Abstract
The main aim of this study was to develop an effective carrier system containing Ag85A-loaded guar gum nanoparticles for oral vaccination against tuberculosis. Nanoparticles were prepared by Nanoprecipitation method. The developed particles with mean diameter 895.5 ± 14.73 nm and high antigen entrapment seem to be optimum for oral vaccine delivery. The acid protection assay, Peyer’s patch uptake study and in-vitro antigen study confirmed that the developed formulations can protect the antigen from harsh gastric environment and can safely deliver the antigen to the intestinal region. In vivo studies data indicated that the developed nanocarriers can induce a strong mucosal as well as systemic immune response. Therefore, the experimental evidence suggests that guar-gum nanoparticle findings indicated that the guar gum nanoparticles can be utilized for safe and effective vaccine delivery via oral route.
Introduction
Tuberculosis (TB) continues to be a serious global health threat and is a major concern till date. The commercially available TB vaccine (BCG) has failed to control the TB epidemic and confers a variable protection (ranging from 0% to 80%) against pulmonary TB in adults (Colditz et al., Citation1994). Cellular immune response is believed to be essential for protection against TB. The interaction of T cells with macrophages further triggers the control of the infection (Garg et al., Citation2012). The stimulation of antigen-specific CD8+ T cells is more challenging than CD4+ T cells stimulation. An effective vaccine for TB should induce both of them (Tascon et al., Citation1998). CD4+ T cells play a central role in the immune response against M. tuberculosis and are involved in the production of cytokines, specifically interferon (IFN)-γ, which is critical for macrophage activation and the subsequent induction of microbicidal mechanisms (Mogues et al., Citation2001; Tascon et al., Citation1998). The nature of antigen-presenting cells involved in antigen presentation can define the kind of immune response. Endocytosis of an antigen by a specific class antigen-presenting cell is essential for the presentation of antigens by respective major histocompatibility complex molecules and subsequent T-cell activation (Lizee et al., Citation2005). It is well-known fact that dendritic cells capture the soluble antigens either via macro pinocytosis or by clathrin-mediated endocytosis (Regnault et al., Citation1999). The clathrin-mediated endocytosis is also termed as receptor-mediated transport (Conner & Schmid, Citation2003). Macro pinocytosis is a non-specific endocytosis but clathrin-mediated endocytosis in DCs is highly specific process which includes the endocytosis of receptors such as Fc receptors, transferrin receptor, macrophage mannose receptor (MMR, CD206), DEC 205 (CD205) and DC-SIGN (Guo et al., Citation2000). In past few years, a huge research has been carried out to target these specific receptors using specific ligands to develop desired immune response. Here, we developed guar gum nanoparticles for targeted antigen delivery to the gut-associated lymphoid tissue via oral route. Guar gum was used for the encapsulation of antigen. Guar gum is natural polysaccharide obtained by ground endosperm of Cyamopsis tetragonolobus. Guar gum is natural nontoxic, biodegradable, mucoadhesive, cost-effective polymer which can encapsulate a higher amount of antigen. It has been widely used for intestinal drug delivery (Prasad et al., Citation1998; Prabaharan, Citation2011). These properties make it an excellent candidate for antigen delivery via oral route (Cummings et al., Citation1978). Moreover, swelling behaviour of guar gum at acidic pH imparts an efficacy to protect the antigen in harsh gastric environment. Guar gum is a water-soluble polysaccharide composed of sugars, such as galactose and mannose. Presence of mannose moiety in guar gum imparts it a great affinity toward mannan receptors (MMR) (Adikwu, Citation2009; Collnot et al., Citation2012). The carbohydrate recognition domains of the MMR show specificity for mannose and acts as an endocytic receptor that binds mannosylated structures and has been shown to mediate the in vitro cellular immune response. The MMR is also expressed on DCs, Langerhans cells, monocytes and endothelial cells (Sallusto et al., Citation1995). The guar gum nanoparticles were developed by solvent precipitation method. This is a simple method rendering safe loading of antigen without any exposure to stringent chemical or mechanical condition which can otherwise alter the structural integrity of the antigen. The selected antigen, Ag85A, induces strong T-cell proliferation and IFN-γ production in most healthy individuals infected with Mtb/M. leprae (Launois et al., Citation1994).
