Abstract
Background: The purpose of the present study was to formulate and characterize Nizatidine-encapsulated microballoons for enhancing bioavailability and increasing the residence time of drug in the gastrointestinal tract.
Methods: Microballoons were prepared using emulsion solvent diffusion method using Eudragit S-100 and HPMC as the polymer. The formulation process was optimized for polymer ratio, drug: polymer ratio, emulsifier concentration, stirring speed, stirring time. Optimized formulation was subjected to scanning electron microscopy, drug entrapment, buoyancy studies, in-vitro drug release and in-vivo floating efficiency (X-ray) study. In-vivo antiulcer activity was assessed by ethanol-induced ulcer in murine model.
Results: The microballoons were smooth and spherical in shape and were porous in nature due to hollow core. A sustained release of drug was observed for 12 h. Examination of the sequential X-ray images taken during the study clearly indicated that the optimized formulation remained buoyant and uniformly distributed in the gastric contents for a period of 12 h. In ethanol-induced ulcer model, drug-loaded Microballoon-treated group showed significant (p < 0.01) ulcer protection index as compared to free drug-treated group.
Conclusion: Nizatidine-loaded floating microballoons may serve as a useful drug delivery system for prolonging the gastric residence time and effective treatment of gastric ulcers.
Introduction
Oral administration suffer from the drawback of having incomplete drug release from device and short residence time of the pharmaceutical dosage form resulting in poor bioavailability of drug in the gastrointestinal tract (GI) (Tayade & Kale, Citation2007). Hence sustained release dosage forms have been designed to both prolong gastrointestinal transit of the dosage form as well as controlled drug release. Several gastrointestinal targeting dosage forms (Moës, Citation1993; Deshpande et al., Citation1997; Hwang et al., Citation1998), including intragastric flotation systems (Yuasa et al., Citation1996; Rouge et al., Citation1998; Lee et al., Citation1999), high-density systems (Hwang et al., Citation1998), mucoadhesive systems, adhesion to the gastric mucosal surface in order to extend gastric residence time (GRT) (Akiyama et al., Citation1995), magnetic systems (Gröning et al., Citation1998), unfoldable, extendible, or swellable systems (Fix et al., Citation1993) and superporous hydrogel systems (Park, Citation1988), have been developed.
One such approach is floating drug delivery systems (FDDS) in which the system floats over the gastric contents and drug is released slowly at the desired rate. These systems can be used for drugs possessing solubility in acidic environment and high rate of absorption in the upper part of the small intestine (Deshpande et al., Citation1997; Arora et al., Citation2005). Both single and multiple unit systems have been developed. High variability of gastrointestinal transit time, due to its all-or-nothing gastric emptying process is the disadvantage associated with single unit system. Thus, a multiple-unit floating system which distributes widely throughout the GI has been developed. Sato et al. (Citation2003) developed a multiple-unit floating system involving hollow microspheres (microballoons) with excellent buoyant properties using o/w emulsion solvent diffusion method. Microballoons are in a strict sense, spherical empty particles without core having internal hollow structure with air inside. Microballoons incorporate a drug dispersed or dissolved throughout particle matrix have the potential for controlled release of drugs (Ojha et al., Citation2006).
Nizatidine is a histamine H2-receptor antagonist that inhibits stomach acid production, and commonly used in the treatment of peptic ulcer disease (PUD) and gastroesophageal reflux disease (GERD). Nizatidine is absorbed from the upper GI and is preferentially localized in parietal cells of gastric mucosa. The short half-life (1–2 h) and rapid clearance of nizatidine suggest it a rationale drug for gastroretentive drug delivery. The high solubility, chemical and enzymatic stability and absorption profile of nizatidine in acidic pH values (of stomach), points to the potential of gastroretentive dosage form. The present works aims to design gastroretentive drug delivery system for nizatidine using microballoons as the carrier system that could give site specific and controlled drug release.
Materials and method
Nizatidine were obtained as gift samples from M/s Dr Reddy’s Labs. Hyderabad. Eudragit S-100 was purchased from Rohm Pharma. Gmbh, Germany and HPMC, Dichloromethane, methanol, polyvinyl alcohol (PVA) and Tween 80 were purchased from Central Drug House, Mumbai (India). All other chemicals used were of analytical grade.
Preparation of microballoons
Microballoons were prepared using the emulsion solvent diffusion method (Kawashima et al., Citation1992). Nizatidine (0.1 g), polymers (1.0 g) and monostearin (0.5 g) were dissolved in a mixture of dichloromethane (8 ml) and ethanol (8 ml) at room temperature. Each solution was introduced into an aqueous solution of PVA (0.75 w/v%, 200 ml) at 40 °C. Resultant emulsion was stirred at 300 rpm with a propeller type agitator for 1 h. The resulting polymeric spheres were dried overnight at 40 °C.
