?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
A novel, self-administration drug delivery system for subcutaneous infusion was developed and evaluated. The device includes two main components: an osmotic tablet controlled gas actuator and a syringe catheter system. The sodium carbonate in the osmotic pump tablet will release into the surround citric acid solution and produce CO2 gas, which will drive the drug solution into subcutaneous tissue. The key formulation factors of the osmotic tablet that would influence the infusion profiles of the device were investigated by single factor exploration. The formulation was optimized via a response surface methodology. With an 18 ± 4 min of lag time, the delivery system was able to infuse at an approximate zero-order up to 5.90 ± 0.37 h with a precision of 9.0% RSD (n = 6). A linear correlation was found for the infusion profile and the fitting equation was Y = 0.014X − 0.004 (r = 0.998). A temperature change of 4 °C was found to modify the flow rate by about 12.0%. In vivo results demonstrated that the present subcutaneous infusion device was similar to the commercial infusion pump, and it could bring a long and constant ampicillin plasma level with minimized fluctuations.
Introduction
Hospitalization is inconvenient for patients and families, and many patients wish to receive treatments at home. The cost of hospitalization and the danger of nosocomial infections are further disadvantages of admitting a patient in hospital. Self-administration at home is a viable and feasible option for ambulatory patients with diseases requiring administration of injection medications. However, home use of intravenous injection entails serious risks and requires close supervision by skilled medical staff (Schen, Citation1997). In such occasion, subcutaneous administration is a useful alternative to intravenous administration because of its safety and ease of operation.
Compared with repeated dosing, continuous subcutaneous infusion has various advantages. It can maintain the blood drug concentration at an optimum level, avoid the under or over dosing, and herein minimize the risk of disadvantages such as side effects, poor therapeutic activity or even adverse effects (Herrlich et al., Citation2012). In addition, the prolonged delivery is required for drugs with short half-lives such as some β-lactam antibiotics and biotech medicine (Roberts et al., Citation2007; Agarwal & Rupenthal, Citation2013). For these drugs, the development of a depot-injectable formulation may take a long time (Chen et al., Citation2014), thus a continuous parenteral delivery via ambulatory infusion pumps is a good option.
Subcutaneous infusion is now a frequently selected infusion route used for opioid drugs in hospitals (Herndon & Fike, Citation2001; Justad, Citation2009), and it is also an effective way in treating diabetes (Hirsch et al., Citation2005; Fujikawa et al., Citation2012), cancer (Leahy et al., Citation1992; Befon et al., Citation2000), bacterial infections (Champoux et al., Citation1996), gastrointestinal obstruction (De Conno et al., Citation1991) and primary immunodeficiency deficiencies (Kirmse Citation2009; Wasserman et al., Citation2012). Such administration way, allowing patients to manage their own infusions at home, is also very suitable for ambulatory outpatients. However, currently there is a lack of cost-effective and easy-to-use devices for continuous delivery of small volume parenteral drugs for the outpatient market. Among the best known are portable, battery driven insulin pumps, which are costly and require extensive patient education (Moulin et al., Citation1991; Pasero, Citation2002). Therefore, economical and use friendly subcutaneous infusion systems, such as MEDIPAD Drug Delivery System actuated with electrochemical gas (Mikkelsen et al, Citation2000) and Acuros portable pump driven by an osmotic actuation (Ehwald et al., Citation2006; Herrlich et al., Citation2012) have been developed. Detailed comparison on several types of subcutaneous infusion devices were summarize in .
Table 1. Comparison on several types of subcutaneous infusion devices.
Osmotic pumps can offer a stable and controllable water flow without electric or mechanical power. There are some successful reports about the development of drug delivery systems by utilizing osmosis (Theeuwes & Yum, Citation1976; Lee & Cima, Citation2011). Previously, our research team has reported an osmotic tablet pump for subcutaneous infusion (Gong et al., Citation2014). However, this infusion system was difficult to maintain its sterility during the preparation process, and the drug content cannot be altered once manufacturing is completed. Moreover, the flow rate provided by this pumps is very small (nL/min) and consequently the drug solution flow has a relatively high drug concentration. However, this high drug concentration may bring in skin irritation for some patients. Combining the advantage of osmotic technology and the gas actuating principle of MEDIPAD Drug Delivery System, we designed a novel subcutaneous infusion system to address the mentioned above problems. The infusion pump is driven by a chemical gas actuation, and the gas actuator is controlled by an osmotic pump. Therefore, such device contains no electronic or mechanical components, reducing manufacturing costs and facilitating operation.
In the present study, an economical and easy-to-use drug delivery system, consisting of a gas actuator and a subcutaneous infusion syringe was developed and evaluated. Ampicillin, one of the broad-spectrum penicillins, was selected as the model drug. The formulation of the osmotic tablet was investigated by single factor exploration and optimized via a response surface methodology. The influence of temperature and back resistance on the in vitro release profile was examined. Finally, the pharmacokinetic profile of this new drug delivery system was determined, compared with a commercial infusion pump in Beagle dogs.
Materials and methods
Materials
Anhydrous citric acid and ampicillin sodium were purchased from Fengyuan Pharmaceutical Co., Ltd. (Anhui, China). Sodium bicarbonate, colloidal silicon dioxide, and magnesium stearate were provided by Liaocheng Pharmaceutical Co., Ltd. (Shandong, China). Lactose monohydrate was purchased from Meggle Group (Tablettose 80, Wasserburg, Germany). Hydroxy propyl methyl cellulose (HMPC), hydroxy propyl cellulose (HPC) and sodium carboxymethylcellulose (CMC) were purchased from Shandong Ruitai Chemical Co. (Shandong, China). Polyethylene glycol (PEG 1500, average molecular weight) was purchased from Sasol Germany (Hamburg, Germany). Cellulose acetate with 39.8% acetylene content (M = 30 000 g/mol) was purchased from Eastman Chemical Company (Kingsport, TN). Polyvinyl pyrrolidone was purchased from International Specialty Products (Plasdone K-29/32, Wayne, NJ). All other chemicals and solvents, which were purchased from Beijing Chemical Agent Co. (Beijing, China), were of analytical or HPLC grade.