Materials and methods
Materials
Ag85A was procured from Genscript, USA. Guar gum, BCA (Bicinchoninic acid) protein estimation kit, fluorescein isothiocyanate (FITC)-BSA (Bovine Serum Albumin), HRP (Horse Radish Peroxidase)-conjugated secondary antibodies and isotyping kits were purchased from Sigma-Aldrich Pvt Ltd., USA. All other chemicals were of analytical grade.
Preparation of guar gum nanoparticles
Guar gum nanoparticles were prepared by solvent precipitation method using ethanol as antisolvent. Varying composition of the solvent with different ethanol and water ratio was added drop wise to the guar gum solution containing antigen. Various formulation parameters including polymeric concentration and solvent composition were optimized on the basis of particle size andparticle size distribution. The formulation was finally spray dried using optimized fixed ratio (data not shown) of mannitol (1% w/v) and l-leucine (0.2% w/v). The formulation was reconstituted in phosphate buffer (0.5 mL) before administration. BSA was used as a model antigen for the complete formulation optimization and in-vitro characterization. The finally optimized formulation was prepared using Ag85A and was subjected to immunization.
Characterization of developed formulation
Morphology
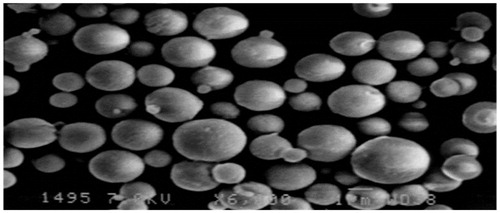
The morphology and surface appearance of the particles were examined by scanning electron microscopy (SEM) (Garg et al., Citation2013). The completely dried samples were coated with a thin gold layer and observed with SEM (Zeiss EVO 50).
Particle size, particle size distribution
The mean diameter and zeta potential of the nanoparticles were determined by Zeta sizer (Beckman Coulter Pvt. Ltd., Delsa Nano C). The zeta potential analysis was performed using samples diluted with 1 mM HEPES (2-[4-(2-hydroxyethyl)piperazin-1-yl]ethane sulfonic acid) buffer (pH 7.4) in order to maintain a constant ionic strength. For each sample, the mean values ± SD of three determinations were established.
Antigen loading efficiency
The amount of free antigen in the supernatant was determined to calculate the entrapment efficiency. The antigen-loaded suspension was centrifuged at 26,000 RCF for 30 min. The protein content was quantified by using BCA protein estimation kit (Sigma, USA) using UV-visible spectrophotometer (Shimadzu, Japan). The blank formulation was treated in the same manner and the supernatant was used to correct the absorbance value. The corrected optical density (OD) value was used to calculate the concentration of protein in the supernatant.
Acid degradation protein assay
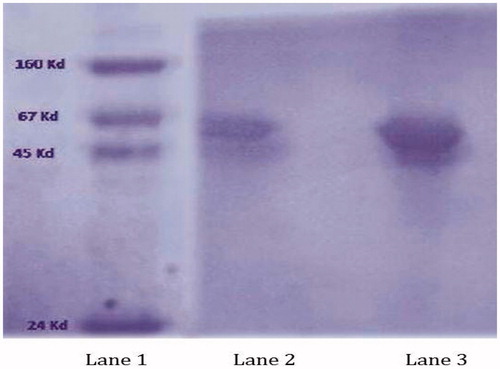
Antigen-loaded particles were resuspended in 0.01 M HCl, for 4 h at 37 °C. The nano-suspension was than centrifuged at 26,000 RCF for 20 min and supernatant was discarded. The pellet was redispersed with 4 mL of PBS 7.2 and the antigen released after 24 h was subjected to SDS-PAGE analysis. The band of pure antigen was used as to evaluate the structural integrity of the released antigen (Li et al., Citation2008).
