Abstract
Context: Increased use of organophosphate insecticides (OPI) and possibility of terror groups using stocks of nerve agents underscore the need to develop effective and safe antidotes. While intramuscular administration of antidotes like atropine sulphate (AS) has certain lacunae, intravenous route may not be always feasible in emergency field conditions.
Objective: Objective was (a) to develop a novel inhalable submicronic-AS respiratory fluid as potential antidote for OPI poisoning, (b) in-vitro and in-vivo evaluation in terms of respiratory fraction, and (c) clinical study to assess drug bioavailability in blood and atropinization pattern post-inhalation.
Methods: Formulation was optimized on the basis of particle size of aerosolized droplets and in-vitro nebulization rate. Anderson cascade impaction (ACI) studies were carried out to validate the advantage of test formulation in terms of respirable fraction. Six healthy volunteers were inhaled the test formulation and blood bioavailability and atropinization were noted serially. Gamma scintigraphy was used to quantify total and regional lung deposition of nebulized AS in-vivo.
Results: The formulation was optimized using 30% ethanol–saline with particle size in the range of 350–500 nm. In-vitro ACI data showed high respirable fraction (82.6 ± 3.1%) for the test formulation. In-vivo scintigraphy suggested whole lung deposition of 80.2 ± 6.8% of the total inhaled dose. Early blood bioavailability and atropinization pattern confirmed that therapeutic concentration of the drug in blood was reached within 5 min.
Conclusions: 3% submicronic-AS respiratory fluid might be used as potential prophylactic/therapeutic option against OPI poisoning with several advantages over intramuscular injection, including early blood bioavailability and atropinization.
Introduction
Organophosphate (OP) poisoning is one of the most common causes of poisoning and is caused by exposure to insecticides like malathion and parathion, and also more importantly, by chemical warfare agents like sarin, soman, tabun or VX (Vesela et al., Citation2008; Rosman et al., Citation2009). Toxicity in these cases is due to severe and excessive systemic cholinergic response, the most important symptom of which is paralysis of respiratory muscles (Bajgar, Citation2004; Pope et al., Citation2005). In any such scenario, antidotes like atropine sulphate (AS) and pralidoxime chloride are the major lifesaving options, which need to be administered at the earliest and through the fastest route. The almost immediate therapeutic concentration of AS in blood is necessary because the toxicant, if inhaled, may reach lethal concentrations quickly. Members of the rescue team are presently provided with a 2 mg intramuscular injection of AS alone or in combination with pralidoxime chloride to be taken as a prophylactic measure before entering the ‘hot’ zone (Dunn et al., Citation1997; Bentur et al., Citation2006). However, the peak plasma concentration (6–8 ng/mL) after giving a standard dose of 2 mg AS intramuscular injection does not occur until 30 min have elapsed, limiting its use as a therapeutic option, more so in case of mass casualty (Kehe et al., Citation1992; Ali et al., Citation2009). In a hospital setting, assuming the patient can be transported there, the intravenous bolus route is the fastest way to introduce and maintain a state of atropinization, with the peak pharmacological concentration occurring within 5–10 min (Berghem et al., Citation1980; Ali-Melkkilä et al., Citation1993; Ali et al., Citation2009). However, in the field conditions or in a pre-hospital setting where giving an intravenous injection may not be always possible, efforts are required to develop and evaluate alternative but effective routes so as to obtain therapeutic range and peak concentration of the drug in blood earlier than 30 min and as close to intravenous range as possible (Albuquerque et al., Citation2006).
In the present study, we describe a novel respiratory formulation of submicronic-atropine sulphate (3% AS in 30% ethanol-saline) in terms of its preparation, in vitro formulation characterization, followed by human experience as part of a phase-I study. Aim was to prepare submicronic atropine sulphate particles in the range of 300–500 nm followed by clinical evaluation of the developed formulation with respect to its safety and pulmonary deposition pattern in healthy volunteers. Lung deposition pattern of nebulized antidote was studied since the amount of drug reaching the lungs shall represent the first line of defense against the inhaled toxicant. Further, clinical utility of AS nebulization in terms of its early bioavailability in blood was also evaluated in healthy human volunteers. Another highlight of the study is the use of gamma scintigraphy in development and evaluation of the present formulation; a technique which we have successfully used for our drug development program in the past (Ali et al., Citation2009; Bhavna et al., Citation2009; Rajpal et al., Citation2009, Citation2010; Kumar et al., Citation2011; Ali et al., Citation2013; Sultana et al., Citation2014). We quantified total and regional lung deposition of nebulized AS using scintigraphy. Both in vitro as well as human studies suggest significant deposition of the antidote at intended sites using the developed formulation.
