Abstract
Release from a transdermal drug delivery system (TDDS) can either be controlled by diffusion in the adhesive, by diffusion processes in the stratum corneum of the skin or a combination of both. In this study, diffusion processes in monolithic type TDDS were investigated using confocal Raman microscopy. An acrylic adhesive (Duro-Tak 180-129a), a rubber adhesive (Duro-Tak H1540) and a silicone adhesive (BIO-PSA 7-4202) were used. Skin permeation of the model drug Paeonol from these adhesives was investigated. Release studies on porcine cadaver skin were carried out. Solubility of Paeonol in the different adhesives was measured. Diffusion coefficients of the drug in the TDDSs were calculated from confocal Raman depth scans, the diffusion coefficient in the stratum corneum was calculated using tape stripping. Solubility of Paeonol in the acrylic adhesive was the highest with 30 g/L among the tested systems. Paeonol had a solubility of 6 and 9 g/L in the silicone and rubber based system. Diffusion coefficient rank order was BIO-PSA 7-4204 > Duro-Tak 180-129a > Duro-Tak H1540. Release on porcine cadaver skin from the silicone was the highest followed by the rubber and the acrylic adhesive. During release studies on porcine skin with Duro-Tak H1540 no concentration gradient of Paeonol could be monitored in the Raman depth profiles, whereas in the stratum corneum an apparent diffusion gradient was detectable. Solubility of a drug in the adhesive dominated the release properties, high-diffusion coefficients of drugs in adhesives do not necessarily lead to high release rates from adhesives.
Introduction
Transdermal drug delivery systems (TDDSs) are a promising alternative to oral drug delivery, which provide several benefits like maintaining a constant drug release during a long period of time and avoiding gastro intestinal mucosa irritation as well as hepatic first-pass metabolism (Prausnitz & Langer, Citation2008; Tanner & Marks, Citation2008). Most common TDDSs have a monolithic-type design: the drug is incorporated in the adhesive itself in a solubilized form. The critical compound in monolithic type TDDSs is the (pressure sensitive) adhesive. Beyond the general requirements for tapes or patches like functional adhesive properties, TDDSs need to provide a sustained drug release. In monolithic type, TDDSs drug delivery rates depend on the drug–polymer interactions. The thermodynamic activity of the drug in the adhesive and the solubility of the drug in the adhesive are important (Sato et al., Citation2001; Li et al., Citation2002). The skin itself as administration site limits the number of drugs available for the transdermal route. Only small molecule of a few hundred Dalton and moderate lipophilicity can penetrate the outermost layer of skin, the stratum corneum. The release from a TDDS can either be controlled by the diffusion in the adhesive, be controlled by the diffusion in the stratum corneum or a combination of both (Snorradottir et al., Citation2011).
When measuring a low drug release rate during penetration tests it is not known, whether this is due to adhesive retarding effects or due to a low diffusity of the drug in the skin (Cantor, Citation1999). Cantor (Citation1999) measured the diffusion coefficients of several compounds in different acrylic adhesives. Here, the diffusion coefficient of the drugs in the adhesives served as indicator for the later release properties on skin. Sato et al. (Citation2001) proposed a solubility factor for the prediction of drug fluxes during application of TDDSs on skin. Roy et al. (Citation1996) used the solubility and the diffusion coefficient to predict the release properties of transdermal delivery systems with fentanyl. Therefore, they investigated acrylic-, rubber- and silicone-based adhesives.
In this study, a model drug was incorporated in three different adhesives Duro-Tak H1540, Duro-Tak 180-129a and BIO-PSA 7-4202. With these three adhesive systems, the three general polymer classes silicones, acrylic adhesives and rubbers were covered. A new method using confocal Raman microscopy for the determination and visualization of diffusion in the adhesives was used. Both the solubility and diffusion coefficients in the adhesive were calculated. Release studies on porcine skin were conducted. The release rates and the diffusion coefficient of the drug in the stratum corneum were measured. In addition, Raman depth scans were taken from the rubber-based adhesive directly after application on porcine skin.
