Abstract
The commercially available alpha-asarone injections (CA-ARE) were frequently found to cause severe anaphylactic reactions by the solubilizer contained in the formulation such as polysorbate 80 and propylene glycol. This study aimed to develop a new ARE injection using Kolliphor HS 15 as solubilizing agent (HS 15-ARE) by the dissolution method to resolve its poor solubility problem and reduce the anaphylaxis of CA-AREs caused by Polysorbate 80. The HS 15-ARE micelle showed a homogeneous round shape with the mean particle size of around 13.73 ± 0.02 nm, polydisperse index (PDI) of 0.19 ± 0.01 and solubilizing efficiency of 95.7% ± 2.4%. In vitro and in vivo studies showed that HS 15-ARE is a stable injection presenting the same pharmacokinetic profile with CA-ARE. Moreover, improved therapeutic effect was observed for HS 15-ARE in treating asthma compared to CA-ARE (p < 0.05) with no anaphylactic reactions observed. These results demonstrate that the new formulation of ARE (HS 15-ARE) has a great potential for replacing CA-AREs injections.
Introduction
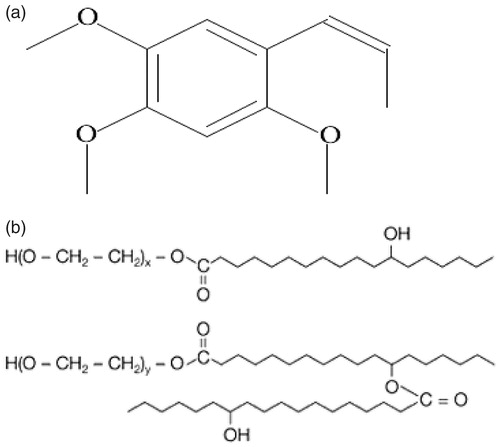
Alpha-Asarone (; ARE, 2,4,5-trimethoxy-1-propenyl benzene) is a biologically active component of the bark extracted from Guatteria gaumeri Greenman (Annonaceae), which is indicated for pneumonia, asthma and grand mal seizures (Xu et al., Citation2007; Zhao et al., Citation2007; Antunez et al., Citation2009). Despite the promising biological effects of ARE, its clinical application is limited due to its great hydrophobicity and low bioavailability (Wu et al., Citation2010). The commercially available ARE injections (CA-ARE) use polysorbate 80 as solubilizer and propylene glycol as solvent which have been applied clinically in China for decades (Wu et al., Citation2010). However, polysorbate 80 may cause anaphylactic reactions (Luo et al., Citation2010) and propylene glycol was known to cause serious adverse reactions such as hemolysis, serum hypertonic, central depression, respiratory failure and hypotension (Zheng et al., Citation2005). The adverse reaction incidence of CA-ARE is as high as 51.5% (Wu et al., Citation2010), endangering patients, especially kids. Therefore, it is of great significance to develop a new formulation for ARE.
Many drugs have failed to be applied successfully in clinic due to their great hydrophobicity and low bioavailability (Gou et al., Citation2008; Wu et al., Citation2010). Nanotechnology provides an important method to solve the problems described above (Chawla & Amiji, Citation2002; Allen & Cullis, Citation2004; Farokhzad et al., Citation2006; Hennenfent & Govindan, Citation2006). Hydrophobic drugs were manipulated to be entrapped into nano-scale particles, which could be well dispersed in aqueous solution to form stable and homogeneous suspension, thus allowing it for better drug solubilization and to be more applicable for clinical use (De et al., Citation2008). In recent years, studies have been conducted to improve the solubility of ARE without using polysorbate 80 in the intravenous formulations, such as freeze-dried powder (Yang, Citation2005), intravenous alpha-asarone emulsion (Cai et al., Citation2007) and lipid emulsions (Guo et al., Citation2008). Though these formulations showed advantages, they still have some shortcomings. The freeze-dried powder is easy to be prepared and transported, but is difficult to be re-dissolved. Other disadvantages with current novel ARE formulations have been reported such as the complexity of preparation, high cost, storage difficulty and high occurrence of pain for intravenous emulsion and lipid emulsions, despite the efficient solubilization of ARE in these formulations. Furthermore, some formulations contain organic solvent that may present potential safety concerns.
Lately, a new surfactant agent named Kolliphor HS 15 has emerged as a good substitute for organic solvent. Kolliphor HS 15 (, HS 15) is a low molecular weight non-ionic surfactant agent for manufacturing aqueous parenteral formulations, which consists of polyglycol mono and diesters of 12-hydroxystearic acid (= lipophilic part) and of about 30% of free polyethylene glycol (= hydrophilic part; BASF, Citation2013). Moreover, Kolliphor HS 15 has been included in the Germany pharmacopoeia, the United States and the European pharmacopoeia. In addition, it has been approved by CFDA as an excipient for injection. The high solubilization capacity, low viscosity, thermo-stability and good biocompatibility make it a very promising solubilizer for injections (Gao et al., Citation2004).