Methods
Atropine sulphate powder (monohydrate) was received from Cipla, Mumbai. All other components of the formulation were of pharmaceutical grade and were purchased from Merck Ltd. (Mumbai, India). Technetium-99 m (Tc-99 m) was obtained from BRIT, BARC, India.
Formulation preparation and characterization
Three millilitre solution of 3% atropine sulphate (AS) in normal saline or in different concentrations of ethanol-saline (10–50%) were taken as the potential test formulation. Ethanol was added to produce aerosol particles and to increase the rate of drug output as per previously reported method (Sultana et al., Citation2011). In vitro nebulization rate and nebulization fraction of potential test formulations was determined by a method previously reported from our laboratory (Mittal et al., Citation2010). For all further experiments, the chosen test formulation was dispensed in sealed sterile colored glass vials after passing through 0.22 micron filter. The formulation was characterized using a particle size analyzer (Lasair II, Particle size measuring systems Inc, Boulder, CO) on the basis of particle size of aerosolized droplets after nebulization. Particle size was measured after 2 min of equilibrium. All samples were analyzed in triplicate.
In vitro estimation of respiratory fraction
Respiratory fraction of the developed formulation was determined using Anderson cascade impactor (ACI; Copley Scientific, Nottingham, United Kingdom). Three milliliter of the chosen test AS formulation was nebulized by jet nebulizer (American Bantex Corp., Alphaneb Plus nebulizer, San Mateo, CA) with/without a spacer that was to be connected to the mouth piece (initiation port) of Anderson cascade impactor (ACI). Nebulization was done for 10 min and the wash solutions from initiation port, preseparator deposit, and various impactor stages were collected and quantified for drug content using High-Performance Liquid Chromatography (Shimazdu, Kyoto, Japan) by the method described by Ali et al. (Citation2009). The in vitro measurements were performed in six replicates. The amount deposited at the various stages was expressed as percentage AS per nebulization. Respirable fraction was calculated as the ratio of percentage of total drug deposited in the lower stages of the ACI (stage 1 to filter) to total theoretical dose, describing the percentage of aerosolized drug deposited deeply in to the lungs. Comparison in particle size attributes was made with and without the use of spacer.
Clinical studies
As part of the Phase-I safety study, the chosen test AS formulation was nebulized in six healthy volunteers. Study protocol was approved by Institutional Human Ethical Committee duly constituted for the purpose (INM/TS/IEC/009/07). Demographic data, age, and weight of the volunteers were recorded in case report form at the time of enrollment. History of any disease, blood pressure abnormalities, heart rate, electrocardiography, and pupil diameter were noted. Routine laboratory investigations for hematology (hemoglobin, total leucocyte count, platelet count, and erythrocyte sedimentation rate) and biochemical investigations including serum glutamic-oxaloacetic transaminase, serum glutamate pyruvate transaminase, serum bilirubin, serum alkaline phosphatase, serum creatinine, blood urea nitrogen, and random blood sugar, and electrocardiogram were done to make sure the volunteer was healthy. The informed-consent form was obtained from the volunteers in vernacular language or English.
Prior to inhalation study, volunteers were trained to inhale from the inhalation device and were specifically instructed to exhale to approximately residual volume, and then inhale rapidly to total lung capacity. The subjects were then nebulized with a single dose of the chosen test AS formulation for 10 min. Various clinical signs and symptoms of atropinization including dry mouth, thirst, blurring of vision, and increase in pulse rate and pupil diameter were recorded before and after AS nebulization at fixed time intervals of 5, 10, 15, 30, 60, and 120 min. Two milliliters of blood were withdrawn from a venous cannula after 5, 15, 30, 60, and 120 min post inhalation for assessing the drug concentration in blood. The pulse rate and blood oxygen level was recorded with the help of a handheld pulse oxymeter. Dry mouth, thirst, and blurring vision were assessed by asking questions about the severity of the symptoms and were recorded. Any other adverse effects, if produced, were recorded. Percentage increase in heart rate and increase in pupil diameter with time were noted. The subjects were in contact with the clinical investigators for a week after the test for recording of any problems or observations. The concentration of drug in blood was assessed using liquid chromatography–mass spectrometry in ng/mL and plotted against time to obtain the bioavailability curve (Xu et al., Citation1995; Rajpal et al., Citation2010).
