?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
The objective of this study was to investigate the potential of spanlastics as an ophthalmic delivery system to improve the corneal permeability and antimycotic activity of itraconazole (ITZ). Spanlastics containing edge activators, including Tween 20 or 80, were produced by modified ethanol injection method and exhibited a particle size of approximately 287 nm and an entrapment efficiency of more than 88%. Less than 13% ITZ was released from spanlastics over 6 h compared to 35% from conventional niosomes. Spanlastics exerted a 1.34-fold increase in the amount of ITZ permeated through excised bovine cornea after 24 h compared to conventional niosomes. Antimycotic study revealed a significant (p < 0.05) increase in the zone of inhibition of Candida albicans culture demonstrated by spanlastics compared to ITZ powder at the same concentration level (10 mg). In vivo Draize test showed no signs of acute ocular toxicity upon application of the selected spanlastic formulation to the rabbit eye. Results revealed that spanlastics loaded with itraconazole could be a potential nanosystem in ocular drug delivery systems.
Introduction
Corneal disease is second to cataracts as the most common cause of blindness worldwide, resulting in more than 1.5 million new cases of vision loss annually (Whitcher et al., Citation2001). Ophthalmic mycosis is emerging as a major cause of vision loss and morbidity and can be life-threatening (Yohai et al., Citation1994; Levin et al., Citation1996). Fungal keratitis is one of the major causes of ophthalmic mycosis (See et al., Citation1998) accounting for more than 50% of proven ophthalmic mycoses in some countries (Hagan et al., Citation1995). Fungal keratitis is characterized by a corneal epithelial defect and inflammation of the corneal stroma. If untreated, fungal keratitis can lead to corneal scarring and vision loss (Whitcher et al., Citation2001). The ultimate goal in the treatment of fungal keratitis is to conserve vision. This requires timely diagnosis of the infection and administration of the appropriate antifungal therapy (Manzouri et al., Citation2001).
The antifungal agents that can be used in fungal keratitis are broadly divided into four main groups: polyenes (amphotericin B, natamycin and nystatin), azoles (ketoconazole, miconazole, econazole, fluconazole, itraconazole, voriconazole and posaconazole), allylamine (terbinafine) and echinocandins (caspofungin) (Al-Badriyeh et al., Citation2010). Itraconazole is a triazole antimycotic agent, which is active against a broad spectrum of fungal species including Cryptococcus, Candida, Aspergillus, Blastomyces and Histoplasma capsulatum var. capsulatum (Saag & Dismukes, Citation1988). Itraconazole is more effective than ketoconazole for treatment of coccidiomycosis (Kerl, Citation2003), with higher response rates (Dunbar et al., Citation1983; Taboda, Citation2000) and for the treatment of histoplasmosis, with fewer adverse effects (Hodges et al., Citation1994). Itraconazole has a similar spectrum of activity as fluconazole but includes Aspergillus. Itraconazole is considered the treatment of choice for blastomycosis (Legendre et al., Citation1996; Kerl, Citation2003) and histoplasmosis (Hodges et al., Citation1994; Wolf, Citation1998). The mechanism of action of itraconazole is related to its binding of fungal cytochrome P-450 with resultant inhibition of ergosterol synthesis and perturbation of membrane-bound enzyme function and membrane permeability (Mohanty et al., Citation2013). Itraconazole is poorly soluble in water (S ≈ 50 ng/ml at neutral pH and S = 1.2 mg/ml in 0.1 mol/l HCl) (Peeters et al., Citation2002). Due to its poor water solubility, itraconazole has low corneal permeation which results in low ocular bioavailability. It is a weak basic drug with a partition coefficient of 8.1 and a pKa of 3.7 (Nakarani et al., Citation2010).
A major drawback of conventional topical ophthalmic drug delivery systems is the rapid and extensive loss of drug caused by the drainage through the nasolachrymal duct and high tear fluid turnover (Bourlais et al., Citation1998). Previous reporters have attempted to increase the corneal permeation of drugs and prolong retention time using various nanosystems such as nanoemulsions (Hagigit et al., Citation2012), liposomes (Li et al., Citation2009), cubosomes (Chen et al., Citation2012) and nanoparticles (Shen et al., Citation2010), which improved the therapeutic effect of the drugs enclosed within. Nanosized carriers have received special attention with the aim of minimizing the side effects and improving the efficacy of drug therapy (Alonso, Citation2004). A number of strategies to deliver itraconazole using nanocarriers were developed to facilitate drug targeting to infected cells such as niosomes (Wagh & Deshmukh, Citation2012), microemulsion (Lee et al., Citation2010), nanosuspension (Nakarani et al., Citation2010), solid–lipid nanoparticles (Mukherjee et al., Citation2009) and nanocrystals (Badawi et al., Citation2011). Elastic vesicular nanocarriers were set up to enhance drug penetration through biomembranes (skin, gastrointestinal mucosa, cornea, etc). Transfersomes®, highly deformable and elastic liposomes, were first introduced by Cevc & Blume (Citation1992). Later, surfactant-based elastic vesicles, mainly consisting of non-ionic surfactants, were introduced (Van den Bergh et al., Citation1999). The term – Spanlastics® – was first set up by Kakkar & Kaur (Citation2011) to express such surfactant-based elastic vesicles. The use of Spanlastics as a technique to enhance corneal permeation of itraconazole has not been attempted before.
Our goal was to improve corneal permeation and prolong the retention time of itraconazole in eye via formulation of spanlastics. The work in this study aims to formulate and evaluate itraconazole-loaded spanlastics containing two edge activators (Tween 20 or 80). Transcorneal permeation was evaluated in an ex vivo diffusion cell model using excised bovine cornea and compared to its permeation from the conventional niosome formulation. The antimycotic activity of the selected spanlastic formulation was compared to that of drug suspension, conventional niosome formulation and reference standard on the market (Itrapex®capsules, MultiApex Pharma, Egypt, 100 mg). Finally, the possible ocular toxicity of the selected spanlastic formulation was tested in rabbits using Draize test.