Animals
Female Beagle dogs (weighing 10.1 ± 0.4 kg) were used as the animal models for in vivo study. The dogs were individually housed in an air-conditioned and light-controlled room at 23 ± 2 °C and at 50 ± 5% relative humidity. The animals accessed food and water, ad lib. All animals were healthy and free of observable ocular abnormalities. The local ethics committees for animal experimentations approved all experiments. All animals were handled according to the code of ethics in research, training and testing of drugs as laid down by the Animal Care and Use Ethics Committee of the 215th Hospital.
Osmotic tablet controlled infusion system design
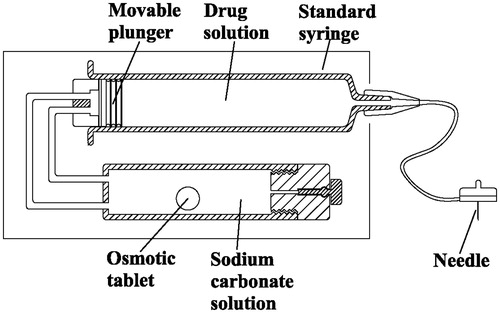
The subcutaneous infusion device () consists of a standard syringe that connected with a catheter, and an osmotic tablet controlled gas actuator.
The syringe (Weigao Medical Tech Co., Ltd, Weihai, China) is 5 mL, 12.5 mm (diameter) × 60 mm (length). The outlet is coupled to an infusion set (Royal Fornia Medical Co., Ltd, Zhuhai, China) with a length of 7 cm and inner diameter 0.4 mm, in the end of which a 27-gauge needle (7.0 mm length) is attached for subcutaneous insertion. The back of the syringe is connected to a gas actuator. In the actuator, the sodium carbonate in the osmotic pump tablet will release into the surround citric acid solution at a zero-order rate, and they will react and generate a small but constant flow of carbon dioxide; then the CO2 gas will go through a silicon vessel into the cavity that is behind the moveable plunger and push the plunger move forwardly, thereby driving the fluid via the needle into subcutaneous tissue continuously.
Preparation of the osmotic tablets
Monolithic osmotic tablets were prepared as follows. Sodium carbonate granules were comminuted and sized through an 80-mesh sieve. All excipients were screened through an 80-mesh sieve separately before blending (Formulations were listed in ). Core tablets were prepared from a wet granulation of sodium carbonate and adhesive agent (1/4 of the binders are dissolved in 75% alcohol and then sprayed onto the powder mixture). The granulation was screened through an 18-mesh sieve and dried for 2 h at 45 °C. The granules were lubricated with magnesium stearate and colloidal silicon dioxide, then they were compressed into convex tablets with 5 mm diameter concave punches by a ZP-5 rotary tablet press machine (Shanghai Tianhe Pharmaceutical Device Co., Ltd., Shanghai, China). The average tablet weight was 70 mg and the average tablet hardness was typically 70-90 N.
The tablets were coated in a pan coater (Jiangsu Taizhou Pharmaceutical Device Co., Ltd., China) with coating solution of cellulose acetate (4.5%, w/v), polyethylene glycol (PEG) and diethylphthalate (DEP) in 95% acetone. The temperature of inlet air was 45 ± 2 °C; spray rate was 2.5 mL/min; pan-rotating rate was 21 rpm. The tablets were coated and then dried at 60 °C for 4 h and stored in a container after drying. A 0.45 mm orifice for the release of citric acid was drilled into the coated tablet by a laser-beam drilling machine (Nanjing Rich Electronic Engineering Technology Industry Co., Ltd., Nanjing, China). In preliminary studies, it was found that the infusion rate did not differ significantly when the size of orifices changed from 0.40 mm to 0.50 mm.
Infusion flow measurement
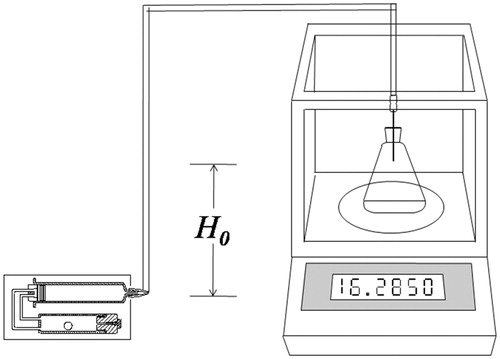
Infusion flow was recorded gravimetrically using an electronic precision balance (Sartorius BA110S, Sartorius AG, Gottingen, Germany) equipped with a data interface (Ehwald et al., Citation2006). The syringe and its outlet tubing were filled with 5 mL sterile water. The water was conducted through a catheter into a water reservoir placed on the balance, where the water volume was covered by a 3 mm layer of silicon oil to prevent evaporation. The increase in mass was recorded with time intervals of 3 min, and the tested period ended when the delivery device had exhausted its fluid reservoir. The vertical distance (H0) between the syringe outlet and the tip of the needle was fixed, so that the hydraulic flow had to overcome the gravity resistance to infuse into the water reservoir (). Here, the gravity resistance is used to simulate the resistance of subcutaneous infusion. During the testing period, the infusion device was soaked in a water bath with the temperature of 32 ± 0.5 °C. The time that drug solution released from the needlepoint was defined as the initial release time (Ti). The complete drug release time was defined as the extend release time (Te). H0 equals 29 mm unless particularly notified.
In vivo study
Six female Beagle Dogs were used in a randomized cross-over study with a 3 × 3 Latin square design. Three treatments were administered to each dog. For osmotic tablet controlled infusion group, the infusion needle was stuck into depilated back of dog and fixed. The infusion pumps were removed 6 h later after the administration. For reference group, dogs were subcutaneously infused by the WZ-50C Micro-infusion pump (Medical Equipment Factory of Zhejiang University, Hangzhou, China) at a rate of 0.83 mL/h. For control group, dogs were injected intravenously. Each group was given with ampicillin sodium injections at a dose of 21.4 mg/kg. The injections were prepared by dissolving 216 mg of ampicillin sodium (Zhong-Nuo Pharmaceutical Ltd., Shijiazhuang, China) in 5 mL waters for injection. A 1-week washout period was provided between each treatment.