Material and methods
Materials
The rubber based (Duro-Tak H5140, based on a styrene–isoprene copolymer) and the acrylic adhesive (Duro-Tak 180-129a, based on 2-Ethylhexyl acrylate) were supplied by Henkel, Düsseldorf, Germany. The silicone-based adhesive BIO-PSA 7-4202 was purchased from Dow Corning, Midland, MI. Isopropyl myristate and decyl oleate were used as penetration enhancers and purchased from BASF Personal Care and Nutrition GmbH, Ludwigshafen, Germany. Porcine skin was obtained from a local slaughter. After the removal of hair and subcutaneous fat, the skin was stored at −20 °C (up to 6 months). The model drug Paeonol was purchased from Xuancheng Baicao Plants Industry & Trade Co., Ltd, Shanghai, China, and had a purity of 99.8%.
Preparation of monolithic type transdermal delivery systems (TDDSs)
Duro-Tak 180-129a
For the preparation of the TDDSs, first the drug was dissolved in isopropyl myristate and decyl oleate. Afterwards the acrylic adhesive solutions were prepared by mixing the drug solution with the PSA solution. This obtained mixture was then casted onto release liner coated with silicone (Poly Slik 111/120, Rexam release, London, UK) using a casting knife. After drying at room temperature over night, the resulting TDDS was either transferred onto a polyethylene backing film for the release experiments or covered with another silicone coated release liner. The final concentration of the drug in the adhesive was 5% (w/w) or 1% (w/w), the final thickness of the TDDS was 100 µm or 300 µm. For all experiments, the adhesives were stamped at 3.1 cm2. A placebo TDDS was prepared for the diffusion measurements in the adhesive. Therefore, the acrylic adhesive solution itself was casted without further adding of drug solution.
BIO-PSA 7-4202
For the preparation of the silicone TDDSs, the drug was either homogeneously dissolved in the adhesive solution (5% w/w, for diffusion coefficient) or dissolved with isopropyl myristate in the adhesive solution (1% w/w, for release experiments) and casted afterwards onto release liner (CLPET S3 10431 release liner, Loparex, Apeldoorn, the Netherlands) using a casting knife. After drying at room temperature overnight, the resulting TDDS was either transferred onto a polyethylene backing film for the release experiments or covered with a release liner (CLPET S3 10431 release liner). The final thickness was 100 µm (for measurement of the diffusion coefficient) or 300 µm (for release study). A placebo TDDS was prepared for the diffusion measurements in the adhesive. Therefore, the silicone PSA solution itself was casted without further adding of drug solution.
Duro-Tak H1540
The Duro-Tak H1540 TDDSs were prepared using a kneading machine (IKA, Staufen, Germany). The hot melt adhesive was heated to 60 °C and the drug solution (Paeonol in isopropyl myristate and decyl oleate) was added. After an appropriate time of mixing the adhesive was coated to 100 or 300 µm thick films between two silicone-coated release liners (Poly Slik 111/120). For the release studies, the resulting TDDS was transferred onto a polyethylene backing film. For all experiments, the adhesives were stamped at 3.1 cm2. A placebo TDDS was prepared for the diffusion measurements in the adhesive without further addition of drug solution.
Drug solubility in adhesives
The drug solubility in the different adhesive matrices was determined with the drug-uptake method described by Kokubo et al. (Citation1991). Briefly a placebo PSA with a surface of 0.79 cm2 was immersed in 2 mL of 1 mg/mL Paeonol in water and 1,2-propanediol (50/50, v/v). The amount of Paeonol absorbed by the adhesive was determined by analyzing the amount of the drug in the solution before and after equilibrium. The experiments were performed in triplicate.
Diffusion measurements in adhesive
The diffusion measurements in the adhesives were performed using confocal Raman microscopy (alpha500, WITec, Ulm, Germany) at room temperature. A placebo TDDS was adhered to a drug loaded adhesive on a microscope slide. At appropriate points of time, a Raman depth scan of 50 µm width and 110 µm depth with a resolution of 50 × 110 pixel were taken from the 100 µm thick placebo TDDS. The diffusion coefficient was determined following Fick's second law of diffusion.
shows the experimental setup and an example for a Raman depth scan of the placebo TDDS.