The formulation process was optimized for polymer ratio, drug: polymer ratio, emulsifier concentration, stirring speed, stirring time (). On the basis of formulation and process variables, the optimized conditions for preparation of microballoons were recorded in .
Table 1. Process variables of nizatidine-loaded microballoons.
Table 2. Formula for the preparation of microballoons after optimization.
Characterization of microballoons
Electron microscopy
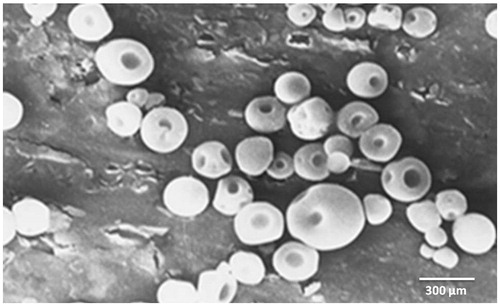
Scanning electron microscopy (SEM, Jeon Scanning Electron Microscpe J.S.-840) was performed to characterize the surface of formed microspheres. The samples for SEM were prepared by lightly sprinkling the microballoons on a double-adhesive tape stuck to an aluminum stub. The stubs were then coated with gold to a thickness of about 300 Å under argon atmosphere using a gold sputter module in a high-vacuum evaporator. The samples were then randomly scanned using a Scanning Electron Microscope and photomicrographs were captured.
Entrapment efficiency
Microballoons containing drug equivalent to 100 mg Nizatidine were digested in a 10 mL mixture of dichloromethane and methanol (1:1 v/v). The mixture was centrifuged at 3000 rpm for 3 min and 1 ml of supernatant was withdrawn and after suitable dilution with distilled water and assayed spectrophotometrically. The percentage drug entrapment is calculated from the equation given below.
In-vitro buoyancy
Microballoons (100 mg) were dispersed in USP dissolution apparatus containing simulated gastric fluid (SGF 900 ml, pH 1.2, 37 °C) containing Tween 20 (0.02% w/v). It was stirred with a paddle at 100 rpm for 12 h. After predetermined time interval, the layer of floating particles was separated from settled particle. Both fractions of particles were dried in vacuum desiccators. Both the fractions of microballoons were weighed and buoyancy was determined by using the formula:
where Wf and WS are the weights of the floating and sinking microballoons, respectively.
In-vitro drug release study
The drug release study was carried out in USP paddle type dissolution apparatus (Veego, DA-6DR USP). Microballoons containing drug equivalent to 100 mg were gently spread over the surface of 900 ml of dissolution media (SGF, pH 1.2). The speed of rotation was maintained at 100 rpm and the temperature of dissolution medium was thermostatically controlled at 37 ± 2 °C. The samples were withdrawn at suitable time interval from the dissolution apparatus. The initial volume of fluid was maintained by adding fresh dissolution fluid after each withdrawal. The samples withdrawn were assayed spectrophotometrically using UV–visible spectrophotometer (Shimadzu 1701, Japan).
In-vivo floating efficiency (X-ray) study
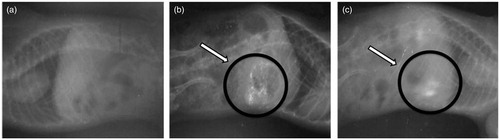
The in-vivo study was carried out by administering floating beads to rats and monitoring them by a radiological method (Rajinikanth & Mishra, Citation2007) with slight modifications. Six healthy albino rats of either sex, weighing 400–500 g were used for the present study. The animals were housed in individual cages, and the experiments were conducted in a sanitized room at a temperature maintained at around 27 °C. Food was withdrawn 12 h prior to the study with water ad libitum. To make the beads radiopaque, 500 mg of barium sulfate was incorporated into polymeric solution (the same optimized formulation composition was used to prepare radiopaque beads) and radiopaque beads were prepared using a similar procedure to that mentioned in the preparation of beads. Beads were administered through oral gastric tube with 2 ml water in fasted state. The animals were not allowed to eat or drink throughout the study (up to 6 h). In total, 1 ml of water was administered to animals every hour throughout the study. The position of bead in the rat’s stomach was monitored by X-ray photographs (Siregraph-B, Siemens, Germany) of the gastric region at varying time intervals (at 1, 4 and 6 h). The protocol of the study was approved by Animals Ethical Committee, Shri Ram Institute of Technology-Pharmacy, Jabalpur, India (Protocol No: SRITP/IEAC/12/05).