Methodology
Materials
Itraconazole (ITZ) was kindly supplied by Adwia Pharmaceuticals Co. (Cairo, Egypt). Span 60 was obtained from Fluka (Newport News, VA). Cholesterol was purchased from Sigma Aldrich Chemie GmbH (Munich, Germany). Tween 20 and 80 were obtained from BDH, Prolabo (London, UK). All other ingredients were of pure analytical grade.
Preparation of itraconazole-loaded spanlastic vesicles and conventional niosomes
ITZ-loaded spanlastics were prepared by ethanol injection method (Kakkar & Kaur, Citation2011), with a slight modification, using Span 60. Two edge activators (EA), either Tween 20 or 80, were used at Span 60: EA ratios of 90:10 or 80:20 (by weight). A mixture of solvents (absolute ethanol and chloroform) in the ratio (1:1 v/v) was chosen as a suitable solvent to dissolve the drug (Zheng et al., Citation2012). The amount of ITZ used to load the vesicles was either 10 or 20 mg. Span 60 and ITZ were dissolved in 2 ml of solvent mixture and injected slowly into 10 ml of preheated (75 °C) aqueous phase (consisting of EA) which was stirred continuously on a magnetic stirrer at 800 rpm for 30 min, then stirring was continued on cold for another 30 min. Finally, the final volume of the formulation was adjusted to 10 ml. The conventional niosome formulation was developed, with Span 60 and cholesterol using film hydration method, for comparison. The composition of different spanlastic formulations and conventional niosome formulation are compiled in .
Table 1. Composition of the prepared ITZ-loaded vesicles.
Characterization of spanlastics
Entrapment efficiency
ITZ loaded spanlastics were separated from the free drug by ultracentrifugation (Megafuge 1.0 R, Heraeus, Germany) at 15 000 rpm and 4 °C for 30 min. The loaded vesicles were disrupted using sufficient quantity of methanol and the amount of drug was determined spectrophotometrically at 263 nm, after comparison to a pre-constructed calibration curve (n = 3, R2 = 0.998). Drug entrapment efficiency was determined three times and the mean ± standard deviation (SD) was calculated according to the following equation (Aggarwal & Kaur, Citation2005):
(1)
(1)
where % EE is the percentage of drug entrapped, ED is the amount of entrapped drug and TD is the total amount of the drug.
Vesicle size
The size of vesicles was determined by dynamic light scattering using Nano ZS Zetasizer (Malvern Instrument, Worcestershire, UK). The system was equipped with 4 mW helium/neon laser at 633 nm wavelength and measured the sample with non-invasive backscatter technology at a detection angle of 173° using DTS Nano version 6.12 software (Malvern Instrument, UK). All measurements were carried out at 25 °C assuming 0.8872 cps and 1.330 as medium viscosity and refractive index, respectively. Samples were diluted with distilled water before measurement. Results were presented as an average diameter of the vesicle suspension (z-average mean) against percent sample volume, and the polydispersity index (PDI), which is a measure of the width of the size distribution, was also deduced. Three size determinations for each formulation was carried out and the mean ± SD was deduced.
Zeta potential
The charges on the vesicular surface were determined using the Zetasizer ZS (Malvern Instruments, UK) at 25 °C for 120 s using a combination of laser Doppler velocimetry and phase-analysis light scattering to measure particle electrophoretic mobility. The average zeta potential and conductivity of three samples of each formulation were determined.
Elasticity measurement of vesicle membrane elasticity
In this study, a fixed amount of the vesicular suspension was extruded through a polycarbonate filter (Whatman®, Nuclepore®, Fischer Scientific (Waltham, MA) of 0.05 μm pore size and 25 mm diameter at a pressure of 2.5 bar (Haug Kompressoren AG; Büchi Labortechnik AG, Flawil, Switzerland), and the time taken for extrusion was recorded. The change in size was also measured. Elasticity of vesicle membrane was expressed in terms of deformability index three times and the mean ± SD was calculated according to the following equation (Schatzlein & Cevc, Citation1995; Gupta et al., Citation2005):
(2)
(2)
where D is the deformability index (ml/s), j is the amount of extruded suspension (ml), t is the time of extrusion (s), rv is the size of vesicles after extrusion (nm), and rp is the pore size of the barrier (nm).
The best-selected spanlastic formula in terms of the above characterization tests was further investigated for its morphology, compatibility, drug release, corneal permeability, antimycotic activity and in vivo toxicity.
Morphology
Optical microscope (LEICA Quin Image Analyser, Q5501W) equipped with Leica DMLB microscope (Cambridge, England, UK) and transmission electron microscope (TEM 1230; JEOL, Tokyo, Japan) were used to characterize the spanlastics for structural attributes such as lamellarity, size, shape and physical stability characteristics, i.e. aggregation and/or irregularity. The samples were inspected at an acceleration voltage of 120 kV.
Differential scanning calorimetry
Samples of 2 mg of pure drug, Span 60, cholesterol, drug-loaded spanlastics and drug-loaded niosomes were placed in a standard aluminum pan and heated from 25 to 400 °C at a constant heating rate of 10 °C/min, under nitrogen with a purging rate of 20 ml/min using a differential scanning calorimeter (Shimadzu, Kyoto, Japan, DSC–60). The thermograms generated were observed/evaluated for any incompatibility (significant shift or disappearance/appearance of new peaks).
