a result of these measurements, it was found that the decay kinetics of echo signals in the presence of AG are significantly shortened compared with pure aqueous solutions of SA and ASA. This indicates a decrease in the rotational mobility of the “guest” molecules and can serve as a proof of the inclusion complex formation. The measurement of the decay kinetics at different concentrations of ASA–AG complex 1:20 (from 0.1% to 2%) allowed us to estimate the relaxation time of ASA protons in the complex (T21) and stability constant (K). shows Benessi–Hildebrand plot of 1/Δkobs versus 1/[AG].
Figure 4. Benessi–Hildebrand plot: the change of relaxation rate of ASA protons in the complex with AG (1:20) as a function of 1/[AG] in D2O at 37 °C. The straight line is linear approximation.
![Figure 4. Benessi–Hildebrand plot: the change of relaxation rate of ASA protons in the complex with AG (1:20) as a function of 1/[AG] in D2O at 37 °C. The straight line is linear approximation.](/cms/asset/3c930c68-16d1-4ceb-b383-8a7151fd5189/idrd_a_884655_f0004_b.jpg)
Here the change of relaxation rate is:
Taking into account that the size of AG macromolecule is much higher than the size of “guest” molecule, we have assumed that the number of binding sites is also higher than the amount of “guest” molecules in the complex. So, for estimation of the parameters K and A1 (Equations (Equation2–3)) we can put constant value of [AG] = [AG]0. Observation of linear dependence 1/Δkobs on 1/[AG] points to the correctness of our assumptions. From the slope and intersection point of Benessi–Hildebrand plot, the values of stability constant K and 1/Δkmax were estimated: 1/Δkmax = 0.22 ± 0.1 s, K = 500 ± 230 M−1, and A1 ∼0.3 at [AG] = 1 mM.
Similar measurements of T2 relaxation times of ASA protons in the complex with ASA–AG ratio 1:10 show an increase in the observed relaxation time (T2(300 °K) = 860 ms) compared with composition 1:20 (T2(300 °K) = 435 ms) (Dushkin et al., Citation2013). This indicates a decrease in the fraction of molecules in the complex with increasing molar ratio of ASA–AG. Simple estimates show that one AG macromolecule can include up to two molecules of ASA, and the further increase in the ratio of ASA–AG in the mixture decreases the binding strength.
The similar dependences were observed for the SA complexes (see and ). It can be seen that the observed relaxation times for SA is much smaller than ASA at equal concentrations of AG. For example, for a 2% solution of the mechanochemically prepared complex 1:20 T2(ASA) = 435 ms and T2(SA) = 240 ms. This indicates an increase in the fraction of drug molecules in the complex. It means the higher stability of SA complex and probably deeper penetration of SA molecules into the macromolecule of AG. A possible reason for this effect is the higher lipophilicity of SA molecules. Thus, the logarithms of the partition coefficients are 2.19 (Machatha & Yalowsky, Citation2005) and 1.18 (Dressman et al., Citation2012) for SA and ASA in the n-octanol–water mixture, respectively. These values differ by one, which indicates a higher lipophilicity of SA. For comparison, shows the observed relaxation times of SA and ASA protons measured at the same temperature of 300 °K and same AG concentration of 1 mM.
Table 4. The relaxation time T2 (in ms) of aromatic protons of SA and ASA in pure form and in AG complexes prepared mechanochemically (m.c.), and by “liquid-phase” (l.p.) method, measured at 300 °K and [AG] = 1 mM.
It should be noted that complexes prepared by the mixture of aqueous solutions of ASA and AG (l.p. in ) are less stable. The observed relaxation time of ASA protons in such a complex is significantly higher than in mechanochemically activated complex (). It can be assumed that the greater stability of the complex prepared mechanochemically is due to deeper penetration of the “guest” molecule into the macromolecules of AG during mechanochemical synthesis. It is important to note that this effect is retained upon dissolution of the complex. In this study, we compared the relaxation rate of ASA protons measured twice, immediately after dissolving the solid dispersion in water (1:20, 1 mM), and 3 d after the establishment of equilibrium. The observed relaxation time increased from 435 ± 20 ms to 540 ± 20 ms after 3 d. However, it is still significantly smaller than the observed relaxation time for the “liquid-phase” complex which equals to 885 ± 35 ms.