Samples of 3 mL blood were taken at 0 (pre-dose), 0.17, 0.5, 1, 1.5, 2, 3, 4, 6, 8, 10, 12, 24, 30 h after administration. Blood samples were centrifuged in heparin vials at 10 000 rpm for 5 min and separated plasma was immediately stored at −20 °C prior to analysis. The plasma samples were prepared and analyzed according to Schulze et al. reported (Schulze et al., Citation2005). Seventy microliters of buffered trichloroacetic acid (TCA), consisting of 1:3 (v/v) citrate phosphate buffer (pH 5.5) and 70% TCA (w/v), was added to 0.7 mL of plasma. The mixture was vortexed for 30 s and centrifuged at 5000 rpm for 5 min (240A, Feige Instrument Company, Shanghai, China). 0.4 mL of supernatant was transferred into a vial containing 10 μL of 5 M sodium hydroxide, which was then sonicated in an ultrasonic water bath for 10 s.
The analysis of samples was performed in an Agilent 1200 HPLC system (Pump G1312B, UV detector G1316B, Auto sampler 1367C, Degasser G1379B, Agilent Technologies, Santa Clara, CA). The UV detector wavelength was set at 210 nm. Aliquots of sample (20 μL) were injected into a reversed phase C18 analytical column (Agela Venusil XBP C18, 4.6 × 150 mm, 5 μm, Agela Technologies, Inc., Boston, MA) at 35 °C. The mobile phase consisted of 50 mM potassium dihydrogen phosphate solution and acetonitrile (94:6, v/v), with a flow rate of 1.0 mL/min.
The pharmacokinetic parameters of ampicillin were processed by WinNonLin (Pharsight Corporation, Mountains View, CA).
Statistical analysis
All the data are shown as mean ± standard deviation (SD) unless particularly outlined. Student's t test or one-way analyses of variance (ANOVA) were performed in statistical evaluation. A p value < 0.05 was considered to be significant. All the testing was done using the SPSS 13.0 (SPSS Inc., Somers, NY).
Results and discussion
Osmotic tablet-controlled infusion system design
The size of this system was approximately 7.40 cm (length) × 3.50 cm (width) × 2.00 cm (height). The total weight of the device containing solution and tablet was less than 42 g. This infusion device can be inconspicuously worn on the body.
Operation of this infusion system
Withdraw drug solution by a 5 mL syringe and attach the needle-catheter part at first, then unscrew the push-rod that connected with the plunger and remove it, assemble the plunger that linked with silicon tube to the back of the syringe and fix the syringe on the basis; fill the solution cartridge with citric acid solution and a sodium carbonate osmotic tablet, screw the seal stop onto the solution cartridge and fix them on the support base. Finally, close the lid of the device and then the infusion system starts to work. An adhesive backing allows the system to be worn on the skin, discreetly under clothing ().
The infusion rate of this system is determined by the Na2CO3 release rate of the osmotic tablet in the cartridge. Osmotic pump tablets with various dose and release time can be prepared according to requirements. It is, however, essential that the mounting direction of the device requires special attention. The syringe should be kept above the solution cartridge to assure that there are enough reaction solutions to soak the whole osmotic tablet. Otherwise, the Na2CO3 release or internal gas pressure cannot be obtained in a short time (≥ 15 min).
Based on switching Na2CO3 release into CO2 gas flow and final infusion flow, this device is easy to obtain a large pumped volume per hour with a flow rate of μL/min. And delivery volume is changeable by withdrawing various bulks of drug solutions.
This delivery system is designed for self-administration by a patient or caregiver in an outpatient or home setting. It offers an alternative to external ambulatory infusion pumps or sustained-release formulations for drugs that maybe difficult to deliver via other means owing to the poor oral bioavailability, short half-life, or a narrow therapeutic window. The main advantages of the demonstrated infusion systems include low cost of manufacture, waiving of electronic driver, and ease of infusion rate or volume change.
Influences of tablet formulation variables on infusion flow
To study the influences of tablet formulation variables on drug release, tablets with various formulations were prepared, subsequently coated with the same coating formulation and a circular orifice with a diameter of 0.45 mm, was drilled on one side of the surface. The tablet and coating formulations are respectively listed in and . The initial release time (Ti) and the extend release time (Te) were employed to evaluate the release profiles of various formulations. The Te value was proposed as 6 h, while Ti value was suggested to be as small as possible.
Table 2. The formulation of core tablets.
Table 3. Coating formulation and tablet weight gain.
Influences of osmotic agents
To obtain the designated infusion profile (5 mL drug solution completed in 6 h), osmotic agents within the core tablets were investigated. As any of the agents (acid as tartaric/citric and base as Na2CO3/NaHCO3) can be compressed in the tablet core, four kinds of osmotic agents were studied. As shown in , the NaHCO3 osmotic tablet (F1, C0) manifested a maximum value both in Ti (63 min) and Te (12.2 h), while the tablet with citric acid (F0, C0) as the osmotic agent had the shortest Ti (6 min) and Te (2.7 h). The Ti and Te value of Na2CO3 osmotic tablet was 18 min and 8.2 h, respectively. Though the initial release times of tartaric acid (F0, C0) and citric acid tablets were relatively short, the hardness of tartaric acid (40–50 N) and citric acid (40-60 N) tablets were low and the core tablets would be easily worn in the coating process. Considering the Ti, Te value and coating feasibility, Na2CO3 was chosen as the final osmotic (reaction) agent for the core tablet.
Figure 3. Influence of the osmotic agents on the infusion flow (n = 6). For tartaric acid and citric acid osmotic tablets, the reaction solution was 5 mg/mL NaHCO3; and for Na2CO3 and NaHCO3 tablets, the reaction solution was 15 mg/mL citric acid. 1* Significant smaller (p < 0.05) when compared with group Na2CO3 and NaHCO3; 2* Significant smaller (p < 0.05) when compared with group NaHCO3; 1△ Significant smaller (p < 0.05) when compared with group Na2CO3 and NaHCO3; 2△ Significant smaller (p < 0.05) when compared with group versus NaHCO3.