Gamma scintigraphy based human volunteer study
As already mentioned, gamma scintigraphy was used to quantify total and regional lung deposition of nebulized AS. For this, Tc-99 m pertechnetate (Tc-99 m) was used as the radiotracer. Tc-99 m is the most widely used radionuclide in gamma scintigraphy for human use because of its short half-life of 6 h, easy availability, and being a pure gamma emitter. Two drops (200–300 μCi) of Tc-99 m–labeled diethylene triamine pentaacetic acid (DTPA) (molecular weight, 393.34 g) were added to AS respiratory fluid for gamma scintigraphic evaluation of the developed formulation as per previously reported method of using Tc-99 m-DTPA for this purpose (Rajpal et al., Citation2009). The formulation was then nebulized to six human volunteers as described above.
Immediately following nebulization of AS respiratory fluid, two-dimensional scintigraphic images of anterior and posterior chest, lateral oropharynx, and nebulization assembly, including spacer were recorded using a dual head gamma camera system (Symbia T2, Siemens, Erlangen, Germany). All images were recorded on a computer system assisted with the inbuilt software (Syngo, Siemens, Erlangen, Germany). Regions of interest were drawn around the oropharynx, esophagus, stomach, and whole lung. The counts within these regions were corrected for background radioactivity, radioactive decay, and tissue attenuation of gamma rays (Pitcairn & Newman, Citation1997). The lung region was subdivided into central, intermediate and peripheral sections which translate predominantly into respiratory tree, mixed and alveolar region, respectively (Newman et al., Citation1989). Visual comparison between the lung images was done to record movement of the deposited drug with time from one compartment to another. Percentage of dose deposited in the oropharynx included activity adhering to the mouth and pharynx together with any swallowed activity detected in esophagus, stomach, and the intestines. Counts for each area were expressed as a percentage of the nominal dose, which was determined from the sum of total body counts in addition to those deposited on nebulizer components, and the exhalation filter.
Statistical analysis
Data are expressed as mean ± S.D. Unpaired t-test was applied for the calculation of significance at p < 0.05 using GraphPad Instat version 3.00 for Windows XP, GraphPad Software (San Diego, CA) www.graphpad.com.
Results
Formulation preparation and characterization
shows the nebulization rates and nebulized fraction of the prospective formulations studied. On the basis of the results obtained and considering the potential throat irritability by ethanol at higher concentrations, 3% AS in 30% ethanol-saline was considered suitable for nebulization.
Table 1. Effect of adding different concentrations of ethanol in 0.9% saline on nebulization rate/min and nebulized fraction (mean ± S.D., n = 6).
depicts the particle size range obtained with and without using a spacer. Since the desired particle size range was achieved by using a large-volume spacer, 3% AS in 30% ethanol-saline to be nebulized through a spacer was chosen as the final formulation.
Table 2. Andersen cascade impactor (ACI) results for AS nebulization with and without spacer at inspiratory flow rate of 28.3 L min−1 (n = 6).
Final composition of the formulation
AS 3% in ethanol–saline
0.9% saline 70%
Ethanol 30%
The formulation was passed through 0.22-micron filter to make it sterile. The pH was set at 7.4. Accelerated stability studies done with the developed formulation as per International Conference on Harmonization (ICH) guidelines (Patel & Poddar, Citation2009) showed high stability of the drug in solution for up to 2 years.