To develop a new injection of ARE with good bioavailability and better safety, in this article, a new formulation HS 15-ARE using HS 15 as solubilizer instead of organic solvent was prepared. The micellar properties, solubilization capacity of HS 15 on ARE, physical stability and morphology of the formulations were investigated in vitro compared to CA-ARE. Furthermore, the pharmacokinetic profiles, tissue distribution, anaphylaxis and therapeutic effect studies were conducted in parallel with CA-ARE.
Materials and methods
Materials
Kolliphor HS15 was supplied by BASF (Shanghai, China). Alpha-asarone (purity > 99.7%) was provided by Harbin SanLian Pharmaceuticals Industry Co. Ltd (China). The commercially available alpha-asarone solutions were purchased from Chengdu LiSiTe Pharmaceuticals Industry Co. Ltd (China). Indometacin was purchased from Huzhou Kangquan Pharmaceuticals Industry Co. Ltd (China). Vitamin C was offered by Sigma(St Louis, MO, USA). All other chemicals used were of analytic grade or better.
Analytic method
The concentration of ARE in vitro and in plasma were determined by HPLC (Agilent 1260,Santa Clara, CA, USA). A reversed-phase column (C18, 150 mm × 4.6 mm, 5 μm) with a corresponding security guard column (C18, 10 mm × 4 mm, 5 μm) was adopted in this study. The mobile phase (pH value was 3.9–4.3 adjusted by phosphoric acid) was a mixture of methanol with ultrapure water (75:25). The drug content of optimized micelles was analyzed in triplicate after dilution with methanol by the HPLC method described above, with the column temperature 35 °C, detection wavelength 313 nm, flow rate 1.0 ml/min and injection volume of the samples 20 µl.
Animals
Male Wistar rats (200 ± 20 g) and guinea pigs were obtained from the Laboratory Animal Center of Sichuan University (Chengdu, China). Free access to food and water was allowed. All animals were raised in a dedicated area with the room temperature and relative humidity kept at 25 °C and 50%, respectively, and would be in quarantine for a week before treatment. All animal experiments were supervised by the Institutional Animal Care and Ethic Committee of Sichuan University.
Methods
Preparation of Kolliphor H S 15-ARE micelles
The new Kolliphor HS 15-ARE micelles were prepared with ARE and Kolliphor HS 15 at a mass ratio of 1:16 by dissolution method. In brief, 18 mg of ARE and 285 mg of Kolliphor HS 15 were placed in a 25 ml round bottom flask, and 4 ml of water was added. The mixture was then stirred with magnetic agitator at 60 °C until it was completely dissolved. After cooling to room temperature, 8 mg of activated carbon was added to the mixture under moderate stirring for 30 min in order to remove pyrogens. The solution was then purified by filtering through a 0.22 μm filter to obtain a clear and homogeneous micelle solution. The filtrate was filled into ampoules and sealed under nitrogen, and then received high-pressure steam sterilization (115 °C for 15 min).
The particle size and polydispersity index (PDI) of HS 15-ARE were measured at 25 °C by Malvern Nano-ZS 90 laser particle size analyzer after equilibration for 10 min. To analyze the stability of HS 15-ARE and CA-ARE, the particle size and zeta potential of samples were measured with no dilution or with a dilution of 50-times by distilled water. All results were shown in the mean of three test runs, and all data were expressed as the mean ± SD.
Determination of drug content, drug loading content and solubilizing efficiency
The drug content of optimized HS 15-ARE micelles was determined by HPLC as described above. The solubilizing efficiency and drug loading content were calculated based on the following equations:
Physical stability
The physical stability of HS 15-ARE under storage conditions was investigated. Freshly prepared ARE solution was placed into a 10 ml tube, then stored at 25 °C for 3 months. The reference parameters for stability evaluation were selected as follows: phenomenon of ARE precipitations, changes in particle size, size distribution and solubilizing efficiency.
Transmission electron microscopy
The morphological characteristics of HS15-ARE micelles were examined by TEM (H-600, Hitachi, Tokyo, Japan). The micelles were diluted with distilled water and placed on a copper grid covered with nitrocellulose. The sample was negatively stained with phosphotungstic acid and dried at room temperature.
Critical micelle concentration determination
The pyrene 1:3 ratio method was used to determine the critical micelle concentration (CMC) of Kolliphor HS 15 in normal saline (Aguiar et al., Citation2003). Briefly Kolliphor HS 15 micelles samples of different concentrations were prepared (ranging from 1 × 10−4% to 7.2%). Pyrene was added to Kolliphor HS 15 micelles to obtain a final concentration of 4 × 10−7 mol/l. After balanced for 24 h in a dark room at 25 °C, the mixtures were plated in 96-well plates for fluorescence measurements (Fluoroskan Ascent FL, Thermo Fisher Scientific, Waltham, MA,USA). Excitation wavelength of Fluorescence was 336 nm with an emission slit of 5 nm. The intensities of I1 and I3 were measured at the wavelengths of 372 and 383 nm, respectively. The ratio of I1 to I3 for pyrene was 1:3. The results were processed using Origin 8.5 software (OriginLab, Northampton, MA, USA). Experiments were performed in triplicate.