Release study
Franz diffusion cells (V = 8 mL, A = 9.39 cm2) were used for the in vitro permeation study. The donor compartment was exposed to room temperature while the receptor half was maintained at 35 °C using an external circulation water bath to ensure a skin temperature of 32 °C. The receiver cell was filled with PBS containing 0.07% gentamicine sulfate and stirred at a constant rate of 600 rpm. Full thickness excised porcine cadaver skin was put on the diffusion cells with the stratum corneum facing the donor compartment. Experiments with drug solution or TDDSs applied to the skin on the donor side over 24 h were performed in triplets. During the release studies adhesives with 1% (w/w) Paeonol were used.
Diffusion measurements in stratum corneum
The diffusion of the model drug in the stratum corneum was measured with tape stripping [as described by Alberti et al. (Citation2001)]. The stratum corneum was sequentially stripped from the treated skin area with a commercially available contact adhesive tape (tesaflim® 4129, tesa, Hamburg, Germany). The tape was pressed to the skin by rubbing it back and forth five times with a spatula. This was repeated up to 20 times while the direction of stripping was alternated. The tapes were individually weighted before and after stripping to determine the removed amount of stratum corneum. With the weights of the removed stratum corneum, assuming that the density of 1 g/cm3 is constant within the stratum corneum (Anderson & Cassidy, Citation1973) and the stripped area is constant, the thickness of the removed stratum corneum layer could be determined. The drug content on the single strips was analyzed with HPLC. Therefore, the drug was extracted from the strips with 2-propanol over night. After filtration with 0.2 µm Spartan filters (Whatman GmbH, Dassel, Germany), the resulting solution was analyzed via HPLC.
Quantification of Paeonol
The drug amount on the tapes (tape stripping) and in TDDSs was analyzed via HPLC. The HPLC analysis was performed using a Merck LaChrome Elite HPLC system with pump L 7100, auto sampler L 7250, diode array detector L 7455 (Merck Hitachi, Pleasanton, Canada). For quantification of Paeonol a reversed-phase Gemini C18 column (4.6 mm × 100 mm, 5 µm, Phenomenex, Torrance, CA) was used. The mobile phase consisted of 55% methanol and 45% water with 0.01% H3PO4. The flow rate was 1 mL/min. The detection wavelength was 275 nm and the average retention time was 7 min.
Diffusion measurements during application of the TDDSs
Release studies with excised porcine cadaver skin were performed. After 2, 6 and 24 h of application the TDDSs were removed from the porcine skin and adhered to a microscope slide for further investigation. Raman depth scans were taken from the TDDS as described in diffusion measurements in adhesive.
Calculation of diffusion coefficients
Diffusion of a drug molecule within a TDDS or the stratum corneum of skin can be described according Fick's second law:
(1)
with cA, the drug concentration in the skin or TDDS, the diffusion coefficient D in skin or TDDS, time t and the depth x.
The concentration can be described with a Gaussian function, which is a solution of Equation (Equation1(1) ) (Baehr & Stephan, Citation2006), with the mass flux of drug in the skin or TDDS
as a function of time and depth.
(2)
(3)
(4)
The experimental data were analyzed using STATISTICA. Only 1D diffusion was evaluated, diffusion in the y direction was not considered. The Raman signals were converted to concentrations of the model drug. Gaussian curves were fitted to the concentration profiles obtained from Raman scans or tape stripping experiments at different time points. The diffusion coefficient D was then calculated from the exponent 1/(4Dt) of the e-function.
Statistical analysis
Normal distribution of the data was checked using the Shapiro–Wilks test. Means of parametric data showing normal distribution like reflectance data were analyzed using two-way ANOVA with post-hoc Turkey HSD test. Differences were regarded statistical significant at a level of p < 0.05.
Results
Experiments with TDDSs based on silicone, acrylic or rubber adhesives were performed. The release of a model drug Paeonol from these transdermal systems on porcine skin was investigated. For the understanding of differences in the release rates from the three adhesive types, the drug solubility in the adhesive matrices and the diffusion coefficients in the adhesives were measured. Furthermore, the diffusion coefficient of Paeonol in porcine stratum corneum was calculated. The investigation of the diffusion processes in the adhesives was performed with confocal Raman microscopy.