In vitro drug release
Drug release from spanlastics was tested using cellulose dialysis tubing (mol. wt. cutoff 12 000–14 000 Da, Spectrapore®, Theodore, AL) in 25 ml of freshly prepared phosphate buffer (pH 7.4) containing 2% sodium dodecyl sulphate (SDS) as the release medium. A known amount of spanlastics encapsulating drug (0.5 ml) was placed inside the cellophane bag which was placed in a shaker at 150 rpm and maintained at 37 ± 1 °C. At predetermined time points, 1 ml sample was withdrawn from the release medium and replaced by freshly prepared medium kept at the same temperature over a period of 6 h. Sink conditions were maintained throughout the study. The samples were suitably diluted and the amount of ITZ within was measured spectrophotometrically at 263 nm in comparison to a pre-constructed calibration curve (n = 3, R2 = 0.998). The experiment was repeated three times and the mean ± SD was calculated.
The release efficiency (% RE) was calculated from the area under the release curve at time t (measured using the trapezoidal rule) and was expressed as a percentage of the area of the rectangle corresponding to 100% release, for the same total time, according to the following equation (Khan, Citation1975):
(3)
(3)
where Y is the percentage of drug released at time t.
Corneal permeability studies
Ex vivo corneal permeability studies were performed using membrane diffusion technique. The studies were conducted within a vertical Franz cell with a diffusional area of 3.14 cm2, maintained at a constant temperature (37 ± 1 °C) and mounted on a magnetic stirrer set at 150 rpm. Freshly excised bovine cornea was used for the study within half an hour of sacrifice of the animals. The excised bovine cornea was mounted in the middle between the donor and the receptor compartments. The spanlastic formulation (0.5 ml) was placed in the donor compartment on the cornea and the receptor medium was 25 ml of freshly prepared phosphate buffer pH 7.4 containing 2% SDS. Aliquots of the medium were withdrawn after fixed time intervals from the sampling port and were replaced with equal quantity of fresh medium to maintain a constant volume. Sink conditions were maintained throughout the study. Samples were analyzed spectrophotometrically at 263 nm. The experiment was repeated three times and the mean ± SD was calculated.
Apparent permeability coefficient (Papp) was calculated using the following equation (Ahuja et al., Citation2008):
(4)
(4)
where ΔQ/Δt (μg/min) is the flux across the corneal tissue, A is the area of diffusion (cm2), Co (μg/cm3) is the initial concentration of drug in donor compartment and 60 is taken as the factor to convert minute into second. The flux across the cornea was obtained from the slope of the regression line obtained from the linear part of the curve between the amount permeated (Q) versus time (t) plot.
In vitro antimycotic study
Antimycotic activity of the selected formulation was compared to reference capsule (Itrapex®, MultiApex Pharma, Cairo, Egypt, 100 mg) adopting agar-cup diffusion method. The study was carried out using cultures of Candida albicans, American Type Culture Collection (ATCC 60193) strain in Sabouraud-dextrose agar. The optical density of an overnight culture suspension of Candida albicans was adjusted to ½ McFarland standard (absorbance 0.125), and then serially diluted with sterile 0.9% NaCl tube to correspond to a final inoculum concentration of 108 CFU/ml. Seeding of culture was performed by swabbing method in which sterile swab was dipped into the culture suspension and excess fluid removed by pressing gently against the wall of test tube. Swab was placed on the edge of the agar plate and move across to the other sides, this was separated to obtain an even spread in sterile conditions. Three seeded agar plates were generated and using a borer (11 mm diameter), four wells were made in every seeded agar plate. An aliquot of 100 µl of the following formulations were used to fill each cup: suspension of 10 ml of the selected spanlastic F5 formulation loaded with 10 mg itraconazole was added to coded well (I); marketed capsule (Itrapex®) suspension (1 mg/ml) in water for injection was added to coded well (II); plain drug suspension in water for injection (1 mg/ml) was added to coded well (III); and spanlastic formulation free of drug was added to coded well (IV). Plates were kept in refrigerator at 4 °C for diffusion for 10–15 min and then placed in incubator for a period of 48 h at 30 °C. Results were expressed as mean diameter of “Zone of inhibition” in cm of three agar plates ±SD.
In vivo eye toxicity Draize test
The Draize rabbit test (Draize et al., Citation1944), developed in the 1940s, is the only eye toxicity test officially accepted in the Organization for Economic Cooperation and Development (OECD) Guidelines (Citation2002) for regulatory purposes in the classification of slightly and moderately irritating chemicals. The experiment was performed in accordance with ethical procedures and policies approved by the Animal Care and Use Committee of the Faculty of Pharmacy, Cairo University, Cairo, Egypt, following the 18th World Medical Association General Assembly, Helsinki, June 1964, and updated by the 59th World Medical Association General Assembly, Seoul, October 2008.
Six male albino New Zealand rabbits, weighing 2–5 kg were used in this study. The rabbits were randomly divided into two groups, each group containing three rabbits as follows: rabbits of group A received the non-medicated vesicle suspension and those of group B received the medicated vesicle suspension. The application was always in the right eye of the rabbits and the contralateral eye was used as the control and received no treatment. Twenty-four hours prior to the application, the animals were allowed to rest overnight, each in a separate cage and received food and water ad libitum. On the first day of the study, two drops of each suspension were instilled once in the conjunctival sac of the right eye (to test single insult challenge). The other eye was left as a control in each animal. The right eye was visually examined at 1, 24 and 72 h after vesicular suspension instillation for any irritation/reaction and scored according to Draize test (Draize et al., 1944). To test the repeated insult challenge, the vesicular suspension was also instilled at 30-min intervals for 6 h in the right eye of each animal after gently pulling the lower lid away from the eyeball to evaluate whether the vesicular system(s) were safe for long-term therapy or not. The right eye was visually examined by the naked eye at 1, 24 and 72 h after vesicular suspension instillation for any irritation/reaction and scored according to Draize test (6). A score for erythema (redness) was given as follows: 0, no reaction; 1, weak spotty or diffuse erythema; 2, weak but well perceptible erythema covering the total eye area; 3, moderate erythema; 4, severe erythema with edema; 5, very severe erythema with defects (vesicles, erosions, etc.).