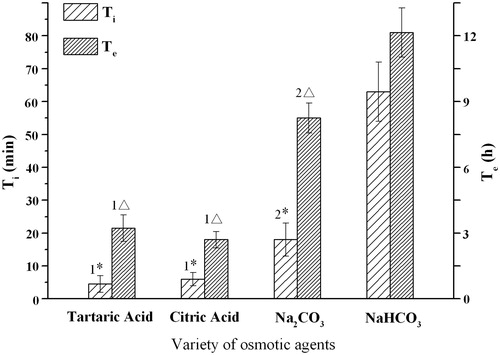
In order to keep consistent with the administration intervals of the marketed ampicillin product (four times a day), the infusion period of the delivery device is designed for 6 h. The structure of elementary osmotic pump (EOP) is simple and its preparation is relatively easy, so EOP was chosen here to release the reaction agent at a zero-order rate. However, the kinetics of EOP drug release is directly related to the solubility of drugs within the core. Agents in EOPs with the solubility of ≥ 0.30 g/cm3 would be released with ≤ 70% zero-order kinetics, and the agents with solubility of ≤ 0.05 g/cm3 would be released with ≥ 95% zero-order kinetics (McClelland et al., Citation1991). The citric acid osmotic tablet had a short time (∼60%) zero-order kinetics due to the high water solubility (64.3 g/100 mL at 30 °C) of citric acid, while the NaHCO3 tablet had a long time (∼90%) zero-order release for the low solubility (10.3 g/100 mL at 30 °C). However, compared with the other three osmotic tablets, the lag time Ti of NaHCO3 tablet (63 min) was too long to be used in the current infusion device.
The onset of drug release often took place at the end of a certain time, which is called lag time. The short lag time can save the onset time of drugs, which is an important consideration for parenteral injections. In most cases, a lag time of 30–60 min (Verma et al., Citation2002) is usually observed in osmotic tablets, as the system hydrates and osmotic pressure builds up in the tablet before the zero-order delivery from the system begins (Ozdemir & Sahin, Citation1997). The short lag time of citric acid tablet may be attributed to the rapid water hydration rate of the core.
Influences of adhesive agents
Adhesive agents in tablet technology mainly serve the purpose of binding small drug or excipient particles together to impart cohesiveness. The binders in the osmotic tablet would absorb water and form a viscous solution to help suspending the core tablet in the coating membrane; thereby, they may influence the release profiles of contents in osmotic pump tablets.
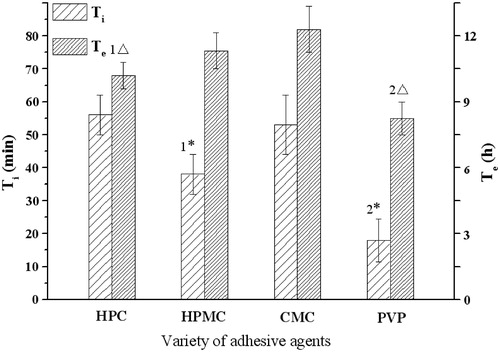
shows the effects of the four usually used binders (F2, C0) on the profiles of infusion flow. The lag time Ti of PVP, HPMC, CMC and HPC were 18, 38, 53 and 56 min, respectively. The extended infusion time Te was at the order of PVP (7.9 h) < HPC (9.8 h) < HPMC (10.9 h) < CMC (11.8 h). Both the Ti and Te values of PVP were different from the other three ones, significantly. Therefore, PVP was chosen as the binder in the tablet formulation for its short lag time and moderate infusion time.
Figure 4. Influence of adhesive agents on the infusion flow (n = 6). 1* Significant smaller (p < 0.05) than group HPC and greater (p < 0.05) than group PVP; 2* Significant smaller (p < 0.05) when compared with group HPC, HPMC and CMC; 1△ Significant smaller (p < 0.05) when compared with group CMC; 2△ Significant smaller (p < 0.05) when compared with group HPMC and CMC.
shows an increase of Ti and an opposite trend of Te as the amount of PVP in the formulation increases. There was no statistical difference between these groups. To maintain a good balance between hardness (> 70 N) and suitable infusion time (∼6 h) of the osmotic pump tablet, 2% PVP amount was chosen and fixed in the subsequent formulations. Moreover, as the components of coating can alter the infusion profiles of osmotic tablets, the Te time (7.9 h) of PVP that is longer than 6 h could be compromised by adjusting the membrane formulation in the final optimization process.
Figure 5. Influence of the amount of PVP on the infusion flow (n = 6). *Significant smaller (p < 0.05) when compared with 3% and 4% PVP; △Significant greater (p < 0.05) when compared with 3% and 4% PVP.
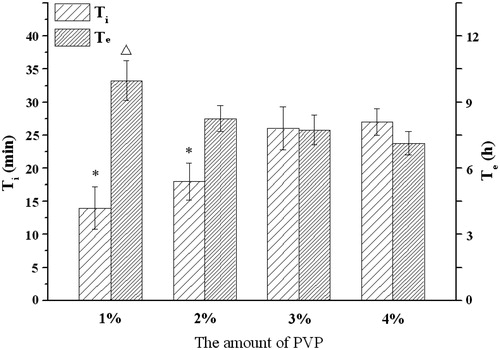
The high hydrating rate and low viscosity of binders may help a rapid drug release. Compared with the other three adhesive agents, PVP is a highly water soluble polymer with a relatively low viscosity (Colombo et al., Citation1996), so it performed well than the others. As the ratio of PVP in formulation increased, a prolonged lag time Ti was seen, likely due to increased time for PVP hydration. The expansion effects of PVP hydration may help the Na2CO3 release, so the Te value decreased as the PVP proportion increased in the tablet.
Influences of plasticizers and pore formers
As a key factor in controlling drug release from osmotic tablets, the influences of the membrane composition such as plasticizers and pore formers on infusion profiles of the delivery system were investigated. The results in displayed that the Ti and Te would increase as the molecular weight of PEG increased (F3, C1). The effects of 1.5, 2.0 and 2.5% (w/v) PEG1500 solutions were further investigated. showed that the increase of PEG level led to an increase of infusion rate. There was an inverse correlation between the PEG1500 amount and Ti or Te: the higher level of PEG1500 was, the shorter time of Ti or Te was.