In vitro estimation of respiratory fraction
shows Andersen cascade impactor (ACI) results for AS nebulization with and without spacer at inspiratory flow rate of 28.3 L min-1 (n = 6). It can be seen that AS nebulization through a spacer showed a significantly higher respirable fraction (82.6 ± 3.1%) as compared to without spacer (65.2 ± 5.2%; p < 0.05). Though no nebulization formulation of AS is previously reported in literature, the respiratory fraction in both instances (without spacer and spacer) was still much higher than that reported for micronized AS dry powder inhalation formulation (42.64 ± 3.10%; Ali et al., Citation2009). The respirable fraction, calculated as ratio of total drug deposited in the lower stages of the ACI (stage 1 to filter) to total theoretical dose, is a measure of the amount of drug reaching the lungs (Sultana et al., Citation2011). These data were also taken into consideration while fixing the dose of AS to be nebulized as 3%.
Clinical studies
Only young adult healthy male volunteers were chosen for the study in view of atropinization, which can be considered a temporary stress but was essential to prove suitability of the formulation. The mean age of volunteers was 28 years, whereas the mean weight and height was 64 kg and 167 cm, respectively.
Atropinization signs and symptoms
No hematological or biochemical changes were noted after AS nebulization in any of the volunteers. The blood pressure remained within the normal range during the study period except in one individual, who showed transient hypotension but did not require any treatment. Hypotension is a known event in atropinization (Rajpal et al., Citation2010). Maximum increase in heart rate was seen at 30 min, although the effect came in early. There was an increase in pupil diameter consistently, but the amplitude and duration showed wide fluctuations. Effect of the single dose remained for 3–4 h. The data are in agreement with the known irregular atropinization effects in different individuals (Ali et al., Citation2009; Rajpal et al., Citation2010).
Early symptoms of atropinization including blurring vision, dry mouth and thirst were evident in all the subjects within 20–30 min post nebulization (). This provided additional evidence that nano-AS nebulization was able to deliver therapeutic dose through the lungs quickly. In comparison, it is reported that peak effect by intramuscular route occurs after 30 min (Kehe et al., Citation1992), while that with oral route occurs only after 60–120 min (Dollery, Citation1999).
Table 3. Signs and symptoms of atropinization in human volunteers after inhalation of submicronic 3% AS respiratory fluid (n = 6).
Drug concentration in blood
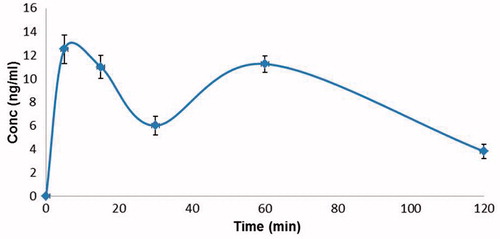
shows the plasma concentrations of 3% nano-AS respiratory fluid in human volunteers (n = 6) in ng/ml at different time intervals after nebulization. The first blood sample drawn within 5 min of inhalation showed the drug concentration of 12.7 ng/ml, suggesting a much earlier bioavailability of the antidote in therapeutic range (6–8 ng/ml) as compared to known values of atropine sulphate given through intramuscular route, where the therapeutic concentration is achieved at 30 min (Kehe et al., Citation1992; Ali et al., Citation2009). At 15 and 30 min interval post inhalation, the concentration of atropine in blood was 11 and 6.1 ng/ml respectively. A second peak occurred at 60 min with a drug concentration of 11.6 ng/ml. Thereafter, the curve followed a slow falling pattern and at 120 min, only 4 ng/ml of the drug was present in blood. The bioavailability of the drug was much more favorable in comparison to that of the known standard intramuscular dose of atropine sulphate (Kehe et al., Citation1992) in terms of amplitude as well as early peaking of the drug concentration curve in blood.
Gamma scintigraphy study
With the help of gamma scintigraphy relative percentage of the drug remaining in various parts of the nebulizer assembly, and that retained in different lung compartments was determined and is shown in . Although nearly 50% of the nebulized drug was retained in nebulizer assembly at the end of nebulization, majority of the inhaled portion reached the lungs. Whole lung deposition was in the range of 80.2 ± 6.8 for nebulized nano AS respiratory fluid, while the rest was deposited in oropharynx compartment, representing oropharynx, esophagus and stomach. It is much less in comparison to that reported for micronized drugs, where nearly two-third of the drug is deposited in the oral region and is not available to the lungs (Bhavna et al., Citation2009). The results indicate that significantly increased levels of the antidote can be targeted to the lungs with this novel formulation. The ratio of peripheral to central lung compartment deposition for submicronic AS respiratory fluid was 0.54. The deposition in intermediate lungs was about 1–2 times higher than central and peripheral regions of lungs. Based on these observations it can be inferred that more than 80% of the inhaled drug reached alveolar regions of the lung using submicronic-AS nebulization. Further, gamma scintigraphic studies in human volunteers clearly showed significant penetration of nebulized AS into the alveolar regions (). Images taken after 2 h showed 50% reduction in radioactivity from lungs, indicating absorption of the drug into the systemic circulation. In comparison, alveolar drug deposition in volunteers nebulized without using spacer was much less ().