Thus, the study of SA and ASA interaction with polysaccharide AG by 1H NMR relaxation allowed us to prove the formation of intermolecular inclusion complexes and to make several important conclusions. First, it was shown that these complexes are in dynamic equilibrium with the “free” molecules in aqueous solution. Second, the complexes prepared by mechanochemical method have higher stability than the complexes formed by mixing the components in solution, i.e. prepared by traditional “liquid-phase” method. Third, AG molecule is able to accommodate a few “guest” molecules, but the stability of inclusion complex decreases with decreasing molar ratio of “host–guest". And, finally, a lipophilic molecule of SA forms more strong complexes. The important role of hydrophobic interaction in the complex stability was suggested earlier from the study of AG complexes of low-soluble drugs (Dushkin et al., Citation2008, Citation2010b, Citation2012).
Pharmacological tests
The anti-aggregation activity of ASA was investigated as the primary pharmacological effect. This action is caused by the ability of ASA blocking thromboxane А2 in platelets. It results in irreversible aggregation suppression (Fuster & Sweeny, Citation2011). Thereby, all new compounds were evaluated on this effect ().
Table 5. Platelets aggregation rate and velocity after intragastric introduction of SA–AG and ASA–AGa complexes.
Complexes with acetylsalicylic acid
The in vivo study of ASA–AG complexes on anti-aggregation effect showed significant decrease in aggregation rate and velocity 3 h after intragastric dosage. In this way, complex with mass ratio 1:10 at a dose 250 mg/kg increased those parameters to 50% and 53%, respectively. ASA at dose 25 mg/kg, that is same to complex, decreased aggregation rate just to 33.5% and did not affect the aggregation velocity. Similarly, ASA–AG effect was achieved only after introduction of a double dose of ASA, i.e. 50 mg/kg.
Complex with higher AG content (ASA–AG 1:20) was studied at a dose of 500 mg/kg and in this case the dose of ASA was also equal to 25 mg/kg. It was found that its anti-aggregation effect does not significantly differ from ASA–AG complex at a dose of 250 mg/kg. When we tried to reduce the dose to half (ASA dose is 12.5 mg/kg), then we obtained the impairment in aggregation effect. AG does not show any anti-aggregation effect at a dose of 250 mg/kg. Thus, we can state that complexation of AG with ASA results in double reduction of the effective dose of the latter.
Complexes with salicylic acid
It is well known that the SA is the primary metabolite of aspirin (Needs & Brooks, Citation1985) and it is responsible for many salicylate properties, however in the literature there are no data about its anti-aggregation effect which is specific for the ASA. Previously, we have shown that by the complexation of drugs with plant metabolites it is possible for enhancing their different pleotropic properties that are not prominent in “blank” compounds (Tolstikova et al., Citation2009). So, it was interesting to find if the complexation with AG facilitates the onset of the SA anti-aggregation effect. Anti-aggregation study of SA complexes was carried out in the same way to ASA complexes.
Platelets aggregation activity after intragastric dosage of the SA–AG 1:10 complex at a dose 250 mg/kg is shown in . As a result of this study, we have found that SA–AG 1:10 complex at a dose 250 mg/kg does not possess anti-aggregation effect and on the contrary reveals the tendency increasing platelet aggregation rate and velocity. SA showed the same effect at a dose of 25 mg/kg, however in this case the action was a little less expressed. We did not carry out the study of the SA–AG 1:20 complex because of no effect in the case of SA–AG 1:10 complex.
