?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Context: Conventional ophthalmic solutions often eliminate rapidly after administration and cannot provide and maintain an adequate concentration of drug in the pre-corneal area.
Objectives: Above problem can be overcome by the use of in situ gel forming systems that are instilled as drops in to the eye and undergo a sol–gel transition in the cul-de-sac.
Methods: An ion sensitive polymer gellan gum was used as gelling agent which formed immediate gel and remained for extended time period. Nanoparticles of moxifloxacin, prepared by solvent evaporation, were separated by freeze drying. The rheological properties and in vitro drug release test of in situ gel loaded with nanoparticles were evaluated and compared with marketed preparation. In vitro release study demonstrated diffusion controlled release for moxifloxacin from formulations over a period of 12 h.
Results: The developed formulation was stable and showed enhanced contact time minimizing the frequency of administration. Confocal microscopy showed clear permeation of drug loaded nanoparticles across L/S of cornea.
Conclusion: The formulation of moxifloxacin was found liquid at the formulated pH and formed gel in the presence of mono or divalent cations. The gel formed in situ showed sustained drug release over a period of 10–12 h. The formulations were less viscous before instillation and formed strong gel after instilling it into cul-de-sac. It is thus concluded that by adopting a systematic formulation approach, an optimum point can be reached in the shortest time with minimum efforts to achieve desirable rheological and in vitro release property for in situ gel forming system.
Introduction
Moxifloxacin is a flouroquinolone analogue, reported for the treatment of conjunctivitis, keratitis and Kerato conjunctivitis. The drug has limited side effects on the normal ocular surface epithelium (Nagarwal et al., Citation2011). Eye is one of the challenging organs for drug delivery because of its unique anatomy; drug absorption is restricted into the deep tissues. Topically applied drugs have lower ocular bioavailability (O’Brien, Citation2012). Topical solution has frequent systemic side effects due to absorption through nasolacrimal duct. Nanoparticles containing drug has targeted action in the ocular tissues with enhanced bioavailability (Rathapon et al., Citation2011). Nanoparticles have various advantages of increasing cellular uptake of the drugs which are poorly permeable (Andreas & Jorg, Citation1995). The cellular and tissue clearance can be reduced and sustain drug delivery can be achieved (Mehrdad et al., Citation2008). Nanoparticles containing moxifloxacin have been developed as particulate system for drug delivery by preparing nanosuspension using solvent diffusion method and freeze drying it (Rosario, Citation2002).
Extensive investigations has been done for development of new systems of ophthalmic drug delivery with prolonged retention time on the eye surface, reduced dosing frequency and improving permeation through trans corneal layers (Taís, Citation2010). Various attempts have been made to deliver drugs to the eye by using polymeric in situ vehicles such as sodium alginate, chitosan and gellan gum (Johan et al., Citation1998). In situ drug delivery systems have polymers that exhibit phase transition sol-to-gel due to changes in their environment can be instilled as drops into the cul-de-sac of the eye and get transformed into a gel or semisolid phase, gellan gum used here is ion sensitive which forms gel in the presence of mono and divalent ions (Hongyi, Citation2007). The particle size, Zeta potential and Poly Dispersity Index (PDI) were characterized. Physicochemical characterization of nanoparticles was performed by Fourier Transform Infrared (FTIR), Differential Scanning Calorimetry (DSC). Encapsulation efficiency was analyzed by using UV spectrophotometry methods. Finally, the potential of this nanoparticulate system was tested for in vitro release (Guana, Citation2011).
Materials and methods
Materials
Moxifloxacin was obtained from BDR Pharmaceuticals Pvt. Ltd., Baroda, India. All other excipients were sourced from standard suppliers and were of A.R grade.
Methods
Solubility study
Solubility study was performed by dissolving one unit of active drug in one unit of solvent. Means, initially 1 mg of drug was dissolved in 1 ml of solvent. After this, if drug was completely dissolved, then more amount of drug was added. If not so, then more amount of solvent is added. Solution was then kept for 24 h to check re-precipitation (Kortejarvi et al., Citation2005).