Antiulcer activity
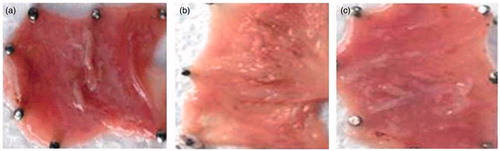
This investigation was conducted on Albino rats, with an average body weight of 400–450 g and ages up to 3 months. Animals were kept in standard cages at constant room temperature 25 ± 1 °C, with circadian rhythm (day/night), and were fed standard laboratory rat feed. Before the experiment, all animals were exposed to a 24-h fasting period prior to treatment with alcohol, but had free access to water. Alcohol stress was induced by intragastric administration of 1 ml of 100% alcohol (Arafa & Sayed-Ahmed, Citation2003). The animals were divided into three groups, each consisting of five rats. First group received normal saline. Nizatidine solution (10 mg/ml) was administered orally to animals of second group. Third group received nizatidine-loaded microballoons (equivalent to 10 mg). Upon treatment, animals were sacrificed, and the abdomen was opened by midline incision, the stomach was removed, opened along the greater curvature, rinsed gently with water, and pinned open for macroscopic examination. Areas with gastric lesions were measured and the ulcer index (UI) was estimated from the formula:
Results
Formulation and optimization of microspheres
Floating microballoons were prepared by emulsion solvent diffusion method using Eudragit S-100 and HPMC. Formulations were optimized by using varying concentration of Eudragit S-100 and fixed concentration of HPMC to evaluate the effect of polymer concentration on the size of microspheres. The mean particle size of the microballoons significantly increased with increasing Eudragit S-100 concentration and was in the range of 268.6 ± 4.6 to 308.5 ± 2.4 µm (). Formulations were optimized in terms of drug: polymer ratio; particle size of microballoon ranged from 266.6 ± 4.3 to 302.8 ± 1.4 µm. Microballoons was also optimized for varying emulsifier concentration (% w/v). Mean particle size of microballoons was larger at low concentration of emulsifier (0.5%; 293.7 ± 3.3 µm) and it decreased with an increase in concentration of emulsifier (1.25%; 249.6 ± 4.2 µm). An increase in stirring speed (700 rpm) promoted formation of small sized microballoons (274.6 ± 3.6). demonstrates the optimized formula.
Electron microscopy
Shape and surface morphology of microballoons was observed by scanning electron microscopy () which confirmed spherical shape and smooth surface of microballoons.
Percent buoyancy
The buoyancy percentage for all batches almost was above 50% which was studied for 12 h.
In-vitro drug release study
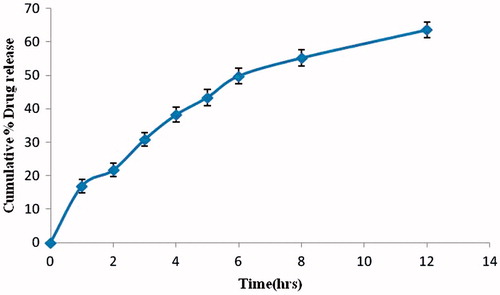
In-vitro drug release studies were performed in simulated gastric fluid pH 1.2 for 12 h. The in-vitro release profile was biphasic with an initial burst release (16.05 ± 0.94%) upto 1.0 h which may be attributed to surface associated drug, followed by a slower release phase as the entrapped drug slowly diffused into the release medium (). Percentage of the drug released up to 12 h was 80.05 ± 0.64. There was sustained release of drug at a constant rate.
In-vivo floating efficiency (X-ray) study
The optimized floating microspheres exhibited good in-vitro buoyancy and controlled release behavior and hence was finally selected for in-vivo radio graphical study. Examination of the sequential X-ray images taken during the study clearly indicates that the optimized formulation remained buoyant and uniformly distributed in the gastric contents for the study period of 6 h ().
Antiulcer activity
In ethanol-induced ulcer model, oral administration of 95% ethanol in control group, produced characteristic lesions in stomach which emerged as elongated bands of broad red lesions. The in-vivo evaluation showed that UI values were 0.64 ± 0.08 for normal saline-treated group, 0.49 ± 0.11 for nizatidine solution and 0.14 ± 0.08 for nizatidine microballoons. Microballoons-treated group showed significant (p < 0.01) ulcer protection index as compared to free drug-treated group ().