Thereby, in the conducted pharmacological study of ASA complexes with AG we have found that complexes with mass ratio 1:10 and 1:20 possess prominent anti-aggregation action at a dose 250 and 500 mg/kg, respectively. In both cases ASA dose was 25 mg/kg. Blank ASA showed the same effect in a double dose, 50 mg/kg, i.e. complexation of the ASA with AG results in half reduction of its effective dose and content of AG does not show influence on its anti-aggregation action. Probably, the reason of pharmacological effect increase is the accelerated absorption of ASA in the gastrointestinal tract specified by complexation of its molecules with AG.
Complexation of the SA with AG does not facilitate the onset of its anti-aggregation effect and it is fitted to the absence of the antithrombotic activity of the initial SA (Paterson & Lowrence, Citation2001).
Conclusion
In the present work, the intermolecular complexes of the “guest–host” type of SA and ASA with polysaccharide AG were prepared in solid state as far as in water solution and investigated by various spectral, structural and analytical methods. Using dynamic 1H NMR spectroscopy, the evidence of complex formation in aqueous solutions was obtained, and stability constant of ASA–AG complex was estimated. It was shown that these complexes are in dynamic equilibrium with the “free” molecules in aqueous solution. The complexes prepared by mechanochemical method have higher stability than the complexes formed by mixing the components in solution, i.e. prepared by traditional “liquid-phase” method. The AG molecule is able to accommodate few “guest” molecules, but the stability of inclusion complex decreases with decreasing molar ratio of “host–guest". A lipophilic molecule of SA forms more strong complexes. A comparative study of ulcerogenic activity and anti-aggregation effect for all samples was performed. The possibility to reduce the therapeutic doses of ASA was demonstrated for mechanochemically prepared inclusion complexes. Probably, the reason of high pharmacological effect of ASA–AG is an accelerated uptake in the gastro-intestinal tract caused by ASA complexation with AG molecules. At the same time, the complex of SA does not show anti-aggregations activity. These results open up the prospect of creating medicines for antithrombotic therapy with low-dose aspirin and reduce the risk of unwanted side effects.
Declaration of interest
This publication is supported by a grant 12-04-31137 from the Russian Foundation for Basic Research. The authors report no declarations of interest.
References
- Awtry EH, Loscalzo J. (2000). Aspirin. Circulation 101:1206–18
- Bilensoy E. (2011). Cyclodextrins in pharmaceutics, cosmetics, and biomedicine: current and future industrial applications. Hoboken, NJ: Wiley
- Campbell CL, Smyth S, Montalescot G, Steinhubl SR. (2007). Aspirin dose for the prevention of cardiovascular disease: a systematic review. J Am Med Assoc 297:2018–24
- Commission of the European Communities: Council Directive of 18 December 1986 on the Lows, regulating of Principles of Good Laboratory Practice and the Verification of Their Applications for Tests on Chemical Substances (87/18/EEC). The Rules Governing Medicinal Products in the European Community, 1991, Vol. 1, 145–6 p
- Davis ME, Brewster ME. (2004). Cyclodextrin-based pharmaceutics: past, present and future. Nat Rev Drug Discov 3:1023–35
- Dressman JB, Nair A, Abrahamsson B, et al. (2012). Biowaiver monograph for immediate-release solid oral dosage forms: acetylsalicylic acid. J Pharm Sci 101:2653–67
- Dushkin AV. (2010). Mechanochemical synthesis of organic compounds and rapidly soluble materials. In: Sopicka-Lizer M, ed. High-energy ball milling. Mechanochemical processing of nanopowders. Oxford: Woodhead Publishing Limited, 249–73