In vitro hemolytic test
Hemolytic test was performed by visual observation method and was conducted in accordance with the technical guidelines of irritating chemicals, allergic and hemolytic researches (FDA Guidance for Industry Skin irritation, sensitization and hemolysis testing of generic transdermal drug products). In brief, the commercially available sheep blood was centrifuged at 3000 rpm for 2 min to collect the blood cells, which was appropriately washed with normal saline and centrifuged until the supernatant was not red. Then, required erythrocytes were diluted to 2% erythrocyte suspensions with normal saline. Three clean glass tubes were numbered as the negative control tube, the positive control tube and the HS 15-ARE tube. The samples were mixed evenly according to . Samples were observed every 15 min for the first hour and then observed once every 1 h for 3 h.
Pharmacokinetic study
Male Wistar rats with an average weight of 200 ± 20 g (Laboratory Animal Center, Sichuan University, China) were randomly divided into two groups (n = 5) and fasted overnight with free access to water before administration.
Both groups were intravenously injected with the HS 15-ARE solution and CA-ARE (with the same mass concentration of ARE) at a dose of 20 mg/kg, respectively. About 0.3 ml of blood was collected from the ocular vein into heparinized tubes at prearranged time after intravenous injection. Plasma samples (100 µl) were collected immediately after centrifugation (4000 rpm, 10 min) and then stored at −20 °C for further use. About 100 µl of plasma samples and 10 µl of internal standard (indometacin at a concentration of 20.0 µg/ml in methanol) were homogeneously mixed by vortex for 2 min. A 200 µl of acetonitrile was added to the obtained mixture and vortexed again for 5 min to precipitate protein. Then the resultant mixture was centrifuged for 10 min at 12 000 rpm and 20 µl of the obtained supernatant was injected directly into the HPLC for assay.
Tissue distribution studies
Forty-eight healthy male Wistar rats (220 ± 20 g) were randomly divided into two groups and fasted overnight with free access to water before administration of doses.
The two groups were intravenously injected with the HS 15-ARE solution and CA-ARE (with the same mass concentration of ARE) at a dose of 20 mg/kg, respectively. At predetermined time intervals (10, 30, 60 and 120 min) after administration, about 0.3 ml of blood was collected from the ocular vein into heparinized tubes followed by centrifugation (4000 rpm, 10 min) to collect plasma samples (100 µl), which were then stored at −20 °C until further analysis. After blood collection, the rats were sacrificed by the neck dislocation and their tissues (liver, spleen, heart, kidney, brain and lung) were collected. Each tissue sample was accurately weighed and homogenized with normal saline at a ratio of 2 ml/g to gain a final homogenate with a concentration of 333.33 mg/ml. Approximately 0.1 g of each homogenate was subjected to HPLC analysis.
Anaphylaxis study
Male guinea pigs (weighing 250–270 g) were raised in a dedicated area with the room temperature and relative humidity kept at 25 °C and 50%, respectively. The guinea pigs were fasted overnight with free access to water before experiments, and 100 mg/ml of vitamin C was added to the standard water.
Twenty male guinea pigs were randomly divided into four groups (n = 5): positive control (10% egg albumen), normal saline group, CA-ARE group and HS 15-ARE group. The anaphylaxis study was designed as shown in (n = 5) according to the previous literatures (Schneider et al., Citation1997; Okunuki et al., Citation2000; Daheshia et al., Citation2001; Underwood et al., Citation2002; Bergeron et al., Citation2006; Ren et al., Citation2011). Animals were injected intraperitoneally with 0.5 ml 10% egg albumen, normal saline, CA-ARE and HS 15-ARE, respectively, on days 1, 3, 5 and were observed for 9 consecutive days. On day 15, four groups were sensitized at the lateral of crus curvilineum with the double dose of their test agents.
All groups were observed in the following 30 min, then 0.5 ml intracardiac blood of each animal was collected. After coagulating at room temperature for 30 min, the blood was centrifuged at 3000 rpm for 30 min to obtain the supernatant serum. The histamine level in serum was determined by ELISA kit (R&D Systems, Minneapolis, MN, USA) according to the manufacturer’s instructions.