Statistical analysis
Data were expressed as mean ± SD. The results were statistically analyzed by analysis of variance (ANOVA) test using Social Package for Statistical Study Software (SPSS 17®, SPSS Inc., Chicago, IL); p values less than 0.05 were considered as significant.
Results and discussion
The goal of our investigation was to formulate an elastic sustained release ophthalmic formulation of itraconazole capable of overcoming the corneal barrier and prolonging the retention time in eye. Composition of ITZ-loaded spanlastics prepared using Span60 and different edge activators in different molar ratios to Span are summarized in .
Entrapment efficiency
The entrapment efficiency is one of the important parameters in the design of vesicular formulations. High entrapment efficiency ensures more bioavailability and also high concentration of drug targeted which may help in the reduction of dose required for therapy and thereby decreasing the dose-related systemic side effects (Rangasamy et al., Citation2008). shows the characterization of spanlastic formulations produced including the entrapment efficiencies. Among all spanlastic and niosome formulations, F1 possessed the highest entrapment efficiency.
Table 2. Characterization of the prepared ITZ-loaded vesicles.
Effect of EA type on entrapment efficiency
From the results in , it was noticed that F1 prepared from the EA Tween 80 showed a significant (p < 0.05) increase in %EE, i.e. 88.58 ± 7.92% when compared to F2 which was prepared from the EA Tween 20, i.e. 78.53 ± 6.06%. The length of the alkyl chain influences the HLB value of the surfactant mixture which by its turn directly influences the drug entrapment efficiency (Raja et al., Citation1994). Spanlastics composed of Tween 80, having the lower HLB values (15), possessed higher entrapment efficiency, compared to those prepared using Tween 20, having the higher HLB values (16.7), using the same amount of the drug to load the spanlastics. Thus, it could be concluded that the entrapment efficiency of spanlastics increased as HLB of Tween was decreased. Our results were similar to those reported by Guinedi et al. (Citation2005) who indicated that the lower the HLB of the surfactant, the higher the entrapment efficiency would be.
Effect of EA amount on entrapment efficiency
The results revealed an increase in ITZ entrapment efficiencies with increasing the EA amount. Statistical analysis showed a significant difference (p < 0.05) between the %EE of F3 spanlastics which was composed of Sp60:Tw80 (90:10; 63.23 ± 6.95%) and F7 which was composed of Sp60:Tw80 (80:20; 72.82 ± 2.28%). There was also a significant difference (p < 0.05) between %EE of F4 that was composed of Sp60:Tw20 (90:10; 62.09 ± 2.6%) and F8 which was composed of higher EA amount, i.e. Sp60:Tw20 (80:20; 76.25 ± 3.92%). These results could be due to the increased fluidity of lipid bilayers by EA leading to increased entrapment efficiency of drug inside the vesicles (Darwhekar et al., Citation2012).
Effect of drug amount on entrapment efficiency
Results in revealed that the entrapment efficiencies decreased as the drug amount used to load the spanlastics increased. Statistical analysis revealed a significant difference (p < 0.05) between the formulations containing 10 mg drug, i.e. F1, F2, F5 and F6 and those containing 20 mg ITZ, i.e. F3, F4, F7 and F8, respectively. This could be due to the saturation of the medium with drug forcing it to be encapsulated into vesicles. When the lipid bilayers became saturated with the drug, any further increase in drug concentration would lead to precipitation (El Zaafarany et al., Citation2010).
Particle size of ITZ-loaded spanlastics
All spanlastic formulations showed vesicle size in the nanorange (158.7–382.1 nm; ) and that the method of preparation used was reproducible as evident by the low polydispersity index (PDI) values (0.0304–0.1285) indicating a homogenous distribution in the formulation (Darwhekar et al., Citation2012). PDI is the ratio of standard deviation to mean vesicle size, so it indicates the uniformity of the vesicle size within the formulation. The higher the polydispersity, the lower the uniformity of the vesicle size in the formulation (Shakeel et al., Citation2007). A PDI value lower than 0.3 indicates a homogenous and monodisperse population (Centis & Vermette, Citation2008). Results also revealed a significant decrease in particle size of spanlastics (p < 0.05) with the increase of the hydrophobicity of the EA used. A decrease in size from 382.1 ± 16.14 nm (F2) to 164.9 ± 21.2 nm (F1) and from 322.2 ± 33.14 nm (F6) to 268.5 ± 15.16 nm (F5) was observed as the hydrophobicity of the surfactant increased, i.e. HLB decreased from 16.7 (Tween 20) to 15 (Tween 80). The use of surfactant with higher hydrophobicity (lower HLB) resulted in decreased surface energy leading to the formation of vesicle with smaller size (Sternberg, Citation1994).
Zeta potential of ITZ-loaded spanlastics
High zeta potential values, either positive or negative, are expected to render stability to the system due to strong electrostatic repulsion (Nava et al., Citation2011). Zeta potential values of the spanlastics ranged from −27.3 to −34 mV. It was observed that increasing the amount of EA led to a significant increase (p < 0.05) in the zeta potential values, i.e. from −29.2 ± 1.4 (F2) to −34 ± 2.1 (F6) and from −29 ± 3.1(F3) to −33.5 ± 2.4 (F7). Many non-ionic surfactants like Tween 80 exhibit a negative zeta potential, leading to repulsion between the vesicle bilayers. The reason behind this low zeta potential value may be due to the chemical structure (Lee et al., Citation2005).