Discussion
Microballoons are one of the innovative gastroretentive drug delivery systems. Their bulk density is less than density of gastric contents which enables them to float in gastric fluid. As microballoons float on gastric contents, drug is released at a desired rate leading to maintenance of drug concentration for a prolonged period of time. After the drug release is completed, residual system is cleared off from the stomach. Thus, such a system provides increased GRT and provides an effective control over fluctuations in plasma drug concentration.
Floating microballoons of nizatidine were prepared by emulsion solvent diffusion method using Eudragit S-100 and HPMC. Microballoons were prepared by using varying concentration of Eudragit S-100 and fixed concentration of HPMC. It is clear from that formulation variables had a great impact on buoyancy, particle size and entrapment efficiency. On increasing Eudragit S-100 concentration, the viscosity of the medium increased resulting in enhanced interfacial tension. Shearing efficiency also diminished at higher viscosities (Reddy et al., Citation1990). This resulted in formation of larger sized particles.
Microballoons were prepared with different drug: polymer ratio (1:1, 1:2, 1:3, 1:4), while other parameters were kept constant. The mean particle size of the microballoons increased significantly by decrease in drug polymer ratio. The drug entrapment efficiency increased initially from 56.8 ± 3.6% to 68.2 ± 4.2% by the decrease in drug polymer ratio up to 1:3 after which it decreased. Since drug entrapment, buoyancy and particle size are dependent on factors like stirring speed and emulsifier concentration, an increase in polymer concentration may have resulted in a shift in the equilibrium between these factors, which was evident by a reduction in drug entrapment and percentage buoyancy. Thus, the optimized drug: polymer ratio was selected as 1:3.
The mean microballoon size, buoyancy and drug entrapment efficiency were found to decrease with an increase in emulsifier concentration. This may be due to the fact that increase in emulsifier concentration resulted in increase in miscibility of ethanol with dichloromethane (processing medium), which may increase the extraction of drug into the processing medium. The buoyancy could have decreased due to tightening of polymeric network, leading to microballoon shrinkage with an increase in concentration of emulsifier. Increased degree of agitation (stirring) resulted in formation of small sized microspheres.
Optimized formulation was subjected to in-vitro drug release studies and floating behavior was observed by X-ray studies. Shape of microballoons was examined by scanning electron microscopy. Microballoons were distinguished as spherical shape enfolded in hard polymer shell. Central cavity of microballoons is formed due to volatilization of dichloromethane. As the polymer and drug solution in dichloromethane and ethanol is dropped in PVA solution, ethanol tends to diffuse in aqueous solution; this leads to formation of a shell and produces a cavity within it which is produced due to volatilization of dichloromethane. Such a phenomenon is responsible for creating a buoyant and floating system that tends to float in gastric fluid (Kawashima et al., Citation1991).
Buoyancy of microparticulate system is dependent on the quantity of polymers, ratio of polymers and nature and type of solvents used in formulation (Streubel et al., Citation2006). In the current study, microballoons continuously floated over dissolution for more than 12 h. The buoyancy of microballoons could be contributed due to presence of pores created due to rapid evaporation of dichloromethane, by which air got entrapped in pores allowing them to float.
Release of nizatidine from HPMC microballoons was evaluated in SGF pH 1.2. A steady drug release () from microballoons was observed, which could be due to diffusion and erosion mechanism. This also demonstrated “no burst effect” from formulation as there was a progressive drug release.
The success of any pharmaceutical formulation could be assessed by biological studies. In the present work, ethanol-induced ulcer model was utilized to determine efficacy of nizatidine-loaded microballoons.
Ethanol produced gastric lesions which are due to stasis in gastric blood flow which causes hemorrhage and tissue necrosis. It rapidly penetrates gastric mucosa and plasma membrane and enhances membrane permeability to water and sodium which in turn augments massive calcium accumulation. This proves to be a major step in pathogenesis of injury of gastric mucosa (Halliwell & Gutteridge, Citation1987; Soll, Citation1990). Along with it, when ethanol is metabolized in body, it causes increased production of in tissues leading to increased cellular free radical concentration, which are ultimately responsible for breaking of DNA strands and protein denaturation (Surendra, Citation1999). Nizatidine microballoons efficiently inhibited ethanol-induced ulcers () demonstrating its cytoprotective effect on gastric mucosa.
Conclusion
In-vitro data obtained for microballoons of nizatidine showed excellent buoyancy. Microballoons of nizatidine floated in SGF for a prolonged period of time and sustained drug release from the beads over a period of 12 h. The in-vivo floating efficiency of beads was satisfactory; beads were retained in rat stomach for extended period. Thus, the microballoons may prove to be promising candidate for obtaining stomach specific drug delivery.