Therapeutic effect on Wistar rats
In this study, Wistar rats were used to test the efficacy of HS 15-ARE and CA-ARE on asthma. Twenty-eight healthy male Wistar rats (200 ± 20 g) were randomly divided into four groups (n = 7) as previously described. Normal group was treated with 1 ml of normal saline by intravenous injection for sensitization, and others were treated with 1 ml of 10% egg albumen. Two weeks later, the rats in model, normal saline, CA-ARE and HS 15-ARE group received their respective formulations (normal saline, normal saline, CA-ARE and HS 15-ARE) at a dose of 10 mg/kg by intravenously injection. Thirty minutes later, ultrasonic atomizing inhalation began. Briefly, each group was placed into a self-made transparent plastic container (). In the following 8 weeks, the saline group was given ultrasonic atomization of normal saline, while other groups were given 2% egg albumen for 30 min, three times a week. Eight weeks later, the administration concentration of egg albumen was raised to 4% in the following 6 weeks with the same treatment. After 24 h of the last ultrasonic atomization, 3% sodium pentobarbital solution was injected intraperitoneally to all rats at a dose of 45 mg/kg. Then, 0.5 ml of intracardiac blood of each animal was collected. The histamine level and Interleukin-5 (IL-5) level in the supernatant serum were analyzed using Rat Histamine (HIS) ELISA kit (R&D Systems, Minneapolis, MN, USA) and Rat interleukin 5 (IL-5) ELISA Kit (R&D Company, USA). Moreover, lungs were removed and lavaged to determine the IL-5 (Interleukin-5) level using Rat interleukin5 (IL-5) ELISA Kit (R&D Company, USA).
Statistical analysis
All data were presented as the mean ± standard deviation (SD). The statistical analysis of the samples was using a SPSS17.0 (SPSS Software Inc., Chicago, IL) t test. A value of p < 0.05 was considered to be significant. The pharmacokinetic parameters were calculated by the computer program DAS 2.0 (Drug and Statistics, Anhui, China).
Results and discussion
Preparation of Kolliphor HS 15-ARE micelles
The optimized formulation by single factor test consisted of 18 mg ARE, 285 mg Kolliphor HS 15, and 4 ml physiological saline that can be prepared by dissolution method.
Characterization of HS 15-ARE micelles
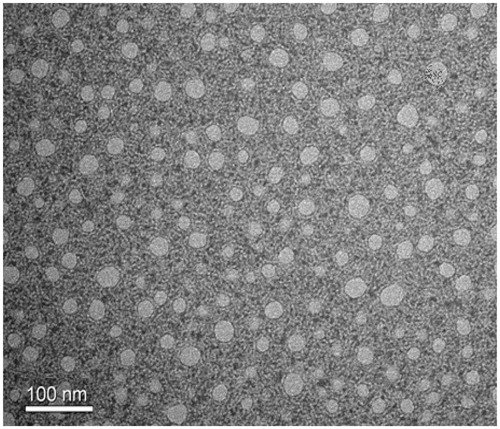
The HS 15-ARE formulation was characterized and the results showed that ARE water solubility was about 4.31 ± 0.16 mg/ml, which was significantly increased compared with free ARE (about 0.10 mg/ml). The solubilizing efficiency and drug loading content were 95.7 ± 2.4% and 5.56 ± 0.02%, respectively. The average particle size was 13.73 ± 0.02 nm with a polydisperse index (PDI) of 0.19 ± 0.01. The TEM image revealed that the micelle prepared were round in shape with particle size around 13 nm ().
showed the physical stability of obtained HS 15-ARE at 25 °C for 3 months. There was not any remarkable changes in the mean particle size, PDI and solubilizing efficiency, and no precipitation of ARE was found during this storage period. In addition, the mean size (nm) and PDI increased from 13.73 ± 0.02 to 14.08 ± 0.02 and from 0.19 ± 0.01 to 0.22 ± 0.01, respectively. Moreover, the drug content and drug loading of HS 15-ARE micelles had a loss of 3.0 and 3.1%, respectively, probably due to the partial decomposition of asarone. The morphology of HS 15-ARE micelles did not show any changes during this period. From these results, it can be concluded that the drug-loaded micelle was physically stable at room temperature for at least 3 months.
Table 1. The stability of the (a) Kolliphor HS 15-ARE at room temperature (25 °C) and (b) Kolliphor HS 15-ARE and CA-ARE after dilution.
To further mimic the in vivo behavior of ARE after injections, the HS 15-ARE micelles and CA-ARE were diluted by distilled water for 50-times. As shown in , the particle size of HS 15-ARE was almost stable (13.7 nm before dilution and 13.2 nm after dilution), while the size of CA-ARE was significantly increased. Moreover, the zeta potential of HS 15-ARE before and after dilution was about −1 mV, however, the zeta potential of CA-ARE after dilution was −9.3 mV compared with 0.9 mV before dilution. One possible reason for this phenomenon is that the formulation of CA-ARE contains solubilizer polysorbate 80 and cosolvent propylene glycol and ethanol, which may cause precipitation after dilution. In contrast, the formulation containing HS 15 remained stable after dilution. The stability of HS 15-ARE may affect its in vivo behavior after administration.
Critical micelle concentration determination
The pyrene 1:3 ratio plots (I1/I3) can be fitted by the Boltzmann-type approach (Aguiar et al., Citation2003; Barbero et al., Citation2009; ) as the following equation:
Figure 4. Plot of the fluorescence of pyrene I1/I3 intensity ratio versus concentration of Kolliphor HS 15 in normal saline at 25 °C (n = 3).