Figure 6. Influence of the PEG on the infusion flow (n = 6). *, △Significant smaller (p < 0.05) when compared with PEG4000 and PEG6000.
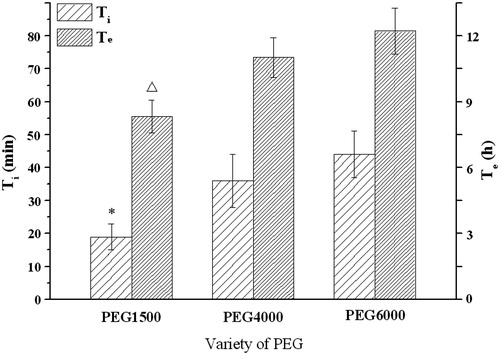
Figure 7. Influence of the level of PEG1500 on the infusion flow (n = 6). *Significant smaller (p < 0.05) when compared with other groups; △Significant greater (p < 0.05) when compared with 2.0%, 2.5% DEP.
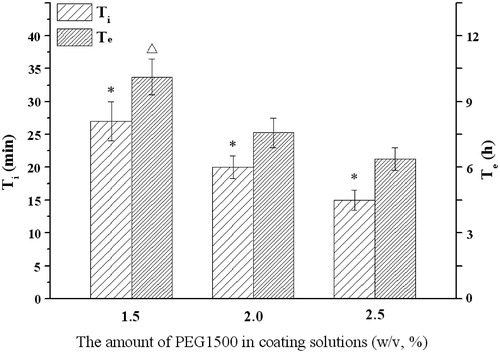
PEG can play a dual role as plasticizer (Guo, Citation1993) and pore former (Rani & Mishra, Citation2004) in the coating membrane. As a hydrophilic polymer, PEG could be leached out easily, thus porous were left behind in the semi-permeable membrane of the osmotic pump tablets. For PEG with the higher molecular weight, it would need more time to moisten and dissolve. This was consistent with the results found in push-pull osmotic pumps (Malaterre et al., Citation2009). Furthermore, the water permeability through a semi-permeable membrane was correlated with the leachable agent proportion in the membrane composition. As the proportion of PEG increased in the membrane, more of PEG would dissolve in water. Hence, the membrane permeability increased and thus increased the drug release rate (Makhija & Vavia, Citation2003). Kumaravelrajan et al. reported that the PEG/cellulose acetate ratio of 1:3 might form appropriate pores after PEG leached in the membrane (Kumaravelrajan et al., Citation2010).
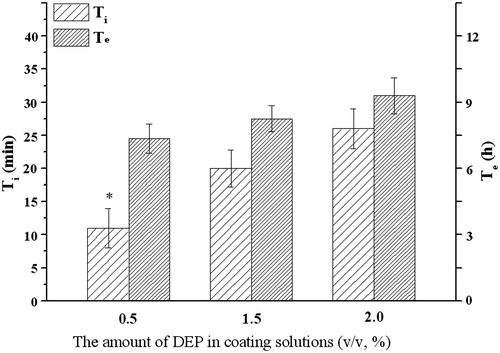
Different from PEG, with the increase of DEP amount in coating solution (F3, C2), the Ti or Te would increase (). DEP was a hydrophobic plasticizer and channeling agent, which was insoluble in water and difficult to leach. Hence, similar hydrophobic plasticizer such as castor oil, DEP would slow the drug release when it was incorporated into the coating membrane (Shokri et al., Citation2008).
Influences of membrane thickness
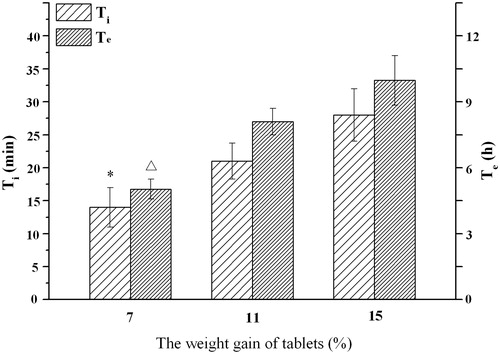
To study the effect of membrane thickness of tablet on the infusion flow, osmotic pump tablets (F3, C0) after coating with the weight gain of 7%, 11% and 15%, respectively, were prepared. shows that the Ti and Te values increased as the weight gain of tablet increased. The rate of water ingress was partially dependent on the permeability of the coating: the thicker coating, the lower water permeability. As the thickness increased, the resistance of the membrane to water diffusion increased and the rate of imbibing water decreased (or to say the lag time become longer), and in turn, the liquification rate of the tablet core decreased, resulting in the drug release rate decreasing (Liu et al., Citation2000; Ofori-Kwakye & Fell, Citation2003).
Optimization of the formulation
Based on the individual factor exploration of the formulation, the amount of PEG1500, DEP and the weight gain of tablet were determined as the key factors for further optimization. Osmotic pump tablets (F3) with various coating formulations were prepared according to Box-Behnken design (). Comprehensive grading method was employed to evaluate the release profiles of various formulations compared with the ideal release profiles. The score of various formulations was calculated by the following equation (Gong et al., Citation2014):
where S is total score of the formulation, t0.6, t3.0 and t6.0 are the cumulative infusion percentage at 0.6, 3.0 and 6.0 h, respectively. The ideal cumulative infusion percentages at 0.6, 3.0 and 6.0 h were supposed to be 10%, 50% and 100%, respectively. Apparently, the smaller the total score, the closer to the ideal infusion profile. The optimization was processed by Design-Expert 7.0.0 (Stat-Easy In., Minneapolis, MN), and the given optimal formulation was 1.50% PEG1500 (w/v) and 1.23% DEP (v/v) in coating solution, and 9.81% weight gain of tablet. The osmotic pump tablets with the optimal formulation were prepared and in vitro infusion rate was tested. The Ti and Te of optimal formulation were 18 ± 4 min and 5.90 ± 0.37 h, respectively.