Permeation properties of model drug in porcine skin
The Diffusion coefficient of Paeonol in porcine stratum corneum was determined using tape stripping after release studies with Franz diffusion cells at room temperature. Depth profiles obtained from the tape strips are shown in . The content of Paeonol in µg/cm2 is plotted against the stratum corneum depth in µm at different time points.
Figure 2. Paeonol distribution [µg/cm2] in the stratum corneum at different time points, after 2 h (⋄),4 h (□),6 h (Δ) and 24 h (+). Data represent means of three different experiments.
![Figure 2. Paeonol distribution [µg/cm2] in the stratum corneum at different time points, after 2 h (⋄),4 h (□),6 h (Δ) and 24 h (+). Data represent means of three different experiments.](/cms/asset/15339ba3-1cec-4f40-a0ea-5755177fd830/idrd_a_889778_f0002_b.jpg)
The concentration profiles of Paeonol within the stratum corneum showed at each sampling point the same trend. With increasing time of incubation more Paeonol could be found in deeper stratum corneum layers. The diffusion coefficient calculated from these depth profiles was 2.85 × 10−16 ± 6.5 × 10−17 m2/s.
Drug solubility
The solubility of Paeonol in the different adhesives was determined by the drug-uptake method. Only 5.9 g/L of Paeonol were soluble in the silicone adhesive BIO-PSA 7-4202. In the rubber based matrix, Duro-Tak H1540 9.6 g/L could be solved. With 30.6 g/L the drug had the highest solubility in the acrylic adhesive Duro-Tak 180-129a. The difference between the solubility of Paeonol in BIO-PSA 7-4202 and Duro-Tak H1540 was not significant (p = 0.81), the difference between the solubility in BIO-PSA 7-4202 and Duro-Tak 180-129a was significant (p = 0.03; ANOVA with post hoc Turkey test).
About 10 g/L of the drug were incorporated in the adhesives for the release studies on porcine skin. Hence, the TDDSs based on Duro-Tak H1540 and BIO-PSA 7-4202 were slightly oversaturated with Paeonol during the release studies, whereas Duro-Tak 180-129a was far away from saturation. No crystallization of Paeonol could be observed at the time of this study. Long period evaluation of crystallization was not yet performed.
Diffusion of model drug in the adhesives
The diffusion of Paeonol inside the three different adhesives was observed using confocal Raman microscopy. The Raman depth scans obtained during these experiments at different time points are shown in . We see the diffusion of Paeonol into the respective placebo TDDSs in cross-sections of the adhesives. Black areas in the scans represent the adhesive without Paeonol present. The Paeonol Raman signals are shown corresponding to their intensity in the depth scans (Scale in ). Clearly visible from the Raman scans Paeonol diffusion in the silicone adhesive BIO-PSA 7-4202 was the fastest, followed by diffusion in the acrylic adhesive Duro-Tak 180-129a and the rubber-based PSA Duro-Tak H1540. From these Raman depth scans, a 3D distribution of Paeonol in the adhesive over time was calculated. The distributions are shown in .
Figure 3. Raman depth scans in (a) Duro-Tak H1540, (b) Duro-Tak 180-129a and (c) BIO-PSA 74202 at different time points. Paeonol distribution within the adhesive is shown according to the scale in arbitrary unit [a.u].
![Figure 3. Raman depth scans in (a) Duro-Tak H1540, (b) Duro-Tak 180-129a and (c) BIO-PSA 74202 at different time points. Paeonol distribution within the adhesive is shown according to the scale in arbitrary unit [a.u].](/cms/asset/a792d614-e3db-464d-8aba-34a7c0b19a93/idrd_a_889778_f0003_c.jpg)
Figure 4. Distribution Paeonol in Duro-Tak H1540, Raman signal [a.u.] of Paeonol over depth [µm] and time [min].
![Figure 4. Distribution Paeonol in Duro-Tak H1540, Raman signal [a.u.] of Paeonol over depth [µm] and time [min].](/cms/asset/a99558e1-b894-46d3-96a0-4b30a55b9b9b/idrd_a_889778_f0004_c.jpg)
Figure 5. Distribution of Paeonol in Duro-Tak 180-129a, Raman signal [a.u.] of Paeonol over depth [µm] and time [s].