Preparation of nanoparticles
As shown in , accurately weighed quantity of Poly Vinyl Alcohol (PVA) was dissolved in 50 ml water. It was allowed to cool to room temperature. Poloxamer 407 and drug were dissolved in aqueous solution of PVA (Nagarwal et al., Citation2011; Singh et al., Citation2014). Eudragit RL100 was dissolved in acetone and this organic solution was added drop wise into the aqueous mixture of PVA, Poloxamer 407 and drug. This solution was then subjected to high speed homogenizer (Silent Crusher M, Heidolph, Instruments GmmH & Co., Schwabach, Germany) at pre-determined rpm and time. Organic solution was evaporated using rotary evaporator (IKA® India Private Limited, Karnataka, India) at 100 °C for 30 min. The solution was then freeze dried in lyophilizer (Ikon Instruments, Delhi, India). The freeze dried product was then passed through sieve to give free flowing particles (Kassem, Citation2007).
Table 1. Formulation table of in situ gel.
Preparation of nanoparticles loaded ophthalmic in situ gel
As described in , required quantity of gellan gum was dissolved in hot deionized water. The solution was cooled at room temperature and the freeze dried particles were dispersed into the in situ gel system. Benzalkonium chloride was added at the end as a preservative (Johan et al., Citation1998).
Table 2. Formulation table for nanoparticles.
Particle size, PDI, zeta potential
The nanosuspension prepared was estimated for particle size and PDI using zeta sizer (Dynamic Light Scattering, Malvern Instruments Ltd, WR14 1XZ, United Kingdom). The particle size was again found after re-dispersion of freeze dried product. The magnitude of zeta potential gives an indication of the potential stability of colloidal system (Xiangrong, Citation2008).
Entrapment efficiency
The entrapment efficiency (EE) of nanoparticles was determined by separation of nanoparticles from the aqueous medium containing free drug by centrifugation at 14 000rpm for 30 min. The absorbance of free drug in the supernatant was measured by UV at 289 nm and the amount of entrapped drug was calculated according to above standard curve. (Guana, Citation2011; Katiyar et al., Citation2014) It was calculated using following equation:
Clarity, appearance and pH
The clarity of the formulation after and before gelling was determined by visual examination of the formulation under light alternatively against white and black backgrounds. The final formulation prepared was checked visually for its color and transparency. The pH of the formulation was measured using calibrated pH meter (Mettler-Toledo India Private Limited, Mumbai, India) (Ibrahim et al., Citation2013a, Citation2013b)
Drug content
The drug content in nanoparticles was assayed UV spectrophotometrically (SHIMADZU Analytical (INDIA) Pvt. Ltd., Mumbai, India) at 289 nm by solubilizing freeze dried product in water (as gellan gum is water soluble, nanoparticles are destroyed to release the drug) and dilution using simulated tear fluid. (Hany et al., Citation2009)
In vitro gelling capacity
The gelling capacity was determined by freshly prepared drop of system in a vial containing 2 ml of freshly prepared simulated tear fluid (STF), sodium chloride 0.67 g, sodium bicarbonate 0.20 g, calcium chloride dehydrate 0.008 g in distilled water q.s. to 100 ml (pH 7.4) and equilibrated at 37 °C. Visual assessment for gel formation was noted. Time required for gelation as well as time taken for the formed gel to dissolve were also noted. Different grades were allotted for gel integrity, weight and rate of formation of gel with respect to time. The grades were given as no gelation (−), gelation after few minutes and remains for few hours (+), gelation immediate and remains few hours (++) and gelation immediate and remains for extended time (+++) (Tayel et al., Citation2013; Lou et al., Citation2014).