As shown in (the 32 °C line), the optimal osmotic pump tablet was able to infuse at an approximately zero-order up to 6 h. The linear fitting equation for the infusion of the optimized formulation was Y = 0.014X − 0.004, with a correlation coefficient of 0.998. The delivery precision (relative standard diversity, RSD) of such device at each point time was less than 9%.
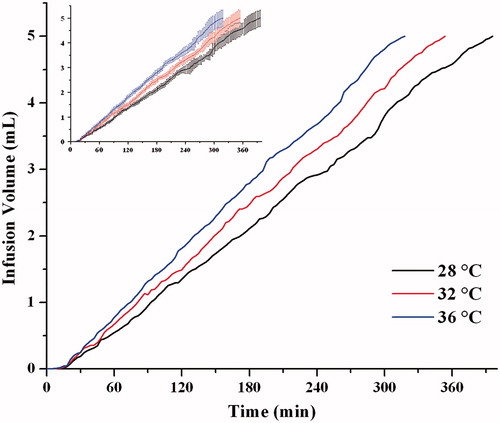
Effect of temperature
To study the effect of temperature on the infusion flow, infusion system with the optimal osmotic pump tablet at different temperatures was tested. As shows that the Ti and Te values decreased as the working temperature increased. The corresponding Ti and Te of 28, 32, 36 °C was 20 ± 5, 18 ± 4, 15 ± 4 min and 6.61 ± 0.44, 5.90 ± 0.37, 5.33 ± 0.39 h, respectively. In the range from 28 to 36 °C, a change with 4 °C modifies the flow rate by about 12%. Therefore, the flow rate may vary over time with changing skin temperature. As Ilfeld et al. reported (Ilfeld et al., Citation2003), when the temperature increased by 4 °C, the infusion rate of elastic- or spring-based and electrical pumps would change less than 15% of their set rates. A 15% to 25% infusion rate change was thought to be clinically insignificant (Patte et al., Citation2013), so the infusion rate accuracy of our developed device (12%) was acceptable in the further clinical practice.
The theoretical thermal effect on the Na2CO3 release and CO2 expansion, which will affect the infusion rate of this device, could be calculated as follows:
The mass of Na2CO3 released over time through the outlet orifice of osmotic pumps Rm, can be described by the following equation (Herrlich et al., Citation2012):
(1)
(1)
where K and A stands for the permeability and surface area of semi-permeable membrane, respectively. π and Care the osmotic pressure and concentration of the Na2CO3, respectively. i Represents the number of moles of solute actually dissolved in a solution per mole of added solid solute. R is the molar gas constant (8314 J mol−1·K−1), and T stands for the absolute temperature (K).
Integrating Equation (1) with t, at time t the release mass of Na2CO3 (mt) is as following:
(2)
(2)
(3)
(3)
According to the ideal gas law (Equation (3)), the CO2 gas volume (V), in the back of the syringe is related with number of moles of CO2 (n), gas pressure (P) and temperature (T). In addition, the drug solution infusion volume equals CO2 gas volume in the syringe. As 1 mol Na2CO3 will generate 1 mol CO2, the number mole of CO2 can be calculated as n = mt/M, M is the molar mass of CO2. After the substitution of n = mt/M and Equation (2) into Equation (3), the drug solution infusion volume Vt at time t can be calculated as:
(4)
(4)
Therefore, the infusion rate (Rt) at time t can be expressed as:
(5)
(5)
(6)
(6)
According to Equation (5), the relative infusion rate diversity generated by temperature fluctuation could be calculated as Equation (6). Here, the temperature fluctuation is ΔT, and T is the working temperature. The temperature fluctuation ΔT is assumed to be 4 °C (K) according to our research, and the working temperature is considered to be 305 K (32 °C, skin temperature) (Ilfeld et al., Citation2003), input ΔT and T value into Equation (6), the obtained theoretical infusion rate diversity is 2.65%.
The above calculated thermal effect (2.65%) on the infusion rate is smaller than the actual one (12%), which implied that there were some other factors that might affect the flow rate. These might include the viscosity of the liquids, the hydrating rate of the semi-permeable membrane, inner osmotic pressure change, liquification rate of the tablet core, and so on. But these factors are complicated and hard to calculate, which needs further study in the future.
To obtain a constant infusion rate, the infusion system should be used at a stable and suitable temperature (∼32 °C). Hence, this delivery system is designed to be worn around the patient's wrist to keep it working in a thermostatic environment.
Effect of resistance pressure
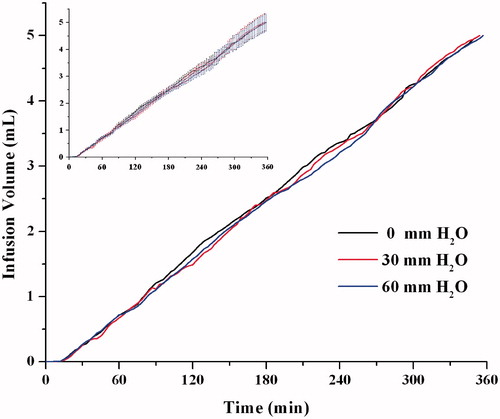
As the user and injection site varies, the tissue resistance pressure (TRP), which is the amount of pressure needed to deliver the liquid into the subcutaneous tissue, will be different and thus may lead to the changes of infusion rate. Different TRPs were simulated by using various hydraulic resistances of H0 in this study (). As seen in , the changes of mean infusion rate during the testing was less than 9%, when the hydraulic resistances H0 varied from 0 to 60 mm H2O.
Figure 11. The effect of resistance pressure on the infusion profiles (n = 6). The same data with error bars (Mean ± SD) was shown in the imbedded figure.
Except for the osmotic pump tablet itself, there are other factors that may influence the infusion profile: TRP, frictional losses of plunger in the syringe and pressure losses in the tubing. The pressure losses due to the elevation and velocity changes could be addressed by following means: (1) put the needle and outlet of the device on the same level to reduce the gravitational difference; (2) keep the flow rate at a low value (5 mL / 6 h = 0.001389 mL/min) to decrease kinetic energy losses. Therefore, there left two factors of TRP and frictional losses to be analyzed.