![Figure 5. Distribution of Paeonol in Duro-Tak 180-129a, Raman signal [a.u.] of Paeonol over depth [µm] and time [s].](/cms/asset/b8523316-fe3b-469e-9a77-cfb5e3ef4bbb/idrd_a_889778_f0005_c.jpg)
Figure 6. Distribution of Paeonol in BIO-PSA 7-4202, Raman signal [a.u.] over depth [µm] and time [s].
![Figure 6. Distribution of Paeonol in BIO-PSA 7-4202, Raman signal [a.u.] over depth [µm] and time [s].](/cms/asset/6e6ca6c2-62ee-4d8b-9af3-6003e278b209/idrd_a_889778_f0006_c.jpg)
Diffusion coefficients calculated from these distributions were determined by fitting curves according to Equation (Equation2(2) ) through these data in time. As already visible from the Raman depth scans, Paeonol diffusion in the silicone adhesive BIO-PSA 7-4202 was the fastest with 1.86 × 10−11 ± 9.69 × 10−12 m2/s among the tested matrices. The Paeonol diffusion in the acrylic adhesive Duro-Tak 180-129a was 10-times lower (2.07 × 10−12 m2/s ± 9.3 × 10−13 m2/s) compared to the silicone system and diffusion in the rubber-based system Duro-Tak H1540 was nearly 100-times lower (4.4 × 10−13 ± 3.45 × 10−14 m2/s). There was a significant difference in diffusion coefficients of Paeonol within the three different transdermal systems.
Release studies on porcine skin
Percutaneous absorption of Paeonol incorporated in the three different PSAs was investigated by performing release studies on porcine cadaver skin using Franz-type diffusion cells. The release of Paeonol from the three different adhesives on porcine skin is shown in .
Figure 7. Release profiles of Paeonol from the different adhesive systems Duro-Tak 180-129a (♦), Duro-Tak H1540 (▪) and BIO-PSA 7-4202 (▴). Percentage of Paeonol (applied on porcine skin with the respective TDDS) absorbed by porcine skin over time of application, data (±standard deviation) represent mean values of three different experiments.
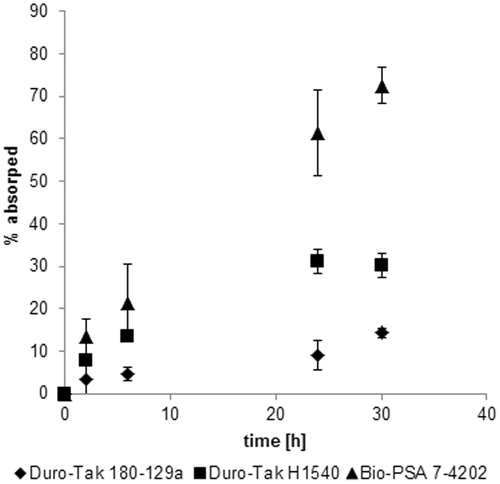
The percentage amount absorbed by the skin is plotted against the time of application. BIO-PSA 7-4202 showed by far the highest release rate of Paeonol on porcine skin. Nearly 80% of the drug incorporated in the adhesive was found in skin and receptor fluid after 30 h of application. For the rubber-based adhesive Duro-Tak H1540, 30% of the drug could be absorbed by the skin. The acrylic adhesive Duro-Tak 180-129a showed that the lowest absorption with only 15%. Significant difference between the three different TDDSs could be observed after 24 and 30 h of application.
Diffusion during the release study
A release study with Duro-Tak H1540 (the adhesive with the lowest diffusion coefficient of Paeonol in the adhesive) on porcine cadaver skin using Franz diffusion cells was performed. Raman depth scans of the TDDSs directly after removal of the TDDS from porcine skin after 2, 6 and 24 h of application were performed. Tape strips from the stratum corneum were collected and analyzed 2, 6 and 24 h after application of the TDDS. The Raman scans and the drug profiles in the stratum corneum are shown in . Again the intensity of the Raman signal of Paeonol is illustrated corresponding to the scale in in the depth scans. The same color code was used for the stratum corneum depth profiles obtained by tape stripping.