In-vitro release studies
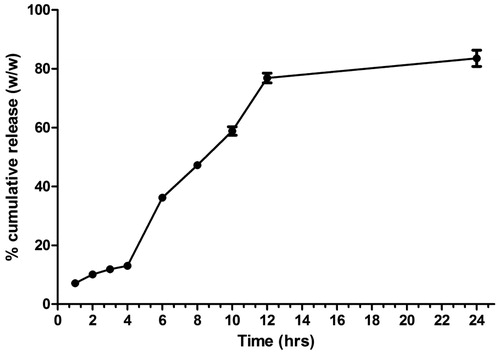
Oral administration follows intestinal path after its passage from the stomach. Therefore, antigen release behaviour was evaluated in simulated gastric fluid (SGF, pH 1.2) for initial 4 h and then transferred to the simulated intestinal fluid (SIF, pH 7.2). The optimized nanoparticles suspension were added to individual vials containing release medium previously equilibrated at 37 °C and placed in incubator shaker at constant 50 rpm. Samples were withdrawn at appropriate time intervals. The amount of free antigen in the supernatant was quantified using BCA kit. Results were expressed as a percentage of total antigen content loaded in the formulation. Supernatant of blank formulation was used as control for the correction of the OD value of the samples analyzed with BCA protein assay. In-vitro release experiments were repeated thrice.
Peyer’s patch uptake study
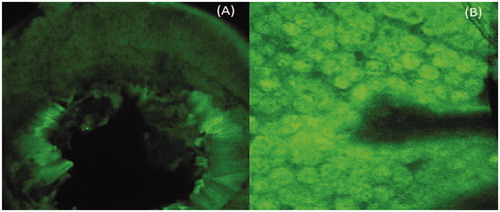
Peyer’s patch uptake study was conducted using confocal microscopy. FITC-BSA was used as fluorescent marker (Van der Lubben et al., Citation2001a). The FITC-BSA-loaded nanoparticles were administered orally to the Balb/c mice. Mice were sacrificed after 4 h of dose administration by cervical dislocation, and intestinal Peyer’s patch were removed using standard procedure. The samples were analyzed under confocal microscope (Nikon, Japan) (Van der Lubben et al., Citation2001b).
In-vivo studies
The in-vivo study protocol was approved by Institutional Animals Ethical Committee and Council for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA). The studies were carried out according to the guidelines of the CPCSEA, Ministry of Social Justice and Empowerment, Government of India (ISF/CPCSEA/IAEC/2013-90). Female Balb/c mice were selected to evaluate the immunogenicity of the developed vaccine formulation. Six animals were included in each group for immunological studies. The mice were immunized by oral route using different formulations containing standard dose (10 µg) of antigen. Each animal received booster dose after 21 days of primary immunization.
Collection of sample
The blood samples were collected from retro-orbital puncture (under mild anaesthesia) at days 0, 14, 28 and 42 after immunization from each animal. The collected blood was allowed to coagulate, so as to separate the serum, which was stored at −40 °C until tested by ELISA for the estimation of IgG titre and IgG isotypes. The various body secretions i.e. nasal, vaginal and salivary secretions were collected at 42nd day to evaluate the IgA titre (Malik et al., Citation2012). Briefly, salivary secretions were induced by injecting 0.2 mL sterile pilocarpine solution (10 mg/mL) intraperitoneal and the saliva was collected using capillary tube after 20 min. Vaginal washes were collected by introducing 50 μL of PBS containing 1% (w/v) BSA (1% BSA–PBS) into the vaginal tract of non-anesthetized mice using a Gilson pipette. A second vaginal wash was collected on the subsequent day and pooled with the first one. The nasal wash was similarly collected by flushing the trachea of the sacrificed mice with 0.5 mL of PBS-B (pH 7.4). These fluids were stored in the presence of 100 mM phenyl methyl sulfonyl fluoride as a protease inhibitor at −40 °C until tested by ELISA for sIgA titre.