Viscosity
The prepared formulations were evaluated for viscosity in order to identify the compositions best suitable for use as in situ gelling systems. Viscosity of the systems was measured using Brookfield viscometer (LV DVII +PRO model, Brookfield Engineering Labs, Inc., Mumbai, India) at 12 rpm using spindle 61 for the purpose of comparative evaluation of viscosity before and after formation of in situ gel (Balasubramanium et al., Citation2003)
In vitro drug release study
The in vitro release studies of moxifloxacin from the formulation were studied using the dialysis membrane of molecular weight cut off 12 000–14 000 kDa. The diffusion medium used was freshly prepared simulated tear fluid (pH 7.4). A dialysis membrane previously soaked overnight in the diffusion medium was tied from both ends and 2 ml volume of the formulation was accurately pipetted into this assembly. The dialysis membrane was suspended in a beaker containing 100 ml of diffusion medium at (37 ± 0.5)°C. Here, 100 ml was selected keeping the sink condition in mind and the formulation remained in the eye for the complete duration of study. Also, in meaning for in vitro release for ocular drug delivery was keeping simulated condition per se. This assembly was kept on magnetic stirrer at 50 rpm. The aliquots were diluted with dissolution medium and analyzed by UV Spectrophotometer at 289 nm (Nagarwal et al., Citation2011).
Antimicrobial efficacy studies
Antimicrobial efficacy was determined by agar diffusion test employing cup plate technique. The microbiological studies ascertained the biological activity of the optimized formulations and marketed eye drops against different microorganism. Cups were made on the solidified agar layer with the help of sterile borer with 4 mm diameter. After allowing diffusion of solution for 2 h, the agar plates were incubated at 37 °C for 24 h. The zone of inhibition (ZOI) of prepared formulation was compared with the marketed formulation (Wavikar et al., Citation2014). The ZOI was for the prepared gel. The results were nearly 2.3 times more than the marketed preparation.
Sterility test
IP method (2007) was followed for the sterility testing of eye drops. Sterility testing was carried out by incubating formulations for not less than 14 days at 30–35 °C in fluid thioglycolate medium to find the presence of bacteria and at 20–25 °C in Soybean-Casein Digest Medium to find the presence of fungi in the formulations.
Effect of sterilization
The selected optimized formulation was packaged and sterilized. The optimized in situ system was packed in glass bottle each fitted with seal, all packs were sterilized by autoclaving at 121 °C for 20 min at 15 psi. After sterilization appearance, viscosity, pH and gelling capacity of the formulation were determined.
Histopathological study
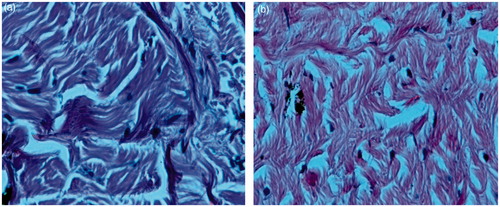
Two eyes of the goat were taken from the slaughter house and immediately preserved in Krebs ringer solution within 30 min. Two Corneal tissue pieces (3 cm2) were separated in histopathological laboratory and stored in 0.9% w/v solution under −80 °C. One was used as control (0.9 saline NaCl). Here, artificial tears or commercial agents were not used because they gave the same iso-tonicity as earlier. The other was processed with 0.6 ml of optimized formulation. The corneal tissues were fixed in 10% neutral carbonate formalin (24 h) and the transverse sections were dehydrated using graded solutions of ethanol. The subdivided tissues were stained with dye. The sections under inverted microscope were photographed at original magnification 40× and analyzed (Paulsson et al., Citation1999).
Confocal microscopy
Eyes of goat were collected from slaughter house and were preserved in freshly prepared Krebs solution. The eyes were then exposed to the formulation containing fluorescein dye for 12 h. Eyes were then preserved in formalin and the longitudinal section (LS) was mounted on slides for further studies. The fixed LS of eye was subjected to confocal microscopy. The slides were dewaxed before the study by treating with xylene and then mounted using mounting medium. The slide was then exposed to confocal microscope (Niederer & McGhee et al., Citation2010; Koichi, Citation2011).