Membrane elasticity of ITZ spanlastics
Elasticity of the vesicle membrane is an important and unique parameter of elastic vesicular formulations because it differentiates elastic vesicles from other vesicular carriers that are unable to cross the mucus membrane. Elastic vesicles can squeeze themselves through intercellular regions under the influence of water gradient based on the membrane bending energy that depends on its composition (Cevc & Blume, Citation1992; Mishra et al., Citation2006). In this study, elasticity of the prepared vesicles was evaluated through determinations of deformability index using polycarbonate filters. Elasticity determinations () showed that spanlastic vesicles and conventional niosome vesicles had the ability to pass through these filters. The elasticity was maximum for spanlastic formulation (F5), prepared from Span60 and Tween 80 in a non-ionic surfactant/EA molar ratio of 80:20, showing the highest deformability index; i.e. DI = 30.57 ml/s.
From the above results, it could be concluded that spanlastic formulation (F5) possessed the most deformable vesicles, besides having reasonable vesicle size and drug entrapment efficiency. Thus, the developed spanlastic formulation F5 was selected for further investigation for in vitro and in vivo studies.
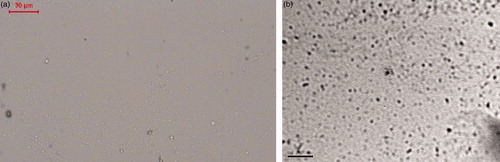
Morphology of ITZ-loaded spanlastics (optical microscope and transmission electron microscopy)
reveals photomicrographs of the selected spanlastics formulation taken by an optical microscope and transmission electron microscopy (TEM), respectively. These micrographs reveal the spherical shape of the prepared spanlastics confirming their vesicular characteristics.
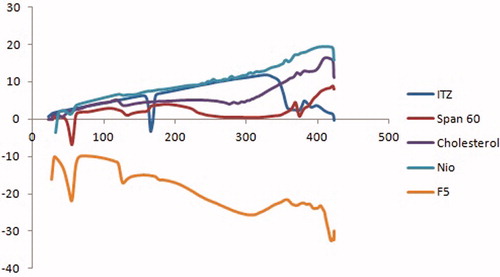
Differential scanning calorimetry
Differential scanning calorimetric (DSC) thermograms of ITZ, Span60, cholesterol as well as ITZ-loaded spanlastics (F5) and conventional niosomes are illustrated in . The DSC thermograms of Span60 and cholesterol revealed endothermic peaks at 55.36 and 130.01 °C, respectively, representing their gel–liquid transition temperatures. The DSC thermogram of ITZ showed an endothermic peak at 166 °C which is the reported melting point for ITZ (Chandraprakash et al., Citation1994). The DSC thermogram of spanlastic formulation (F5) revealed the disappearance of the melting endotherm of ITZ and shifting of the endothermic peak of Span60 to 49.83 °C. The DSC thermogram of conventional niosomes revealed the disappearance of the melting endotherm of ITZ and a slight shifting of the endothermic peak of Span 60 to 52.36 °C. The disappearance of the characteristic endothermic peak of drug and shifting and/or broadening of the endotherms of surfactant bilayers suggest possible interaction of drug with bilayer components and can account for the enhanced entrapment of ITZ into these vesicles (Chandraprakash et al., Citation1993; Parthasarathi et al., Citation1994).
In vitro drug release studies
Spanlastic formulations studied (F1–F8) released 12–34% of their drug contents after 6 h. Formulations that showed least entrapment efficiencies (F3, F4, F7 and F8) were characterized by having the lowest values for release efficiencies as well (). revealed that the release of drug from either spanlastics or niosome vesicles was biphasic, with an initial faster release followed by a period of slow release. This biphasic release pattern seemed to be a characteristic of bilayered vesicles (El-Ridy et al., Citation2012). Rapid drug leakage was observed during the initial phase where about 6–27% of the entrapped drug was released from various formulations in the first half hour. This could be explained by the fact that the lipophilic drug was mainly incorporated between the fatty acid chains in the lipid bilayers of spanlastics or niosomal vesicles which led to rapid release upon dispersing vesicles in buffer until reaching equilibrium (Fang et al., Citation2001; Mokhtar et al., Citation2008). However, during the following 5.5 h, only further 5–12% of ITZ was released from different spanlastic preparations. In general, the drug adsorbed on the vesicular surface would be released rapidly. After that, the entrapped drug would show a sustained release profile.
Figure 3. In vitro release profiles of different ITZ-loaded spanlastics and conventional niosome formulation (nio).
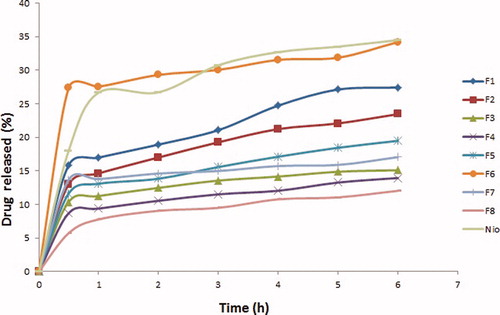
Almost the entire amount of loaded drug was not released from the vesicles. This might be due to entrapment of the drug in the lipophilic region of the vesicles (Ruckmani & Sankar, Citation2010). Differences in the in vitro release profiles might be due to vesicle size, lamellarity and membrane fluidity as a function of chain length of surfactant (Weiner et al., Citation1989).
Effect of EA type on release efficiency
Results showed that spanlastic formulation prepared using Tween 20 (F6) yielded a significant (p < 0.05) increase in release efficiency, i.e. 29.04 ± 13.28%, compared to spanlastics (F5) prepared using Tween 80, i.e. 15.05 ± 0.165%. This could be explained by the fact that vesicles exhibited an alkyl chain length of EA-dependent release and that the higher the chain length, the lower the release rate (Devaraj et al., Citation2002). It is worth mentioning that Tween 20 possesses 12 saturated carbons in the alkyl chain while Tween 80 possesses 18 carbons in the alkyl chain and is unsaturated.