Measurement of IgG and IgA response
Antibody titres in immunized animals were measured by using microplate ELISA using standard protocol (Dou et al., Citation2005). Briefly, 100 µL Ag85A (10 µg/mL) was used to coat 96-well plate overnight at 4 °C followed by three times washing with PBS-Tween 20 (0.5%, v/v) (PBS-T). Afterward, blocking was done with PBS-BSA (3% w/v) for 2 h at 37 °C, followed by washing with PBS-T. The serum was serially diluted with PBS and 100 μL of each sample was added to the respective wells. The plates were incubated for 2 h at room temperature and again washed three times with PBS-T. Peroxidase-labelled goat anti-mouse IgG (100 µL) (1:1000 dilution; Sigma, USA) was added to each well. The plates were incubated for 1 h at room temperature, and the plates were covered. Hundred microliters of tetramethylbenzidine solution was added to each well and plate was incubated for 30 min at a dark place and 50 μL of 1 N HCl was added to stop the reaction. The plate was read at 450 nm (Bio-Rad, USA). End point titers were expressed as the log of the reciprocal of the last dilution, which gave an OD at 450 nm above the OD of negative controls.
Statistical analysis
All data were expressed as mean ± SD. Significance was tested using two-way analysis if variance (ANOVA) test followed by Bonferroni post-test to compare all formulations versus control. A p value less than 0.05 were considered as statistical significant for all comparisons.
Results and discussion
Optimization of guar gum concentration
The effect of variable guar gum concentration was evaluated on the particle size and distribution ().
Table 1. Optimization of guar gum concentration.
The other parameters such as the solvent composition, rate of stirring, temperature and rate of addition were kept constant. It was observed that polymer concentration can significantly affect the particle size of the formulation. There was a slight (p > 0.05) increase in the mean particle size with increase in the guar gum concentration. However, we observed a highly significant (p < 0.05) and uncontrolled (higher polydispersity index (PDI)) increase in particle size above 0.3% guar gum concentration. It may be attributed to increase in the viscosity of the polymeric solution at its higher concentration. At higher polymer concentration, there may be limited reduction in the end groups resulting in uncontrolled entanglement of the polymeric chain due to scarcity of the solvent. The optimized guar gum concentration was used for future trials.
Optimization of solvent composition
The composition of solvent used for precipitation shows a significant effect on the formulation characteristics. Undiluted ethanol resulted in the formation of a large clump of the polymer and we were not able to control the precipitation. We exposed the polymer to different proportions of ethanol ().
Table 2. Optimization of Solvent composition.
The addition of more diluted ethanol resulted in the formation of the particles with minimum mean diameter (∼880 nm) with high uniformity. There was a significant increase in the particle size with increase in alcohol content and again resulted in the formation of large aggregate (film-like structure) at higher alcohol content. It may be due to rapid precipitation of the polymer which further results in the formation of larger aggregates by Ostwald ripening and the added surfactant concentration was not sufficient to control the growth of the particles.
Optimization of antigen content
The antigen concentration was varied to ensure the maximum content which can be loaded within fixed polymer content under specified condition, i.e. solvent composition, rate of stirring and temperature. The effect of change in antigen content was evaluated on the particle size and loading efficiency. It was observed that increase in the antigen content resulted in slight increase in the particle size. This may be due to incorporation of the antigen within the polymeric particles. Moreover, there was continuous decrease in the loading efficiency which may be attributed to saturation of free sites on the polymer with increasing the amount of antigen. Addition of antigen beyond certain limit and more amount of antigen resulted in a drastic fall in loading efficiency ().
Table 3. Optimization of antigen content.
Particle size, morphology and entrapment efficiency
Finally, optimized formulation were suitable for oral delivery with mean particle diameter around 900 nm and the PDI was 0.291 (). The SEM studies clearly indicate spherical shape of the spray dried guar gum nanoparticles (). There was an increase in the particle size after spray drying from 900 nm to 1.85 µm, which was still optimum for oral drug delivery.
In-vitro release studies
Orally administered formulations encounter both simulated gastric and SIFs. Guar gum nanoparticles were designed to retard the antigen release at harsh acidic content in the stomach due to its swelling nature at low pH. The results of the in-vitro release study confirmed that guar gum protects the antigen from its acidic degradation and there was merely 12% of antigen released in SGF after 4 h. However, the release was quite high and sustained in SIF. It may be due to quick erosion of the particles at higher pH ().