Figure 2. Human scintigraphy images of submicronic 3% AS respiratory formulation in two healthy volunteers at 5 min and 2 h showing distribution of the drug into oral cavity, tracheobronchial tree, lungs and stomach. While (a) represents distribution of nebulized drug using a spacer, (b) represent distribution of drug without a spacer.
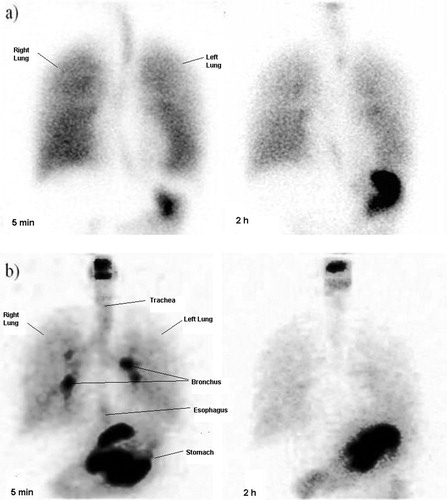
Table 4. Gamma scintigraphy based regional deposition of AS aerosols after inhalation of submicronic 3% AS respiratory fluid containing tracer quantity of 99mTc-DTPA (200–300 μCi) in human volunteers (n = 6).
Discussion
The present work describes (a) development and optimization of a novel submicronic AS respiratory formulation, (b) its in vitro characterization using Anderson cascade impaction studies, (c) gamma scintigraphy in human volunteers to assess pulmonary drug deposition pattern with respect to developed formulation, followed by (d) human phase-I trial yielding pharmacokinetic and atropinization data.
Inhalation route is increasingly being used for systemic delivery of drugs Due to the presence of an extremely large surface area and thin epithelium barrier, the pulmonary route offers a means of rapid biotransfer, a fact well described in the literature for several drugs (Ali et al., Citation2009; Bailey & Berkland, Citation2009). The present work probably describes the first nebulization formulation of atropine sulphate for systemic action used on humans to obtain atropinization and bioavailability data. By enhancing respiratory fraction and deep lung penetration, which becomes feasible by using submicronic particles, it is possible to optimize the inhalation route for faster systemic delivery of drugs (Sultana et al., Citation2011). Submicronic-AS respiratory fluid was therefore developed with characteristics favoring deeper penetration and retention in the lung spaces. In vitro ACI data showed that use of a large volume spacer resulted in further improvement in respirable fraction of the submicronic AS formulation (82.6 ± 3.1%) as compared to when no spacer was used (65.2 ± 5.2%), suggesting higher lung penetration potential and drug bioavailability.
Human scintigraphy studies further highlighted the advantage of submicronic test formulation with lung scintigrams showing nearly 80% of the inhaled drug reaching the pulmonary vasculature, which is much more in comparison to that reported for micronized drugs, where nearly two-third of the drug is deposited in the oropharyngeal region and is not available to the lungs (Bhavna et al., Citation2009). The blood bioavailability curve with respect to AS post nebulization in humans was favorable by way of a much earlier bioavailability of the antidote in the therapeutic range (6–8 ng/ml) as compared to known values of AS given through intramuscular route, where the therapeutic concentration is achieved at 30 min (Kehe et al., Citation1992; Ali et al., Citation2009). The therapeutic concentration was reached within 5 min and two peaks were observed. The first peak may have resulted entirely due to biotransfer of drug from the lungs. A significant second peak occurring at 60 min probably resulted from absorption through GIT after swallowing of the drug portion by mucociliary action in the throat and due to delayed resorption of atropine from intermediate and central hilum area of the lungs. Moreover, the signs and symptoms of atropinization were clearly evident within 20 min indicating that the therapeutic concentration would have been achieved much earlier.