Effect of EA amount on RE
Statistical analysis of the release results revealed that the amount of EA used had an insignificant effect (p > 0.05) on the release efficiencies of ITZ from spanlastics.
Corneal permeability studies
Spanlastics showed a significant (p < 0.05) improvement in the apparent permeation coefficient (Papp) of ITZ (5.354 × 10−6 cm/s) compared to conventional niosomal formulation (2.062 × 10−6 cm/s; ). There was a significant difference (p < 0.05) between spanlastics and conventional niosomes in terms of the total amount of ITZ permeated through bovine cornea after 24 h, i.e. 423.01 ± 16.28 µg for spanlastics and 315.65 ± 27.35 µg for conventional niosomes, and rate of permeation (0.762 ± 0.035 µg/h for spanlastics and 0.293 ± 0.011 µg/h for conventional niosomes; and ). Thus, it could be concluded that spanlastics were significantly better than conventional niosomes in enhancing penetration of ITZ through eye cornea.
Figure 4. Percent drug permeated through cornea versus time (h) data of spanlastics (F5) and conventional noisome formulation (nio).
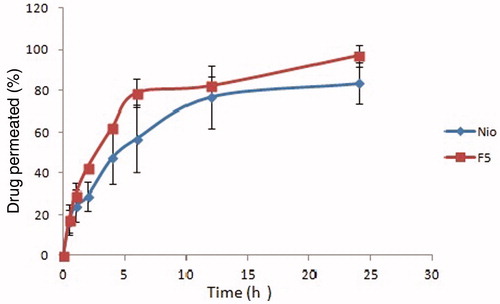
Table 3. Comparison of developed spanlastics F5 and niosome formulation in terms of total amount of drug permeated after 24 h, rate of drug permeation and the apparent permeability coefficient (Papp) from ex vivo permeation studies (n = 3).
In vitro antimycotic study
The zones of inhibition were labeled as I, II, III and IV. The results () revealed that Itrapex® reference standard capsule (100 mg ITZ in 100 ml solvent) (II) showed the highest antimycotic effect, revealing a zone of inhibition of 2.925 ± 0.095 cm, with a significant difference from the rest of formulations (p < 0.05). This was followed by spanlastics F5, containing 10 mg ITZ (I), which showed a zone of inhibition of 2.475 ± 0.05 cm. Statistical analysis revealed a significant (p < 0.05) difference in zones of inhibition of (I) compared to both the ITZ powder (10 mg; III; zone of inhibition:1.850 ± 0.057 cm) and plain spanlastics formula (IV; zone of inhibition: 2.00 ± 0.00 cm), indicating a pronounced and significant antimycotic effect of ITZ spanlastics.
Table 4. Zone of inhibitions of I: spanlastics F5 containing 10 mg itraconazole in 10 ml sterile water; II: Itrapex® reference standard capsule (MultiApex Pharma) 100 mg in 100 ml sterile water; III: itraconazole suspension 10 mg in 10 ml sterile water; and IV: plain spanalstic formula, through the fungal cell wall.
It should be noted that plain spanlastics formulation (IV) showed a zone of inhibition of 2 cm although it did not enclose an antifungal drug within, indicating it exhibited an antimycotic effect of its own. This may be due to the presence of Tween 80 in the plain F5 spanlastics. It was reported that the antifungal activity of rosemary oil against Candida albicans can be enhanced by the addition of Tween 80 (Matsuzaki et al., Citation2013).
In vivo eye irritancy Draize test
Acute irritation or toxicity study (single insult challenge) showed that both the medicated (F5) and plain spanlastic formulations displayed no signs of inflammation or irritation in the two groups of rabbits after 1, 24 and 72 h when compared to control eye. On the other hand, the repeated insult challenge showed well perceptible erythema or inflammation and tears in both groups after 1 h. This inflammation resolved to weak or diffusive erythema after 24 h and completely disappeared after 72 h ( and ). In fact, being a controlled-release dosage form, spanlastics would not be instilled in eyes following the same time regime used in the irritation study (repeated insult challenge). The frequency of administration would be less, so fear of causing inflammation is much diminished.
Figure 5. In vivo eye irritancy study showing photos of the eyes of the rabbits (a) control; (b) after 1 h (single insult challenge); (c) after 24 h (single insult challenge); (d) after 1 h (repeated insult challenge); (e) after 24 h (repeated insult challenge), from instilling the medicated spanlastics (F5).
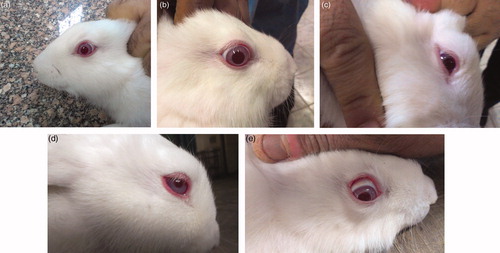
Table 5. Evaluation of corneal toxicity by scoring method according to Draize test after single and repeated insult challenges of both plain and medicated spanlastics in rabbits’ eyes.
Conclusion
This study showed that spanlastic formulations could be a promising delivery system for the antifungal drug itraconazole. It provided successful preparation with efficient encapsulation efficiency. These formulations provide a great deal of flexibility as the size and zeta potential of the formulations can be tuned to suit the need using scalable and robust methodologies. The elasticity of the vesicles assists to serve as a potential drug delivery system for both the anterior and posterior eye diseases. The spanlastic formulations proved to be safe and non-irritant to the eyes of rabbits using Draize test. Further histopathological studies need to be carried out to assess the biocompatibility of these spanlastic formulations.