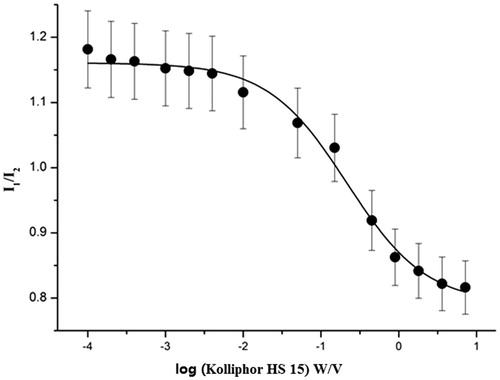
The variable y in the equation is I1/I3, while the independent variable (χ) stands for the total concentration of Kolliphor HS 15. χ0 represents the center of the sigmoid and Δχ is directly related to the independent variable occurs. A1 and A2 correspond to the upper and lower limits of sigmoid, respectively. According to , the Boltzmann-type model was consistent with the shape of the plot (R2 = 0.99), and the values for A1, A2, χ0 and Δχ in the equation were 1.15, 0.81, −0.63 and 0.37, respectively. χ0 should be the CMC value since the value of χ0/Δχ was no more than 10(Aguiar et al., Citation2003). As a consequence, the CMC value of Kolliphor HS 15 was 0.23 mg/ml.
In vitro hemolytic test
In vitro hemolytic test of the micelles was performed using 2% sheep erythrocyte suspension by visual observation method. As shown in , neither the HS-ARE nor the negative control tube caused hemolysis within 3 h, while the positive control tube showed hemolysis in 0.25 h.
Table 2. Panel a: Design of the hemolysis assay of the Kolliphor HS 15-ARE and Panel b: the hemolysis assay of the Kolliphor HS 15-ARE.
Pharmacokinetic study
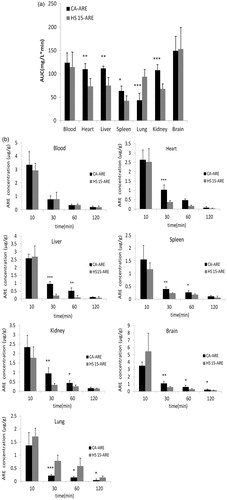
The mean plasma concentration–time curves of the HS 15-ARE and CA-ARE after intravenous administration were shown in . The two formulations showed that the same trend and the ARE was eliminated from blood sharply within the first 30 min and extremely fast within the first 15 min, followed by a very slow elimination phase. This elimination behavior might be attributed to the quick release of ARE from both formulations after intravenous injection into the blood. The results were shown in . It was found that there were no significant differences in the main pharmacokinetic parameters between HS 15-ARE and CA-ARE injections, which indicated that HS 15-ARE was bioequivalent with CA-ARE.
Figure 5. Mean plasma concentration–time curves of ARE in plasma after intravenous injections of HS 15-ARE and CA-ARE. Each value represents the mean±standard deviation (n = 5).
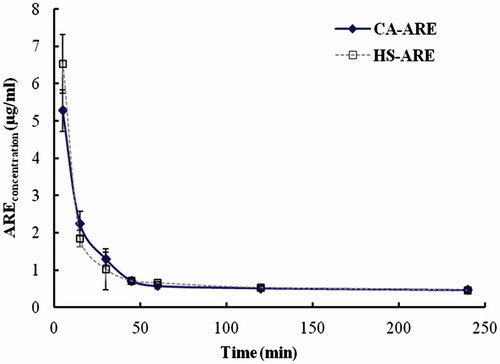
Table 3. The main pharmacokinetic parameters of HS 15-ARE and CA-ARE in rats (n = 5).
Tissue distribution study
The tissue distribution results of ARE for both groups are shown in . In , the ARE concentration in the lung treated with HS 15-ARE was significantly higher than that with CA-ARE (p < 0.05) at all predetermined time intervals (10, 30, 60 and 120 min). No significant difference in blood and brain was observed between the two formulations. Both groups displayed comparable levels of ARE concentration in the blood over time. At 10 min after administration, HS 15-ARE group displayed significantly higher levels of ARE distribution in brain than CA-ARE group, then lower at other time points. Moreover, the distribution of ARE in HS 15-ARE group in heart, liver, spleen and kidney was significantly lower than that of CA-ARE group (p < 0.05). It is inferred that the HS 15-ARE formulation may relatively reduce the uptake of ARE by the reticuloendothelial system (RES) compared to CA-ARE. After intravenous injection, the formulation is likely to be diluted by serum. In our previous in vitro study, the particle size and zeta potential of diluted HS 15-ARE and CA-ARE were analyzed. The results indicated that the size of CA-ARE formulation was significantly increased after dilution (), which could substantially enhance the chance to be uptaken by RES after i.v. injection. By comparison, the size of HS 15-ARE was relatively stable, avoiding the uptake by RES and thus increasing the chance to distribute in lung. In addition, the higher drug accumulation in lung might be beneficial to achieve better therapeutic effect of lung diseases, such as asthma.