Based on the data reported by Patte et al. (Patte et al., Citation2013), a power-law relationship was found between median TRP (y, mbar) and infusion rate (x, mL/min), y = 87.388·x0.5211, (R2 = 0.9985). Put the proposed infusion rate (0.001389 mL/min) into this power function, the corresponding TRP of 2.835 mbar (or 29 mm H2O) is obtained. Therefore, H0 of 29 mm H2O, which represented the median TRP was used in the previous testing.
The dynamic friction of plunger in the syringe was 1.79 ± 0.16 N, divided by the cross-sectional area of the plunger (1.227 × 10−4 m2), the frictional pressure loss was 14.6 ± 1.3 kPa. As the TRP of 60 mm H2O (0.59 kPa) was much smaller than 1.3 kPa, the influence of TRP on infusion rate could almost be ignored. Overall, among the three factors that may influence the flow profiles, the friction force of the plunger was the predominant one, which needs special control to assure an accurate delivery rate. In addition, assumed the frictional pressure loss was the main resistance force, according to Equation (3), the theoretical mole number of CO2 to complete the infusion process was 2.88 × 10−5 mol.
As vibration may affect the infusion behavior, a vibration test for this infusion system where device was put in a water bath shaker with various shaking frequencies (0–50 rpm) was carried out, and the variation of infusion behavior was less than 9%. It implies that this device worn by stationary or motion patient might be able to work rightly.
A gas-actuated delivery system must not be subjected to the changes in barometric pressure. Thereby, in the future, the current developed device needs a barometric-pressure-compensating design and test, which can maintain the delivery accuracy at a reasonable range across a broad range of barometric pressures.
In vivo study
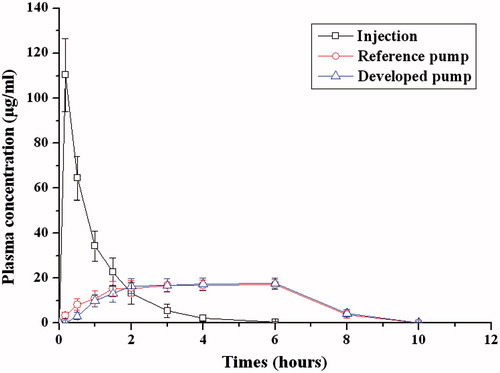
The in vivo performance of the infusion device was evaluated in dogs. The mean ampicillin plasma concentration profiles following subcutaneous infusion and intravenous injection are depicted in , and the pharmacokinetic parameters of both administration means are shown in . The peak plasma concentration of ampicillin reached as soon as the intravenous injection and the Cmax was 134.01 ± 16.38 μg/mL, then the plasma concentration of ampicillin decreased rapidly and remained about 10% of Cmax after about two hours. However, for both subcutaneous infusion pumps, the mean ampicillin plasma concentrations rose gradually, and then they reach the plateau level at about 1.5 and 2 h for reference pump group and developed pump group, respectively. This difference may be attributed to the lag release character of the developed infusion system. The Cmax of the developed and reference pump was 18.3 ± 3.15 and 17.14 ± 2.77 μg/mL, respectively, which was remarkably lower than that of intravenous injection. After 10 h the ampicillin plasma concentration for both infusion pumps was below the limit of quantization (0.2 μg/mL). The fluctuation of plasma concentration for the developed pump was similar to the reference commercial pump. Steady drug plasma levels produced by this delivery device would result in the desired pharmacodynamic efficacy and mild adverse effects (Wright et al., Citation2001). The AUC(0–t) of developed pump and reference one was 111.60 ± 18.65 and 104.72 ± 16.49 μg·h/mL, respectively. The F ratio for developed pump compared with reference pump was 107 ± 17% for AUC(0–t), which suggested that the osmotic pump controlled subcutaneous infusion administration was equivalent to the commercial pump in terms of bioavailability.
Table 4. Results of Box-Behnken design.
Table 5. Pharmacokinetic parameters of administration means.
Conclusion
A new portable and self-service subcutaneous infusion system was developed and evaluated in this paper. The innovation of this drug delivery system lies in the controlled infusion of drug solution under the driving force of CO2 gas that generated by the sustained release of Na2CO3 from an osmotic pump tablet. The predetermined in vitro release profile of this system was achieved by screening and optimizing the formulation composition of the Na2CO3 osmotic pump tablet. Besides osmotic tablets, working temperature and friction force of the plunger were found to be the main factors that would influence the infusion profiles. Compared with intravenous injection, constant drug plasma levels with minimized fluctuations could be achieved with this infusion system. The pharmacokinetic results demonstrated that this infusion device was almost equivalent to the commercial infusion pump that driven by electronic power. Overall, these results bring the hope that this cost-effective and easy-to-use drug delivery system may be as a potential platform for the constant subcutaneous delivery of drugs.
Declaration of interest
The authors report no declarations of interest. The authors are grateful to the financial support from the National Natural Science Foundation of China (Grant No. 81202466 and 81402874) and the Important National Science & Technology Specific Projects (Grant No. 2012ZX09301003-001-009) of China.