Acknowledgements
The authors would like to thank Associate Professor Amal Abd El-Halim and Dr. Eslam Mohamed Kamal, Department of Microbiology and Immunology, Faculty of Pharmacy, Cairo University for the help provided in the in vitro antimycotic study.
Declaration of interest
There is no declaration of interest
References
- Aggarwal D, Kaur IP. (2005). Improved pharmacodynamics of timolol maleate from a mucoadhesive niosomal ophthalmic drug delivery system. Int J Pharm 290:155–9
- Ahuja M, Singh G, Majumdar DK. (2008). Effect of formulation parameters on corneal permeability of ofloxacin. Sci Pharm 76:505–14
- Alonso MJ. (2004). Nanomedicines for overcoming biological barriers. Biomed Pharmacother 58:168–72
- Al-Badriyeh D, Neoh CF, Stewart K, Kong DCM. (2010). Clinical utility of voriconazole eye drops in ophthalmic fungal keratitis. Clin Ophthalmol 4:391–405
- Badawi AA, El-Nabarawi MA, El-Setouhy DA, Alsammit SA. (2011). Formulation and stability testing of itraconazole crystalline nanoparticles. AAPS PharmSciTech 12:811–20
- Bourlais CL, Acar L, Zia H, et al. (1998). Ophthalmic drug delivery systems-recent advances. Prog Retin Eye Res 17:33–58
- Centis V, Vermette P. (2008). Physico-chemical properties and cytotoxicity assessment of PEG-modified liposomes containing human hemoglobin. Colloids Surf B Biointerfaces 65:239–46
- Cevc G, Blume G. (1992). Lipid vesicles penetrate into intact skin owing to the transdermal osmotic gradients and hydration force. Biochim Biophys Acta 1104:226–32
- Chandraprakash KS, Udupa N, Pillai GK. (1994). Pharmacokinetic evaluation of surfactant vesicle entrapped methotrexate in tumour bearing mice. Int J Pharm 61:R1–3
- Chandraprakash KS, Udupa N, Umadevi P, Pillai GK. (1993). Formulation and evaluation of methotrexate niosomes. Drug Dev Ind Pharm 19:1331–42
- Chen Y, Lu Y, Zhong Y, et al. (2012). Ocular delivery of cyclosporine A based on glycerylmonooleate/poloxamer 407 liquid crystalline nanoparticles: preparation, characterization, in vitro corneal penetration and ocular irritation. J Drug Target 20:856–63
- Darwhekar G, Jain DK, Choudhary A. (2012). Elastic liposomes for delivery of neomycin sulphate in deep skin infection. Asian J Pharm Sci 7:230–40
- Devaraj GN, Parakh SR, Devraj R, et al. (2002). Release studies on niosomes containing fatty alcohols as bilayer stabilizers instead of cholesterol. J Colloid Interface Sci 251:360–5
- Draize JH, Woodard G, Calvery HO. (1944). Methods for the study of irritation and toxicity of substances applied topically to the skin and mucous membranes. J Pharmacol Exp Ther 82:377–90
- Dunbar MJ, Pyle R, Boring J, McCoy C. (1983). Treatment of canine blastomycosis with ketoconazole. J Am Vet Med Assoc 182:156–7
- El Zaafarany GM, Awad GAS, Holayel SM, Mortada ND. (2010). Role of edge activators and surface charge in developing ultradeformable vesicles with enhanced skin delivery. Int J Pharm 397:164–72
- El-Ridy MS, Badawi AA, Safar MM, Mohsen AM. (2012). Niosomes as a novel pharmaceutical formulation encapsulating the hepatoprotective drug silymarin. IJPPS 4:549–59
- Fang JY, Yu SY, Wu PC, et al. (2001). In vitro skin permeation of estradiol from various proniosome formulations. Int J Pharm 215:91–9
- Guinedi AS, Mortada ND, Mansour S, Hathout RM. (2005). Preparation and evaluation of reverse-phase evaporation and multilamellar niosomes as ophthalmic carriers of acetazolamide. Int J Pharm 306:71–82
- Gupta PN, Mishra V, Rawat A, et al. (2005). Non-invasive vaccine delivery in transfersomes, niosomes and liposomes: a comparative study. Int J Pharm 293:73–82
- Hagan M, Wright E, Newman M, et al. (1995). Causes of suppurative keratitis in Ghana. Br J Ophthalmol 79:1024–8
- Hagigit T, Abdulrazik M, Valamanesh F, et al. (2012). Ocular antisense oligonucleotide delivery by cationic nanoemulsion for improved treatmentof ocular neovascularization: an in-vivo study in rats and mice. J Control Release 160:225–31
- Hodges R, Legendre A, Adams L, et al. (1994). Itraconazole for the treatment of histoplasmosis in cats. J Vet Intern Med 8:409–13
- Kakkar S, Kaur IP. (2011). Spanlastics – a novel nanovesicular carrier system for ocular delivery. Int J Pharm 413:202–10
- Kerl M. (2003). Update on canine and feline fungal diseases. Vet Clin North Am Small Anim Pract 33:721–47
- Khan KA. (1975). The concept of dissolution efficiency. J Pharm Pharmacol 27:48–9
- Lee E, Balakrishnan P, Song CK, et al. (2010). Microemulsion-based hydrogel formulation of itraconazole for topical delivery. J Pharm Invest 40:305–11
- Lee EH, Kim A, Oh YK, Kim CK. (2005). Effect of edge activators on the formation and transfection efficiency of ultra deformable liposomes. Biomaterials 26:205–10
- Legendre AM, Rohrbach B, Toal R, et al. (1996). Treatment of blastomycosis with itraconazole in 112 dogs. J Vet Intern Med 10:365–71