Anaphylaxis study
To evaluate the safety of the HS 15-ARE for clinical use, anaphylaxis study was performed. The anaphylactic reaction, which is classically mediated by histamine release, is an acute IgE-mediated, life-threatening, allergic reaction (Burks & Sampson, Citation1999). In general, when an immunogen is intraperitoneally injected into an animal, an intensive IgE response is induced and an allergic response appears after the challenging injection. Once the animal was challenged via intravenous injection, an immediate anaphylaxis might occur along with the release of histamine (Ren et al., Citation2011). Histamine is the most important inflammatory mediator released by mast cells and basophiles when challenged during an allergic reaction, in which tachypnea, expiratory dyspnea and even spasm were usually observed, and histamine plays a crucial role in anaphylaxis and the cause of death (Varma et al., Citation1985; Lee et al., Citation1996; Tattersfield et al., Citation2002). In addition, it has been reported that the increased release of histamine caused by Polysorbate 80 has been demonstrated to be the mechanism of inducing anaphylaxis (Varma et al., Citation1985; Tattersfield et al., Citation2002). Therefore, the immediate hypersensitivity reactions can be evaluated by the level of histamine released into the blood after the challenge injection.
The results of allergy symptoms of four groups observed within 30 min after challenging were presented in . In the positive control group (10% egg albumen), four animals died in 2 min and another two died in 5 min. In CA-ARE group, five guinea pigs showed typical allergy symptoms such as restlessness, trembling, scratching the nose, shortness of breath and gait disorder. Two of them became syncope after the challenging injection and woke up about 5 min later. Half an hour later, the allergy symptoms of five animals have been greatly alleviated. As expected, the HS 15-ARE group did not show any allergy symptom, same as the normal saline group. The histamine levels of various groups are presented in . It is obvious that the content of serum histamine release in the HS 15-ARE group was almost the same as the normal saline group and was significantly lower than that of the positive control group and CA-ARE group (p < 0.05), nearly 30.5% compared with the CA-ARE group. The results of these histamine levels were consistent with animal behaviors described above. The lower histamine levels in HS 15-ARE group might be attributed to the absence of polysorbate 80. Furthermore, no report is available about sensitization induced by Kolliphor HS 15, which was also verified in this study. Thus, this study further demonstrated that the HS 15-ARE could be a safe and promising substitute for CA-ARE for clinical application.
Figure 7. The histamine levels of different groups of guinea pigs in anaphylaxis study. ***p < 0.001 (compared with CA-ARE).
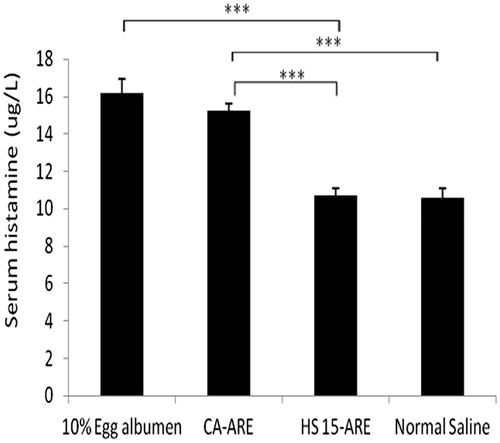
Table 4. The results of allergy symptoms of four groups of guinea pigs in anaphylaxis study.
Therapeutic effect study of Wistar rats
Ascertaining whether asthma is present in human is usually based on the presence of clinical symptoms such as wheezing, episodic cough and breathlessness (Masini et al., Citation1985). Furthermore, numerous epidemiologic studies have demonstrated a relationship between levels of IgE and asthma. IgE contributes to mast cell degranulation and subsequent pulmonary eosinophilic inflammation playing a crucial role in asthma pathogenesis through the release of proinflammatory and cytotoxic molecules (Lichtenstein et al., Citation1973; Burrows et al., Citation1989; Pollart et al., Citation1989; Sears et al., Citation1991; Busse et al., Citation1993; Naclerio et al., Citation1993; Reed, Citation1994). Therefore, the histamine levels can be used to quantify the efficacy of HS 15-ARE on asthma.
We compared the effect of the HS 15-ARE and CA-ARE using Wistar rats to test their efficacy on asthma. In this study, animals in the model group showed obvious asthmatic reaction, indicating the asthma model was successfully established, while others did not. Moreover, three animals in model group died in the activation process, the same mortality appeared in the CA-ARE group; one and zero died in HS 15-ARE group and normal saline group, respectively. presented the histamine levels of various groups. It is obvious that the content of serum histamine in the model group was significantly higher than that of other three groups (p < 0.05), which was consistent with the results of asthmatic reaction described above. The histamine level released into blood in the HS 15-ARE group was significantly lower than that of the model group and almost the same as normal saline group. Most importantly, the histamine release in the HS 15-ARE group was reduced by 23% compared with the CA-ARE group, which is consistent with the lung distribution result of the tissue distribution study.