Acidic degradation protein assay
The stability of proteins is very susceptible against harsh conditions and it losses activity in the presence of the same. To develop an effective antigen carrier based on guar gum that can effectively avoid the acidic degradation of encapsulated antigen before reaching the intestinal region, the formulation was exposed to the acidic medium (pH 2.0) first and then was transferred to SIF (pH 7.2). The released antigen was compared with the bands of the molecular marker (lane 1) and the naive antigen (lane 2) using SDS-PAGE. Appearance of identical bands () clearly indicated that guar gum nanoparticles could effectively protect BSA from hydrolysis/degradation at acidic medium.
Peyer’s patch uptake study
Confocal fluorescence results confirmed the targeting potential of guar gum nanoparticles to the Peyer’s patch region. A higher intensity of fluorescence was observed in case of mice administered with guar gum nanoparticles. It may be attributed to the fact that the M cells present in the Peyer’s patch region are specialized for antigen sampling from gut lumen and are even exploited by many pathogens as a route of host invasion (Gebert et al., Citation1996). They possess high transcytosis capacities and have an ability to transport a broad range of materials, including nanoparticles (Frey et al., Citation1996; Frey & Neutra, Citation1997). This may also be attributed to the specificity of guar gum nanoparticles toward MMRs which are over expressed by the dendritic cells and in the mouse FAE (Follicle-associated epithelium) (). This is consistent with other reports stating that targeting to the mannose receptor can induce a strong immune response due to receptor-mediated endocytosis and hence better uptake (Apostolopoulos et al., Citation2000; Engering et al., Citation1997a,Citationb). It is well-known fact that the mannose receptor functions as a potential antigen receptor in the dendritic cells. Moreover, dendritic cells in the Peyer’s patch region (LysoDC) extended the dendrites through M-cell-specific transcellular pores to the gut lumen to sample antigenic material. Therefore, the DCs based sampling may be an additional contributor for increased uptake of the guar gum nanoparticles via Peyer’s patch region (Lelouard et al., Citation2012).
Immunological studies
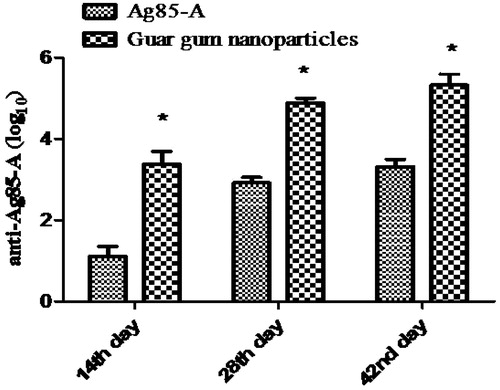
Immunization studies were carried out to determine the immune adjuvant capability of the developed guar gum nanoparticles. Results were compared with the immune response induced by naïve antigen administered via oral route. Each group contained six animals. Both groups received booster dose on 21st day of primary immunization. Blood sample were collected by retro-orbital plexus, and IgG and IgG isotyping was estimated by ELISA (Sigma, USA). IgG titre was measured at different time intervals. Immunological outcomes clearly indicate the highly significantly improved (p < 0.001) immune response in mice after the guar gum nanoparticles formulation administered orally () as compared to the plain antigen.
Figure 5. IgG response. (Each data represents mean ± SD (n = 6). Significance was tested using two-way ANOVA and Bonferroni post-test.) Data compared with antigen alone as control denoted by *. p value less than 0.5 was considered as significant.
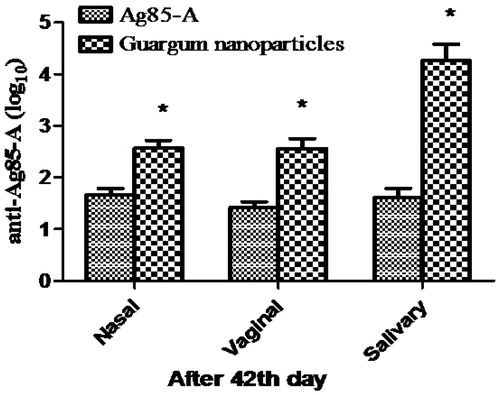
This could be attributed to the protective action of the guar gum at acidic pH and targeting potential of guar gum nanoparticles to the mannose receptor present on the immune cells resulting in better antigen presentation. Similarly, there was a significant increase in the IgA (p < 0.001) than the plain antigen in the salivary, nasal and vaginal secretions after 42nd day of primary immunization (). Mucosal IgA plays a significant role in protection against enteropathogens and imparts nonspecific defence mechanism and restricts the entry of pathogen via mucosal route. Thus, orally administered polymeric carrier systems have capability to provoke mucosal immune response. Hence have an additional advantage of mucosal immunity.