- Levin L, Avery R, Shore J, et al. (1996). The spectrum of orbital aspergillosis: a clinicopathological review. Surv Ophthalmol 41:142–54
- Li N, Zhuang C, Wang M, et al. (2009). Liposome coated with low molecular weight chitosan and its potential use in ocular drug delivery. Int J Pharm 379:131–8
- Manzouri B, Vafidis G, Wyse R. (2001). Pharmacotherapy of fungal eye infections. Expert Opin Pharmacother 2:1849–57
- Matsuzaki Y, Tsujisawa T, Nishihara T, et al. (2013). Antifungal activity of chemotype essential oils from rosemary against Candida albicans. OJST 3:176–82
- Mishra D, Dubey V, Asthana A, et al. (2006). Elastic liposomes mediated transcutaneous immunization against Hepatitis B. Vaccine 24:4847–55
- Mohanty B, Mishra SK, Majumdar DK. (2013). In vitro permeation characteristics of itraconazole from oil drops and ophthalmic ointment through excised goat and sheep corneas. JPBS 5:19–26
- Mokhtar M, Sammour OA, Hammad MA, Megrab NA. (2008). Effect of some formulation parameters on flurbiprofen encapsulation and release rates of niosomes prepared from proniosomes. Int J Pharm 361:104–11
- Mukherjee S, Ray S, Thakur RS. (2009). Design and evaluation of itraconazole loaded solid lipid nanoparticulate system for improving the antifungal therapy. Pakistan J Pharm Sci 22:131–8
- Nakarani M, Misra AK, Patel JK, Vaghani SS. (2010). Itraconazole nanosuspension for oral delivery: formulation, characterization and in vitro comparison with marketed formulation. DARU 18:84–90
- Nava G, Piñón E, Mendoza L, et al. (2011). Formulation and in vitro, ex vivo and in vivo evaluation of elastic liposomes for transdermal delivery of ketorolac tromethamine. Pharmaceutics 3:954–70
- Organization for Economic Cooperation and Development (OECD). (2002). Guidelines for the testing of chemicals No. 405: acute eye irritation/corrosion. Paris, France, 1–14
- Parthasarathi G, Udupa N, Umadevi P, Pillai GK. (1994). Niosome encapsulated of vincristine sulfate: improved anticancer activity with reduced toxicity in mice. J Drug Target 2:173–82
- Peeters J, Neeskens P, Tollenaere JP, et al. (2002). Characterization of the interaction of 2-hydroxypropyl-b-cyclodextrin with itraconazole at pH 2, 4 and 7. J Pharm Sci 91:1414–22
- Raja RAN, Pillai GK, Udupa N, Chandrashekar G. (1994). Anti-inflammatory activity of niosome encapsulated diclofenac sodium in arthritic rats. Indian J Pharmacol 26:46–8
- Rangasamy M, Ayyasamy B, Raju S, et al. (2008). Formulation and in vitro evaluation of niosome encapsulated acyclovir. J Pharm Res 1:163–6
- Ruckmani K, Sankar V. (2010). Formulation and optimization of zidovudine niosomes. AAPS PharmSciTech 11:1110–27
- Saag MS, Dismukes WE. (1988). Azole antifungal agents: emphasis on new triazoles. Antimicrob Agents Chemother 32:1–8
- Schatzlein A, Cevc G. (1995). Skin penetration by phospholipid vesicles, transfersomes, as visualized by means of the confocal laser scanning microscopy. In: Cevc C, Paltauf F, eds. Phospholipids, characterization, metabolism, and novel biological applications. Champaign, IL: AOCS Press, 191–209
- See J, Wong T, Yeo K. (1998). Trends in the pattern of blindness and major ocular diseases in Singapore and Asia. Ann Acad Med Singapore 27:540–6
- Shakeel F, Baboota S, Ahuja A, et al. (2007). Nanoemulsions as vehicles for transdermal delivery of aceclofenac. AAPS PharmSciTech 8:304–10
- Shen J, Sun M, Ping Q, et al. (2010). Incorporation of liquid lipid in lipid nanoparticles for ocular drug delivery enhancement. Nanotechnology 21:025101
- Sternberg YT. (1994). Preparation and properties of vesicles (niosomes) of Sorbian monoesters (Span 20,40,60,80) and a Sorbitan trimester (Span 85). Int J Pharm 105:1–6
- Taboda J. (2000). Systemic mycoses. In: Ettinger S, Feldman E, eds. Textbook of veterinary internal medicine. Philadelphia: WB Saunders, 453–76
- Van den Bergh BAI, Bouwstra JA, Junginger HE, Wertz PW. (1999). Elasticity of vesicles affects hairless mouse skin structure and permeability. J Control Release 62:367–79
- Wagh VD, Deshmukh OJ. (2012). Itraconazole niosomes drug delivery system and its antimycotic activity against Candida albicans. ISRN Pharm (2012) 653465. doi:10.5402/2012/653465
- Weiner ND, Williams N, Birch G, et al. (1989). Topical delivery of liposomally encapsulated interferon evaluated in a cutaneous herpes guinea pig model. Antimicrob Agents Chemother 33:1217–21
- Wolf A. (1998). Histoplasmosis. In: Green C, ed. Infectious diseases of the dog and cat. Philadelphia: WB Saunders, 378–83
- Zheng W, Fang X, Wang L, Zhang Y. (2012). Preparation and quality assessment of itraconazole transfersomes. Int J Pharm 436:291–8
- Whitcher J, Srinivasan M, Upadhyay M. (2001). Corneal blindness: a global perspective. Bull World Health Organ 79:214–21
- Yohai R, Bullock J, Aziz A, Markert R. (1994). Survival factors in rhino-orbital cerebral mucormycosis. Surv Ophthalmol 39:3–22