Figure 8. (a) The histamine level released into blood in Wistar rats. *p < 0.05 (compared with CA-ARE). (b) The IL-5 content in the serum of each group in Wistar rats. *p < 0.05 (compared with CA-ARE). (c) The IL-5 content in alveolar lavage fluid in Wistar rats. *p < 0.05 (compared with CA-ARE).
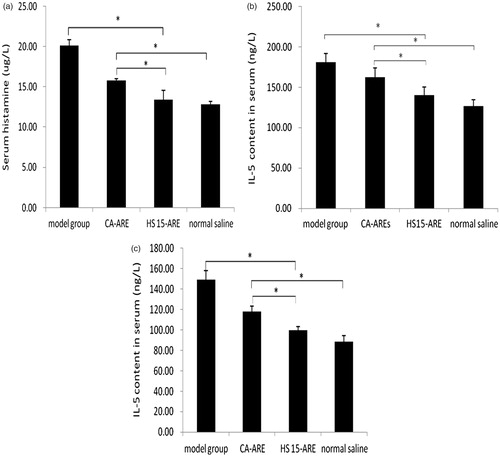
IL-5 is a pleiotropic cytokine that promotes differentiation and survival of eosinophil, and thus is considered as an important factor in asthma pathogenesis (Lalani et al., Citation1999). Moreover, IL-5 expression is elevated in patients with asthma, so IL-5 might directly induce bronchial hyper responsiveness (Gleich, Citation2000). Therefore, the amount of IL-5 could also be used to quantify the efficacy on asthma of HS 15-ARE. showed the comparison of IL-5 content in the serum of each group. IL-5 content in asthma model control group was obviously higher than that of normal group with statistically significant difference, indicating a pathological state. IL-5 content in the HS15-ARE group was reduced much more than that of the CA-ARE group. The amount of IL-5 in alveolar lavage fluid () shared almost the same trend as in the serum.
Conclusion
A novel formulation composed of ARE and Kolliphor HS 15 was successfully developed with suitable physicochemical properties and stability. In our study, Kolliphor HS 15 was proven an appropriate solubilizer for ARE that did not cause hemolysis. The HS 15-ARE displayed similar in vivo pharmacokinetic profiles compared with CA-ARE injections. Moreover, HS 15-ARE did not induce anaphylactic reactions and was shown to provide improved therapeutic effect on asthma. In general, the Kolliphor HS 15-ARE could be an excellent replacement for commercially available CA-ARE containing polysorbate 80 for intravenous administration.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article. This work was supported by grants from the National Basic Research Program of China (973 program, No: 2013CB932504), the National S&T Major Project of China (Grant No: 2012ZX09304004) and the National Natural Science Foundation of China (No. 81273443).
References
- Aguiar J, Carpena P, Molina-Bolívar JA, Carnero Ruiz C. (2003). On the determination of the critical micelle concentration by the pyrene 1:3 ratio method. J Colloid Interface Sci 258:116–22
- Allen TM, Cullis PR. (2004). Drug delivery systems: entering the mainstream. Science 303:1818–22
- Antunez-Solis J, Hernandez-Derramadero F, Aquino-Vega M, et al. (2009). 2,4,5-Trimethoxycinnamic acid: the major metabolite of alpha-asarone, retains most of the pharmacological properties of alpha-asarone. J Enzyme Inhib Med Chem 24:903–9
- Barbero N, Quagliotto P, Barolo C, et al. (2009). Characterization of monomeric and gemini cationic amphiphilic molecules by fluorescence intensity and anisotropy. Part 2. Dyes Pigments 83:396–402
- BASF. (2013). Kolliphor HS 15. Available from: http://www.pharma-ingredients.basf.com [last accessed 22 May 2013]
- Bergeron C, Boulet LP. (2006). Structural changes in airway diseases: characteristics, mechanisms, consequenees and pharmacologic modulation. Chest 129:1068–87
- Burks AW, Sampson HA. (1999). Anaphylaxis and food allergy. Clin Rev Allergy Immunol 17:339–60
- Burrows B, Martinez FD, Halonen M, et al. (1989). Association of asthma with serum IgE levels and skin-test reactivity to allergens. N Engl J Med 320:271–7
- Busse WW, Calhoun WF, Sedgewich JD. (1993). Mechanism of airway inflammation in asthma. Am Rev Res Dis 147:S20–4
- Cai ZQ, Liang Z, Jin H, Mao SJ. (2007). Preparation and quality evaluation of intravenous α-asarone emulsion. West China J Pharm Sci 22:611–13
- Chawla JS, Amiji MM. (2002). Biodegradable poly (e-caprolactone) nanoparticles for tumor-targeted delivery of tamoxifen. Int J Pharm 249:127–38
- Daheshia M, Friend DS, Grusby MJ, et al. (2001). Increased severity of local and systemic anaphylactic reactions in Gp49b1-deficient mice. J Exp Med 194:227–34
- De M, Ghosh P, Rotello V. (2008). Applications of nanoparticles in biology. Adv Mater 20:4225–41