References
- Agarwal P, Rupenthal ID. (2013). Injectable implants for the sustained release of protein and peptide drugs. Drug Discov Today 18:337–49
- Befon S, Mystakidou K, Lyra M, et al. (2000). Continuous subcutaneous octreotide in gastrointestinal cancer patients: pain control and beta-endorphin levels. Anticancer Res 20:4039–46
- Champoux N, Dusouich P, Ravaoarinorom M, et al. (1996). Single-dose pharmacokinetics of ampicillin and tobramycin administered by hypodermoclysis in young and older healthy volunteers. Br J Clin Pharmacol 42:325–31
- Chen Z, Liu D, Wang J, et al. (2014). Development of nanoparticles-in-microparticles system for improved local retention after intra-articular injection. Drug Deliv 21:342–50
- Colombo P, Bettini R, Santi P, et al. (1996). Analysis of the swelling and release mechanisms from drug delivery systems with emphasis on drug solubility and water transport. J Control Release 39:231–7
- De Conno F, Caraceni A, Zecca E, et al. (1991). Continuous subcutaneous infusion of hyoscine butylbromide reduces secretion sin patients with gastrointestinal obstruction. J Pain Symptom Manage 6:484–6
- Ehwald M, Adleff H, Geggier P, et al. (2006). A long-term stable and adjustable osmotic pump for small volume flow based on principles of phloem loading. Biotechnol Bioeng 94:37–42
- Fujikawa T, Imbe H, Date M, et al. (2012). Severe insulin allergy successfully treated with continuous subcutaneous insulin infusion. Diabetes Res Clin Pract 97:e31–3
- Gong W, Ma R, Mei D, et al. (2014). A novel subcutaneous infusion delivery system based on osmotic pump: in vitro and in vivo evaluation. Drug Deliv 21:1–7
- Guo JH. (1993). Effects of plasticizers on water permeation and mechanical properties of cellulose-acetate-antiplasticization in slightly plasticized polymer film. Dr Dev Ind Pharm 19:1541–55
- Herrlich S, Spieth S, Messner S, et al. (2012). Osmotic micropumps for drug delivery. Adv Drug Deliv Rev 64:1617–27
- Herndon CM, Fike DS. (2001). Continuous subcutaneous infusion practices of United States hospices. J Pain Symptom Manage 22:1027–34
- Hirsch IB, Bode BW, Garg S, et al. (2005). Continuous subcutaneous insulin infusion (CSII) of insulin aspart versus multiple daily injection of insulin aspart/insulin glargine in type 1 diabetic patients previously treated with CSII. Diabetes Care 28:533–8
- Ilfeld BM, Morey TE, Enneking FK. (2003). Portable infusion pumps used for continuous regional analgesia: delivery rate accuracy and consistency. Reg Anesth Pain Med 28:424–32
- Justad M. (2009). Continuous subcutaneous infusion: an efficacious, cost-effective analgesia alternative at the end of life. Home Healthcare Nurs 27:140–7
- Kirmse J. (2009). The nurse's role in administration of intravenous immunoglobulin therapy. Home Healthcare Nurs 27:104–11
- Kumaravelrajan R, Narayanan N, Suba V, et al. (2010). Simultaneous delivery of nifedipine and metoprolol tartarate using sandwiched osmotic pump tablet system. Int J Pharm 399:60–70
- Leahy MG, Pitfield D, Popert S, et al. (1992). Phase I study comparing continuous infusion of recombinant interleukin-2 by subcutaneous or intravenous administration. Eur J Cancer 28:1049–51
- Lee H, Cima MJ. (2011). An intravesical device for the sustained delivery of lidocaine to the bladder. J Control Release 149:133–9
- Liu L, Ku J, Khang G, et al. (2000). Nifedipine controlled delivery by sandwiched osmotic tablet system. J Control Release 68:145–56
- Makhija SN, Vavia PR. (2003). Controlled porosity osmotic pump-based controlled release systems of pseudoephedrine I. Cellulose acetate as a semipermeable membrane. J Control Release 89:5–18
- Malaterre V, Ogorka J, Loggia N, et al. (2009). Approach to design push-pull osmotic pumps. Int J Pharm 376:56–62
- McClelland GA, Sutton SC, Engle K, et al. (1991). The solubility-modulated osmotic pump: in vitro/in vivo release of diltiazem hydrochloride. Pharm Res 8:88–92
- Mikkelsen Lynch P, Butler J, Huerta D, et al. (2000). A pharmacokineticand tolerability evaluation of two continuous subcutaneous infusionsystems compared to an oral controlled-release morphine. J Pain Symptom Manage 19:348–56
- Moulin DE, Kreeft JH, Murray-Parsons N, et al. (1991). Comparison of continuous subcutaneous and intravenous hydromorphone infusions for management of cancer pain. Lancet 337:465–8
- Ofori-Kwakye K, Fell JT. (2003). Biphasic drug release from film-coated tablets. Int J Pharm 250:431–40
- Ozdemir N, Sahin J. (1997). Design of a controlled release osmotic pump system of ibuprofen. Int J Pharm 158:91–7
- Pasero C. (2002). Subcutaneous opioid infusion. Am J Nurs 102:61–2
- Patte C, Pleus S, Wiegel C, et al. (2013). Effect of infusion rate and indwelling time on tissue resistance pressure in small-volume subcutaneous infusion like in continuous subcutaneous insulin infusion. Diabetes Technol Ther 15:289–94
- Rani M, Mishra B. (2004). Comparative in vitro and in vivo evaluation of matrix, osmotic matrix, and osmotic pump tablets for controlled delivery of diclofenac sodium. AAPS Pharm Sci Tech 5:153–9
- Roberts JA, Paratz J, Paratz E, et al. (2007). Continuous infusion of β-lactam antibioticsin severe infections: a review of its role. Int J Antimicrobial 30:11–8
- Schen R. (1997). Administration of fluid by subcutaneous infusion: revival of a forgotten method. Harefuah 132:716–17
- Schulze JDR, Peters EE, Vickers AW, et al. (2005). Excipient effects on gastrointestinal transit and drug absorption in beagle dogs. Int J Pharm 300:67–75
- Shokri J, Ahmadi P, Rashidi P, et al. (2008). Swellable elementary osmotic pump (SEOP): an effective device for delivery of poorly water-soluble drugs. Eur J Pharm Biopharm 68:289–97
- Theeuwes F, Yum SI. (1976). Principles of the design and operation of generic osmotic pumps for the delivery of semisolid or liquid drug formulations. Ann Biomed Eng 4:343–53
- Verma RK, Krishna DM, Garg S. (2002). Formulation aspects in the development of osmotically controlled oral drug delivery systems. J Control Release 79:7–27
- Wasserman RL, Melamed I, Stein MR, et al. (2012). Recombinant human hyaluronidase-facilitated subcutaneous infusion of human immune globulins for primary immunodeficiency. J Allergy Clin Immunol 130:951–7
- Wright JC, Leonard ST, Stevenson CL, et al. (2001). An in vivo/in vitro comparison with a leuprolide osmotic implant for the treatment of prostate cancer. J Control Release 75:1–10