Figure 8. (a) Raman depth scans in TDDS over the time of application (0, 2, 6 and 24 h) on porcine skin and (b) depth profiles of Paeonol in stratum corneum of porcine skin obtained by tape stripping 2, 6 and 24 h after application of the TDDS with Paeonol.
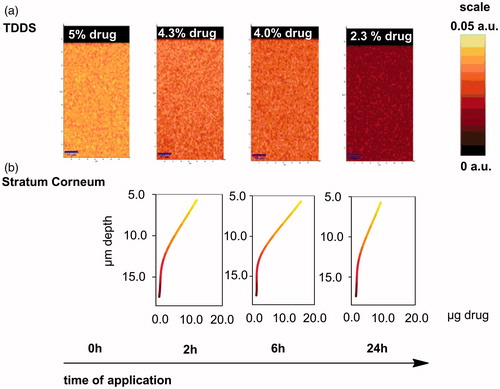
No diffusion gradient in the depth profile of the adhesive Duro-Tak H1540 could be observed throughout the release study. Nevertheless, in the stratum corneum of the porcine skin, a clear concentration gradient was detected. Hence the release was dominantly controlled via the stratum corneum of the skin, not via the diffusion processes in the adhesive itself.
Discussion
Confocal Raman microscopy gives us an insight in the diffusion processes in adhesives of monolithic type TDDSs. One can very nicely monitor concentration gradients of the drugs within the adhesive matrix, even during the application of the TDDS. The diffusion coefficients calculated from the Raman depth scans together with the solubility experiments give us an understanding of the different release rates into skin of an acrylic, a rubber-based and a silicone adhesive loaded with the same drug.
Diffusion coefficients calculated for the model drug Paeonol in the three different adhesives were in the range of 4.4 × 10−13 to 1.9 × 10−11 m2/s. Already published data by Cantor (Citation1999) and Roy et al. (Citation1996) were in the same order of magnitude (). The diffusion coefficient in the silicone adhesive BIO-PSA 7-4202 of Paeonol was the highest followed by the diffusion coefficient in the acrylic adhesive. The lowest diffusion coefficient was observed for the rubber-based matrix. The order of silicone > acrylate > rubber was also reported by Roy et al. (Citation1996) for the diffusion of fentanyl. Paeonol diffusion measured in porcine stratum corneum was much lower than in the adhesives with a coefficient of 2.85 × 10−16 m2/s. This value again was in the same order of magnitude as previously published data for diffusion coefficients of various molecules in skin ().
Table 1. Diffusion coefficients of various molecules in adhesives reported in literature and in this study.
Table 2. Diffusion coefficients of various molecules in skin reported in literature and this study.
The Raman scans of the rubber-based adhesive directly after application on porcine skin showed at no time point a gradient of Paeonol within the matrix. In-depth profiles of porcine stratum corneum, however, a clear concentration gradient of Paeonol was observed. This together with the comparison of the diffusion coefficients in the adhesive and the stratum corneum indicate that the rate limiting step for the release of Paeonol from the rubber-based TDDS Duro-Tak H1540 into skin was the skin barrier itself.
Solubility of Paeonol measured in the three adhesives was between 5.9 and 30.6 g/L. The highest amount of Paeonol could be solved in the acrylic TDDS Duro-Tak 180-129a, followed by the rubber-based matrix Duro-Tak H1540. The lowest solubility of Paeonol was observed in the silicone adhesive BIO-PSA 7-4202. Acrylic, rubber-based and silicone adhesives are chemically highly different. Due to (amongst others) the varying functional groups within the different adhesives Paeonol shows this diverse behavior in its solubility. The TDDSs used for release studies with porcine skin had a Paeonol content of 10 g/L. Hence, the silicone adhesive was oversaturated with the model drug. This TDDS may not be stable under long time conditions, but during the time of this study, no crystallization of Paeonol was observed.