Strength of submicronic AS respiratory fluid (3%) was fixed on the basis of in vitro scintigraphy experiments and previously reported work on atropine formulation from our laboratory and elsewhere (Volans, Citation1996; Ali et al., Citation2009), taking into account drug wastage, including GIT absorption post nebulization. Calculations based on in vitro and in vivo scintigraphy experiments suggest that nebulization of 3% submicronic AS respiratory fluid through a spacer will lead to transfer of 2–2.1 mg of drug into the bloodstream from lungs. Clinical data in six healthy volunteers confirmed that the dose fixed on the basis of scintigraphy data was quite appropriate and was able to generate clinical atropinization and blood bioavailability curve satisfactorily.
Although intravenous administration of atropine offers the fastest route of absorption (Aaltonen et al., Citation1984; Ali-Melkkilä et al., Citation1993) and probably the most efficient line of treatment in OP poisoning, this novel respiratory formulation has been developed mainly for prophylactic local action in the lungs and as an alternate for delivering therapeutic amount of the antidote quickly in a pre-hospital set-up or in ambulances for mass treatment of casualties, where giving an intravenous injection may not be always possible due to non-availability of a paramedic and other logistical reasons. It is also worth mentioning here that although inhalation through dry powder inhalation (DPI) technology may be more suitable for emergency field use than nebulization, stability of submicronic DPI formulations is still an unresolved issue. Our lab had previously developed a nano-DPI formulation of atropine sulphate (Ali et al., Citation2009) but its stability is not more than 3 months, after which the particles tend to aggregate, thereby changing the pulmonary drug deposition pattern of the DPI formulation and therefore its potential efficacy. From a pharmacokinetic point of view, pulmonary absorption is rapid, which should provide a faster onset of action as compared to oral/intramuscular administration. Additional advantages are that lung drug delivery is needle-free, patient friendly, and painless. Given the known safety profile of atropine (Volans, Citation1996), a critical patient may be nebulized twice in the first hour to produce sufficient atropinization, enough for him to be carried to the hospital for conventional treatment, which is the intravenous bolus route. Since systemic pharmacokinetics of any inhaled drug reflects its deposition in distal pulmonary regions (Dequin et al., Citation2001; Kumar et al., Citation2011), systemic absorption of nebulized AS proves that its absorption is also occurring mainly at the alveolar level, giving an additional proof of antidote’s deep penetration into the lung parenchyma, and emphasizing its role in neutralizing inhaled toxicant in the lungs itself.
Conclusion
The results obtained suggest that the novel submicronic 3% AS respiratory fluid could be a promising prophylactic and therapeutic option for neutralizing the adverse effects of inhaled OP toxicants by ensuring rapid systemic delivery of the drug during a cholinergic crisis. Although the present novel drug delivery system has been made specifically for treatment of nerve agent poisoning in a pre-hospital set-up, other therapeutic applications are not ruled out.
Declaration of interest
The authors report no declaration of interests.
References
- Aaltonen L, Kanto J, Iisalo E, Pihlajamäki K. (1984). Comparison of radioreceptor assay and radioimmunoassay for atropine: pharmacokinetic application. Eur J Clin Pharmacol 26: 613–17
- Albuquerque EX, Pereira EF, Aracava Y, et al. (2006). Effective countermeasure against poisoning by organophosphorus insecticides and nerve agents. Proc Natl Acad Sci USA 103:13220–5
- Ali-Melkkilä T, Kanto J, Iisalo E. (1993). Pharmacokinetics and related pharmacodynamics of anticholinergic drugs. Acta Anaesthesiol Scand 37:633–42
- Ali R, Jain GK, Iqbal Z, et al. (2009). Development and clinical trial of nano-atropine sulfate dry powder inhaler as a novel organophosphorous poisoning antidote. Nanomedicine NBM 5:55–63