Results
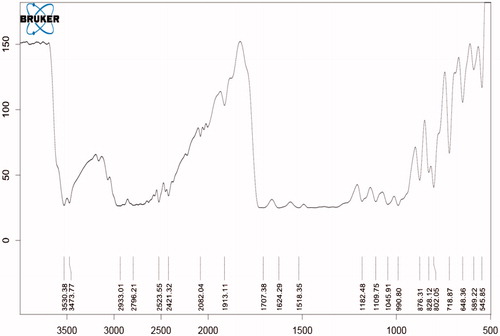
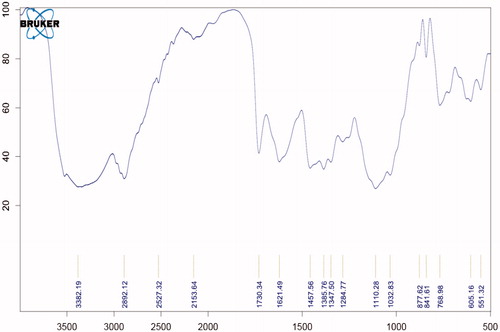
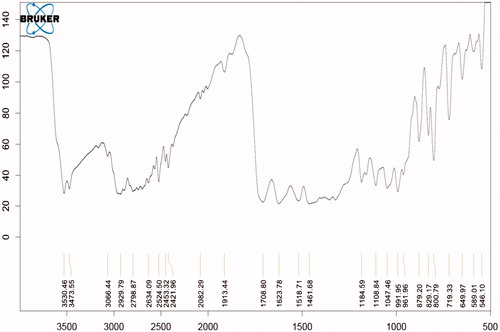
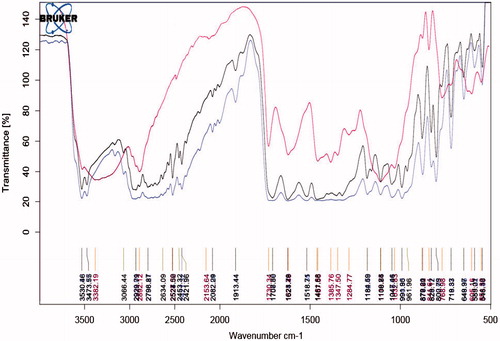
Compatibility study with FTIR
show that all the standard peaks for functional group are at their place indicating compatibility of drug with all other excipients. Drug and/or excipients were crushed in 1:1 ratio and compressed with KBr to form pellets, which were used to analyze. shows characteristic peaks at 1706 cm−1 due to carboxylic acid C = O stretching, C − N stretching at 1340 cm−1, aromatic C = C stretching at 1628 cm−1, 1518 cm−1 and 1461 cm−1 and C−H bending for the substituted benzene at 1913 cm−1.
Particle size, PDI, zeta potential, entrapment efficiency, clarity and pH
From , it can be observed that F1 formulation had lower particle size as well as entrapment efficiency was also higher than rest. Lesser PDI proved that prepared nanoparticles have uniform size distribution.
Table 3. Particle size, PDI, zeta potential, % EE, clarity and pH.
Drug content, gelling capacity and viscosity
shows that F1 formulation had higher drug content and there was no significant difference between viscosity and gelling capacity of both the batches.
Table 4. Drug content, gelling capacity and viscocity.
Antimicrobial efficacy test
The zone of inhibition was observed in each petridish containing organisms like Bacillus subtilis, Candida albicans, Escherichia coli, Staphylococcus aureus. The zone of inhibition was highest observed in B. subtilis as compared to other organisms. Data are shown in .
Table 5. Antimicrobial effect.
Sterility test
Sterility test included sterilization and incubation. Results, documented in , showed that formulation was clear.
Table 6. Sterility test.
Effect of sterilization
The selected optimized formulation was undergone for sterilization. Data for sterilization before and after are shown in .
Table 7. Effect of sterilization.
Contact time study
The study conducted showed minimal difference in enhanced contact time between gel and the marketed preparation.
Discussion
Preliminary studies
Preliminary study on drug solubility, drug excipient compatibility study and UV spectroscopy showed results as follows.