In release studies on porcine skin, the silicone adhesive showed by far the highest liberation of Paeonol with 61% in 24 h. For the rubber-based Duro-Tak H1540 matrix, 30% release and for the acrylic TDDS only 10% release was observed in the same time period. The oversaturation of the silicone adhesive with Paeonol led to the very high release on porcine skin. The important role of oversaturation for release properties of transdermal systems was reported by Hadgraft (Citation2001). Release rates on porcine skin followed the order of solubility of Paeonol in the adhesives, but they did not follow the order of diffusion coefficients ().
Table 3. Drug solubility, release on porcine skin and diffusion coefficients of Paeonol in adhesive.
Conclusion
Confocal Raman microscopy is a good method for the investigation of diffusion processes in adhesives matrices of transdermal delivery systems. Diffusion processes can be easily monitored and diffusion coefficients in the adhesives of transdermal systems are calculated from the Raman depth scans. Furthermore, the diffusion and partition processes during application of a TDDS on skin can be observed in real time. Combining this method of investigation with solubility measurements of a model drug in the different adhesives and release studies on porcine skin led to following conclusions. The solubility dominates the release properties of Paeonol from different adhesive systems on porcine skin. Higher diffusion coefficients of drugs in adhesives do not necessarily lead to higher release rates. The partition of the drug from the adhesive to the stratum corneum is the rate limiting step during the release from a transdermal system.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
References
- Alberti I, Kalia YN, Naik A, et al. (2001). In vivo assessment of enhanced topical delivery of terbinafine to human stratum corneum. J Control Release 71:319–27
- Anderson RL, Cassidy JM. (1973). Variation in physical dimensions and chemical composition of human stratum corneum. J Invest Dermatol 61:30–2
- Baehr HD, Stephan K. (2006). Wärme- und Stoffübertragung, vol. 5. : Springer Verlag
- Cantor AS. (1999). Drug and excipient diffusion and solubility in acrylate adhesives measured by infrared-attenuated total reflectance (IR-ATR) spectroscopy. J Control Release 61:219–31
- Hadgraft J. (2001). Skin, the final frontier. Int J Pharm 224:1–18
- Kokubo T, Sugibayashi K, Morimoto Y. (1991). Diffusion of drug in acrylic-type pressure-sensitive adhesive matrices. I. Influence of physical property of the matrices on the drug diffusion. J Control Release 17:69–78
- Li J, Masso JJ, Rendon S. (2002). Quantitative evaluation of adhesive properties and drug–adhesive interactions for transdermal drug delivery formulations using linear solvation energy relationships. J Control Release 82:1–16
- Prausnitz MR, Langer R. (2008). Transdermal drug delivery. Nat Biotechnol 26:1261–8
- Roy SD, Gutierrez M, Flynn GL, Cleary GW. (1996). Controlled transdermal delivery of fentanyl: characterizations of pressure-sensitive adhesives for matrix patch design. J Pharm Sci 85:491–5
- Sato K, Mitsui N, Hasegawa T, et al. (2001). Potential usefulness of solubility index for prediction of the skin permeation rate of 5-ISMN from pressure-sensitive adhesive tape. J Control Release 73:269–77
- Snorradottir BS, Gudnason PI, Thorsteinsson F, Másson M. (2011). Experimental design for optimizing drug release from silicone elastomer matrix and investigation of transdermal drug delivery. Eur J Pharm Sci 42:559–67
- Sugibayashi K, Todo H, Oshizaka T, Owada Y. (2010). Mathematical model to predict skin concentration of drugs: toward utilization of silicone membrane to predict skin concentration of drugs as an animal testing alternative. Pharm Res 27:134–42
- Tanner T, Marks R. (2008). Delivering drugs by the transdermal route: review and comment. Skin Res Technol 14:249–60
- Yamaguchi K, Morita K, Mitsui T, et al. (2008). Skin permeation and metabolism of a new antipsoriatic vitamin D(3) analogue of structure 16-en-22-oxa-24-carboalkoxide with low calcemic effect. Int J Pharm 353:105–12

![Figure 1. Experimental setup, Paeonol distribution within the adhesive is shown according to scale in arbitrary unit [a.u.], dimensions of the adhesive are given in µm (−10 µm).](/cms/asset/70755683-8cf8-45a5-b01c-dae1f5a0f546/idrd_a_889778_f0001_c.jpg)