Acknowledgements
Authors are thankful to Rewa Shiksha Samiti for constant support during studies.
Declaration of interest
The authors declare that there was no conflict of interest.
References
- Akiyama Y, Nagahara N, Kashihara T, et al. (1995). In vitro and in vivo evaluation of mucoadhesive microspheres prepared for the gastrointestinal tract using polyglycerol esters of fatty acids and a poly(acrylic acid) derivative. Pharm Res 12:397–405
- Arafa HM, Sayed-Ahmed MM. (2003). Protective role of carnitine esters against alcohol-induced gastric lesions in rats. Pharmacol Res 48:285–90
- Arora S, Ali J, Ahuja A, et al. (2005). Floating drug delivery systems: a review. AAPS PharmSciTech 19:E372–90
- Deshpande AA, Shah NH, Rhodes CT, Malick W. (1997). Development of a novel controlled-release system for gastric retention. Pharm Res 14:815–19
- Fix JA, Cargill R, Engle K. (1993). Controlled gastric emptying, Part 3. Gastric residence time of a nondisintregrating geometric shape in human volunteers. Pharm Res 10:1087–9
- Gröning R, Berntgen M, Georgarakis M. (1998). Acyclovir serum concentrations following peroral administration of magnetic depot tablets and the influence of extracorporal magnets to control gastrointestinal transit. Eur J Pharm Biopharm 46:285–91
- Halliwell B, Gutteridge A. (1987). Free radicals in biology and medicine. Oxford: Clarendon Press. 247 p
- Hwang SJ, Park H, Park K. (1998). Gastric retentive drug delivery systems. Crit Rev Ther Drug Carrier Syst 15:243–84
- Kawashima Y, Niwa T, Takeuchi H, et al. (1991). Preparation of multiple unit hollow microspheres (microballoons) with acrylic resin containing tranilast and their drug release characteristics (in vitro) and floating behavior (in vivo). J Contr Rel 16:279–89
- Kawashima Y, Niwa T, Takeuchi H, et al. (1992). Hollow microspheres for use as a floating controlled drug delivery system in the stomach. J Pharm Sci 81:135–40
- Lee JH, Park TG, Choi HK. (1999). Development of oral drug delivery system using floating microspheres. J Microencapsul 16:715–29
- Moës AJ. (1993). Gastroretentive dosage forms. Crit Rev Ther Drug Carrier Syst 10:143–95
- Ojha G, Tanwar YS, Chauhan CS, Naruka PS. Floating microspheres: development, characterization and application. pharmaceutical information for you, May 2006. Available at: www.pharmainfo.net/.../floating-microspheres-development-characterization-and-applications-United States [last accessed 14 July 2008]
- Park K. (1988). Enzyme-digestible swelling hydrogels as platforms for long-term oral drug delivery: synthesis and characterization. Biomaterials 9:435–41
- Rajinikanth PS, Mishra B. (2007). Preparation and in vitro characterization of gellan based floating beads of acetohydroxamic acid for eradication of H. pylori. Acta Pharm 57:413–27
- Reddy BP, Dorle AK, Krishna DK. (1990). Albumin microspheres: effect of process variables on the distribution and in vitro release. Drug Dev Ind Pharm 16:1781–803
- Rouge N, Cole ET, Doelker E, Buri P. (1998). Buoyancy and drug release patterns of floating minitablets containing piretanide and atenolol as model drugs. Pharm Dev Technol 3:73–84
- Sato Y, Kawashima Y, Takeuchi H, Yamamoto H. (2003). Physicochemical properties to determine the buoyancy of hollow microspheres (microballoons) prepared by the emulsion solvent diffusion method. Eur J Pharm Biopharm 55:297–304
- Soll AH. (1990). Pathogenesis of peptic ulcers and implication for therapy. New Eng J Med 322:909–16
- Streubel A, Siepmann J, Bodmeier R. (2006). Gastroretentive drug delivery system. Expert Opin Drug Deliv 3:217–33
- Surendra S. (1999). Evaluation of gastric antiulcer activity of fixed oil of tulsi and possible mechanism. Ind J Exp Biol 36:253–57
- Tayade PT, Kale RD. (2007). A multiple unit floating drug delivery system of Piroxicam using Eudragit polymer. Ind J Pharm Sci 69:120–3
- Yuasa H, Takashima Y, Kanaya Y. (1996). Studies on the development of intragastric floating and sustained release preparation. I. Application of calcium silicate as a floating carrier. Chem Pharm Bull 44:1361–6