- Ali R, Mittal G, Ali R, et al. (2013). Development, characterisation and pharmacoscintigraphic evaluation of nano-fluticasone propionate dry powder inhalation as potential antidote against inhaled toxic gases. J Microencapsul 30:546–58
- Bailey MM, Berkland CJ. (2009). Nanoparticle formulations in pulmonary drug delivery. Med Res Rev 29:196–212
- Bajgar J. (2004). Organophosphates nerve agents poisoning: mechanism of action, diagnosis, prophylaxis and treatment. Adv Clin Chem 38:151–216
- Bentur Y, Layish I, Krivoy A, et al. (2006). Civilian adult self injections of atropine-trimedoxime (TMB4) auto-injectors. Clin Toxicol 44:301–6
- Berghem L, Bergman U, Schildt B, Sörbo B. (1980). Plasma atropine concentrations determined by radioimmunoassay after single-dose i.v. and i.m. administration. Br J Anaesth 52:597–601
- Bhavna, Ahmad FJ, Mittal G, et al. (2009). Nano-salbutamol dry powder inhalation: a new approach for treating broncho-constructive conditions. Eur J Pharm Biopharm 71:282–91
- Dequin PF, Faurisson F, Lemarie E, et al. (2001). Urinary excretion reflects lung deposition of aminoglycoside aerosols in cystic fibrosis. Eur Respir J 18:316–22
- Dollery C. (1999). Therapeutic drugs. 2nd ed. New York: Churchill Livingstone
- Dunn MA, Hackley BE, Sidell FR. (1997). Pretreatment for nerve agent exposure. In: Sidell FR, Takafuji ET, Franz DR, ed. Medical aspects of chemical and biological warfare. Washington: Borden Institute, 181–96
- Kehe CR, Lasseter KC, Miller NC, et al. (1992). Comparative absorption of atropine from a metered dose inhaler and an intramuscular injection. Ther Drug Monitor 14:132–4
- Kumar N, Soni S, Jaimini A, et al. (2011). Edetate calcium disodium nanoparticle dry powder inhalation: a novel approach against heavy metal decorporation. Int J Pharm 416:376–83
- Mittal G, Kumar N, Rawat H, et al. (2010). A radiometric study of factors affecting drug output of jet nebulizers. Ind J Pharm Sci 72:31–8
- Newman SP, Clark AR, Talalee N, Clarke SW. (1989). Pressurised aerosol deposition in the human lung with and without an open spacer. Thorax 44:706–10
- Patel RS, Poddar SS. (2009). Development and characterization of mucoadhesive buccal patches of salbutamol sulphate. Curr Drug Deliv 6:140–4
- Pitcairn GR, Newman SP. (1997). Tissue attenuation corrections in gamma scintigraphy. J Aerosol Med 10:187–98
- Pope C, Karanth S, Liu J. (2005). Pharmacology and toxicology of cholinesterase inhibitors: uses and misuses of common mechanism of action. Environ Toxicol Pharmacol 19:433–46
- Rajpal S, Ali R, Bhatnagar A, et al. (2010). Clinical and bioavailability studies of sublingually administered atropine sulphate. Am J Emerg Med 28:143–50
- Rajpal S, Mittal G, Sachdeva R, et al. (2009). Development of atropine sulphate nasal drops and its pharmacokinetic and safety evaluation in healthy human volunteers. Environ Toxicol Pharmacol 27:206–11
- Rosman Y, Makarovsky I, Bentur Y, et al. (2009). Carbamate poisoning: treatment recommendations in the setting of a mass casualties event. Am J Emerg Med 27:1117–24
- Sultana S, Bhatnagar A, Rawat H, et al. (2014). Pulmonary delivery of nanosized alendronate for decorporation of inhaled heavy metals: formulation development, characterization and gamma scintigraphic evaluation. Pharm Dev Technol 19:623–33
- Sultana S, Singh T, Ahmad FJ, et al. (2011). Development of nano alpha-ketoglutarate nebulization formulation and its pharmacokinetic and safety evaluation in healthy human volunteers for cyanide poisoning. Environ Toxicol Pharmacol 31:436–42
- Vesela S, Kuca K, Jun D. (2008). Daphnia intoxicated by nerve agent tabun can be treated using human antidotes. Environ Toxicol Pharmacol 25:329–33
- Volans AP. (1996). Sarin: guidelines on the management of victims of a nerve gas attack. J Accid Emerg Med 13:202–6
- Xu A, Havel J, Linderholm K, Hulse J. (1995). Development and validation of an LC/MS/MS method for the determination of l-hyoscyamine in human plasma. J Pharm Biomed Anal 14:33–42