Figure 6. IgA response at 42nd day in different body fluids. (Each data represents mean ± SD (n = 6). Significance was tested using two-way ANOVA and Bonferroni post-test.). Data compared with antigen alone as control denoted by *. p value less than 0.5 was considered as significant.
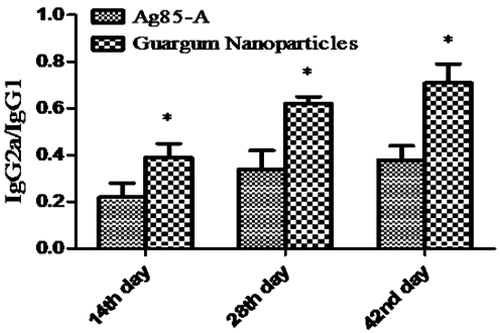
To ensure complete protection, the systems should be able to induce both humoral as well as cellular immune response. In this study, the IgG1and IgG2a isotypes were determined at day 42 and presented as a ratio of cellular to humoral immune response. Although there was a dominant humoral immune response throughout the study, the guar gum nanoparticles were able to generate a balanced humoral and cellular immune response. It may be due to the targeting potential of the guar gum to the mannose receptor which preferably results in the induction of Th1 immune response (). It is consistent with the previous reports stating that targeting to mannose receptors on the dendritic cells significantly improved the T cell response (Apostolopoulos et al., Citation2000).
Conclusion
Carbohydrates are involved in a vast variety of biological process. A long list of safe, nontoxic, biodegradable, biocompatible polymers is available. A number of polysaccharides have been utilized for drug/vaccine delivery. Here, we develop guar gum nanoparticles for safe and effective vaccine delivery via oral route. The guar gum is nature-gifted polymer which is equipped with mannose moiety having potential to target mannose receptor. The guar gum nanoparticles resulted in the elicitation of a strong systemic as well as mucosal immune response. Therefore, guar gum nanoparticles can be a promising tool for needle-free targeted vaccination delivery. It can be utilized for safe and effective vaccine delivery via oral route.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
Acknowledgements
Authors Dr. Amit K. Goyal and Dr. Goutam Rath are thankful to the Department of Biotechnology (DBT), New Delhi, Mr. Tarun Garg is thankful to PTU, Jalandhar and Mr. Basant Malik is thankful to CSIR, New Delhi, India, for providing financial assistance to carry out research on the development of novel nanocarriers for mucosal vaccine delivery.
References
- Adikwu MU. (2009). Biopolymers in drug delivery: recent advances and challenges. Bentham Science Publishers, 1–198. (DOI: 10.2174/97816080507891090101)
- Apostolopoulos V, Barnes N, Pietersz GA, McKenzie IF (2000). Ex vivo targeting of the macrophage mannose receptor generates anti-tumor CTL responses. Vaccine 18:3174–84
- Colditz GA, Brewer TF, Berkey CS, et al. (1994). Efficacy of BCG vaccine in the prevention of tuberculosis. Meta-analysis of the published literature. JAMA 271:698–702
- Collnot E-M, Ali H, Lehr C-M. (2012). Nano-and microparticulate drug carriers for targeting of the inflamed intestinal mucosa. J Control Release 161:235–46
- Conner SD, Schmid SL. (2003). Regulated portals of entry into the cell. Nature 422:37–44
- Cummings J, Branch W, Jenkins D, et al. (1978). Colonic response to dietary fibre from carrot, cabbage, apple, bran, and guar gum. Lancet 311:5–9
- Dou J, Chen JS, Wang J, et al. (2005). Novel constructs of tuberculosis gene vaccine and its immune effect on mice. Cell Mol Immunol 2:57–62
- Engering AJ, Cella M, Fluitsma D, et al. (1997a). The mannose receptor functions as a high capacity and broad specificity antigen receptor in human dendritic cells. Eur J Immunol 27:2417–25