- Dushkin AV, Meteleva ES, Tolstikova TG, et al. (2008). Mechanochemical preparation and pharmacological activities of water-soluble intermolecular complexes of arabinogalactan with medicinal agents. Russ Chem Bull 6:1299–307
- Dushkin AV, Chistyachenko Yu S, Tolstikova TG, et al. (2013). Pharmacological and physicochemical properties of mechanochemically synthesized supramolecular complexes of acetylsalicylic acid and polysaccharide arabinogalactan from larches Larix Sibirica and Larix Gmelinii. Dokl Biochem Biophys 451:180–2
- Dushkin AV, Meteleva ES, Tolstikova TG, et al. (2010a). Complexation of pharmacons with glycyrrhizic acid as a route to the development of the preparations with enhanced efficiency. Chem Sustain Dev 18:517–25
- Dushkin AV, Meteleva ES, Tolstikova TG, et al. (2010b). Mechanochemical preparation and properties of water-soluble intermolecular complexes of polysaccharides and β-cyclodextrin with pharmaceutical substances. Chem Sustain Dev 18:719–28
- Dushkin AV, Tolstikova TG, Khvostov MV, Tolstikov GA. (2012). Complexes of polysaccharides and glycyrrhizic acid with drug molecules. Mechanochemical synthesis and pharmacological activity. In: Karunaratne DN, ed. The complex world of polysaccharides. Rijeka, Croatia: InTech, 573–602
- Emsley JW, Freeney J, Sutcliffe LH. (1965). High resolution nuclear magnetic resonance spectroscopy. Oxford: Pergamon Press
- Fuster V, Sweeny JM. (2011). Aspirin: a historical and contemporary therapeutic overview. Circulation 123:768–78
- Kornievskaya VS, Kruppa AI, Polyakov NE, Leshina TV. (2007). Effect of glycyrrhizic acid on lappaconitine phototransformation. J Phys Chem B 111:11447–52
- Krishnaiah YSR. (2010). Pharmaceutical technologies for enhancing oral bioavailability of poorly soluble drugs. J Bioequiv Bioavailab 2:28–36
- Lanas A. (2001). Cyclo-oxygenase-1/cyclo-oxygenase-2 non selective non-steroidal anti-inflammatory drugs: epidemiology of gastrointestinal events. Dig Liver Dis 33:S29–34
- Lieserowitz L. (1976). Molecular packing modes. Carboxylic acids. Acta Crystallogr B 32:775–802
- Machatha SG, Yalkowsky SH. (2005). Comparison of the octanol/water partition coefficients calculated by ClogP, ACDlogP and KowWin to experimentally determined values. Int J Pharm 294:185–92
- Marchessault RH, Ravenelle F, Zhu XX. (2006). Polysaccharides for drug delivery and pharmaceutical applications, V.934, ACS Symposium Series
- Needs CJ, Brooks PM. (1985). Clinical pharmacokinetics of the salicylates. Clin Pharmacokinet 10:164–77
- Paterson JR, Lawrence JR. (2001). Salicylic acid: a link between aspirin, diet and the prevention of colorectal cancer. QJM 94:445–8
- Polyakov NE, Leshina TV. (2011). Glycyrrhizic acid as a novel drug delivery vector: synergy of drug transport and efficacy. Open Conf Proc J 2:64–72
- Polyakov NE, Leshina TV, Meteleva ES, et al. (2009). Water soluble complexes of carotenoids with arabinogalactan. J Phys Chem B 113:275–82
- Popova MV, Tchernyshev YS, Michel D. (2004). NMR investigation of the short-chain ionic. Langmuir 20:632–6
- Roderick PJ, Wilkes HC, Meade TW. (1993). The gastrointestinal toxicity of aspirin: an overview of randomised controlled trials. Br J Clin Pharmacol 35:219–26
- Rodriguez LAG, Hernandez-Diaz S, de Abajo FJ. (2001). Association between aspirin and upper gastrointestinal complications: systematic review of epidemiologic studies. Br J Clin Pharmacol 52:563–71
- Tolstikova TG, Khvostov MV, Bryzgalov AO. (2009). The complexes of drugs with carbohydrate-containing plant metabolites as pharmacologically promising agents. Mini-Rev Med Chem 9:1317–28