- Farokhzad OC, Cheng J, Teply BA, et al. (2006). Targeted nanoparticle-aptamer bioconjugates for cancer chemotherapy in vivo. Proc Natl Acad Sci USA 103:6315–20
- Gao Z, Fain HD, Rapoport N. (2004). Ultrasound-enhanced tumor targeting of polymeric micellar drug carriers. Mol Pharm 1:317–30
- Gleich GJ. (2000). Mechanisms of eosinophil-associated inflammation. J Allergy Clin Immunol 105:651–63
- Gou M, Dai M, Li X, et al. (2008). Preparation and characterization of honokiol nanoparticles. J Mater Sci Mater Med 19:2605–8
- Guo DD, Hou SX, Mao SJ, et al. (2008). Comparison of kinetic behavior in both plasma and tissue after intravenous administration of α-asarone in lipid emulsion and aqueous solution in rats and mice. China J Chin Mater Med 33:46–50
- Hennenfent KL, Govindan R. (2006). Novel formulations of taxanes: a review: Old wine in a new bottle. Ann Oncol 17:735–49
- Lalani T, Simmons RK, Ahmed AR. (1999). Biology of IL-5 in health and disease. Ann Allergy Asthma Immunol 82:317–33
- Lee HK, Lee HH, Park YM, et al. (1996). Anti-IL-4 antibody inhibits antigen specific IgE response but fails to prevent chicken gamma globulin-induced active systemic anaphylaxis: evidence for the involvement of IgG antibodies. J Korean Med Sci 11:111–17
- Lichtenstein LM, Ishizaka K, Norman PS, et al. (1973). IgE antibody measurements in ragweed hay fever. Relationship to clinical severity and the results of immunotherapy. J Clin Invest 52:472–82
- Luo X, Wang Q, Zhou L, et al. (2010). Study on relationship between Tween-80 contained in injections of Chinese herbal medicine and anaphylactic reactions. Adv Drug Reactions J 12:160–5
- Masini E, Planchenault J, Pezziardi F, et al. (1985). Histamine-releasing properties of Polysorbate 80 in vitro and in vivo: correlation with its hypotensive action in the dog. Agents Actions 16:470–7
- Naclerio RM, Adkinson NF, Creticos PS, et al. (1993). Intranasal steroids inhibit seasonal increases in ragweed-specific immunoglobulin E antibodies. J Allergy Clin Immunol 92:717–22
- Okunuki H, Teshima R, Sakushima J, et al. (2000). Induction of active systemic anaphylaxis by oral sensitization with ovalbumin in mast-cell-deficient mice. Immunol Lett 74:233–7
- Pollart SM, Chapman MD, Fiocco GP, et al. (1989). Epidemiology of acute asthma: IgE antibodies to common inhalant allergens as a risk factor for emergency room visits. J Allergy Clin Immunol 83:875–82
- Reed CE. (1994). The importance of eosinophils in the immunology of asthma and allergic disease. Ann Allergy 72:376–80
- Ren CJ, Gong T, Sun X, et al. (2011). Alpha-Asarone incorporated in mixed micelles suitable for intravenous administration: formulation, in-vivo distribution and anaphylaxis study. Pharma 66:1–6
- Schneider T, van VD, Moqbel R, et al. (1997). Kinetics and quantitation of eosinophil and neutrophil recruitment to allergic lung inflanunation in a brown Norway rat model. Am J Respir Cell Mol Biol 17:702–12
- Sears MR, Burrows B, Flannery EM, et al. (1991). Relation between airway responsiveness and serum IgE in children with asthma and in apparently normal children. N Engl J Med 325:1067–71
- Tattersfield AE, Knox AJ, Britton JR, Hall IP. (2002). Asthma. Lancet 360:1313–22
- Underwood SL, Haddad E, Birrell MA, et al. (2002). Functional characterization and biomarker identification in the brown Norway model of allergic airway inflammation. Br J Phannaeol 137:263–75
- Varma RK, Kaushal R, Junnarkar AY, et al. (1985). Polysorbate 80: a pharmacological study. Arzneimittel Forschung 35:804–8
- Wu X, Rao WW, Ou Y, Liang ZD. (2010). A brief analysis on ADR monitoring situation of Asarone injection. Chin J Modern Drug Appl 16:9–10
- Xu GY, Zhou Y, Zuo GL, Jiang YJ. (2007). An improved procedure for synthesis of alpha-asarone. Org Prep Proc Intern 39:514–17
- Yang LP. (2005). Preparation of alpha-Asarone proliposomes by freeze-drying method. China Pharmacist 8:570–2
- Zhao LH, Wu JM, Wu FL. (2007). A review of recent ten-year study on alpha-asarone. Zhongguo Zhong Yao ZaZhi 32:562–5, 650
- Zheng C, Mei D, Wang L. (2005). Focused on the adverse effects of formulation excipients. China J Chin Mater Med 40:644–9