- Engering AJ, Cella M, Fluitsma DM, et al. (1997b). Mannose receptor mediated antigen uptake and presentation in human dendritic cells. Adv Exp Med Biol 417:183–7
- Frey A, Giannasca KT, Weltzin R, et al. (1996). Role of the glycocalyx in regulating access of microparticles to apical plasma membranes of intestinal epithelial cells: implications for microbial attachment and oral vaccine targeting. J Exp Med 184:1045–59
- Frey A, Neutra MR. (1997). Targeting of mucosal vaccines to Peyer's patch M cells. Behring Institute Mitteilungen 98:376–89
- Garg T, Singh O, Arora S, Murthy R. (2012). Scaffold: a novel carrier for cell and drug delivery. Crit Rev Therap Drug Carrier Systems 29:1–63
- Garg T, Singh S, Goyal AK. (2013). Stimuli-sensitive hydrogels: an excellent carrier for drug and cell delivery. Crit Rev Therap Drug Carrier Systems 30:369–409
- Gebert A, Rothkotter HJ, Pabst R. (1996). M cells in Peyer's patches of the intestine. Intern Rev Cytol 167:91–159
- Guo M, Gong S, Maric S, et al. (2000). A monoclonal antibody to the DEC-205 endocytosis receptor on human dendritic cells. Human Immunol 61:729–38
- Launois P, DeLeys R, Niang MN, et al. (1994). T-cell-epitope mapping of the major secreted mycobacterial antigen Ag85A in tuberculosis and leprosy. Infect Immun 62:3679–87
- Lelouard H, Fallet M, de Bovis B, et al. (2012). Peyer's patch dendritic cells sample antigens by extending dendrites through M cell-specific transcellular pores. Gastroenterology 142:592–601
- Li X, Kong X, Shi S, et al. (2008). Preparation of alginate coated chitosan microparticles for vaccine delivery. BMC Biotechnol 8:89
- Lizee G, Basha G, Jefferies WA. (2005). Tails of wonder: endocytic-sorting motifs key for exogenous antigen presentation. Trends Immunol 26:141–9
- Malik B, Goyal AK, Markandeywar T, et al. (2012). Microfold-cell targeted surface engineered polymeric nanoparticles for oral immunization. J Drug Target 20:76–84
- Mogues T, Goodrich ME, Ryan L, et al. (2001). The relative importance of T cell subsets in immunity and immunopathology of airborne Mycobacterium tuberculosis infection in mice. J Exp Med 193:271–80
- Prabaharan M. (2011). Prospective of guar gum and its derivatives as controlled drug delivery systems. Int J Biol Macromol 49:117–24
- Prasad YV, Krishnaiah YS, Satyanarayana S. (1998). In vitro evaluation of guar gum as a carrier for colon-specific drug delivery. J Control Release 51:281–7
- Regnault A, Lankar D, Lacabanne V, et al. (1999). Fcgamma receptor-mediated induction of dendritic cell maturation and major histocompatibility complex class I-restricted antigen presentation after immune complex internalization. J Exp Med 189:371–80
- Sallusto F, Cella M, Danieli C, Lanzavecchia A. (1995). Dendritic cells use macropinocytosis and the mannose receptor to concentrate macromolecules in the major histocompatibility complex class II compartment: downregulation by cytokines and bacterial products. J Exp Med 182:389–400
- Tascon RE, Stavropoulos E, Lukacs KV, Colston MJ. (1998). Protection against Mycobacterium tuberculosis infection by CD8+ T cells requires the production of gamma interferon. Infect Immun 66:830–4
- Van Der Lubben I, Konings F, Borchard G, et al. (2001b). In vivo uptake of chitosan microparticles by murine Peyer's patches: visualization studies using confocal laser scanning microscopy and immunohistochemistry. J Drug Target 9:39–47
- Van der Lubben I, Verhoef J, Van Aelst A, et al. (2001a). Chitosan microparticles for oral vaccination:: preparation, characterization and preliminary in vivo uptake studies in murine Peyer's patches. Biomaterials 22:687–94