Compatibility study
FTIR result showed peaks of moxifloxacin and polymers (Poloxamer 407, PVA, Eudragit RL 100) are compatible with no interaction.
UV-spectroscopy
For UV spectroscopy, the spectrum of pure moxifloxacin (10 μg/ml) in simulated tear fluid was scanned in spectrum basic mode in the range of 200–400 nm against blank. Maximum absorbance was found to be 289 nm.
Solubility study
Solubility of Moifloxacin was found to be 7 mg/ml in distilled water, 3 mg/ml in ethanol and practically insoluble in acetone and chloroform.
Preparation
The in situ gel was prepared using ion sensitive polymer gellan gum which undergoes sol-to- gel transition in presence of ions like Ca++, Na++ upon instillation into cul-de-sac of the eye. The gel formed in situ showed pH independent timed drug release for more than 10 h due to the presence of nanoparticles containing moxifloxacin. The system is easy to instill into eye because of its low viscosity at non-physiological condition.
Viscosity
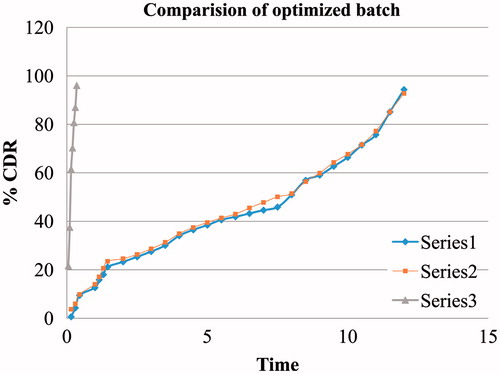
All the formulated systems exhibited linearity in viscosity as shown in . Prepared nanoparticles of moxifloxacin were loaded onto the previously prepared gel which retained its in situ gelling property. Nanoparticles were prepared by solvent diffusion method resulting in nanosuspension using Eudragit RL100 as the main polymer.
Evaluation
Nanosuspension was subjected to freeze dried for gaining free flowing nanoparticles. They were stored for further use, i.e. to disperse into in situ gel system. The optimization of the formulation, with respect to dispersion, was done on the basis of trial and error method, with different grades of Eudragit (Eudragit L100, Eudragit S100, Eudragit RS100 and Eudragit RL100) and solvent (methanol, acetone and ethanol).
They were further evaluated for particle size, PDI and entrapment efficiency. The best batch was found with Eudragit RL100 as polymer and acetone as solvent. Eudragit showed good solubility in acetone and hence, no precipitation was observed upon dispersion.
Optimization
Nanoparticles (NPs), with the optimized formula, were prepared using PVA as a stabilizer and compared with NPs without PVA. Both these batches were compared with a marketed eye drop of moxifloxacin. NPs were larger in size when PVA was used. PVA prevents nanoparticles from adhering to each other (Dengning, Citation2010).
Evaluation of optimized batch
NPs without PVA showed chances of aggregation thereby increasing the size. The surface charges play an important role in keeping the particles apart and if the distance/size is changed, there are all possibilities of particles aggregating. But, this is not supported by zeta potential value. There is minimum difference in zeta potential values. However, we can propose that the particles will not aggregate because of the viscosity of the polymer sol in which they are dispersed. The viscosity is much higher than normal aqueous vehicles and this elevated viscosity will prevent movement of particles within the medium thereby not allowing aggregation. PDI of the particles with PVA and of the trial batches was higher than the NPs prepared without PVA. PDI is the indication of the particulate system whether mono-dispersed or poly-dispersed. The value indicates that the particles were of similar size without variation within a large group of particles for NPs without PVA. Therefore, it can be assumed that PVA produced particles of uneven size may be by improper deposition on the surface of the NPs (Dengning, Citation2010).
Entrapment efficiency of NPs of trial batches when compared with batches of NPs prepared with Eudragit RL 100 and RL 100 with PVA showed a lot of variation. NPs of trial batches showed very less entrapment efficiency, ranging from 10% to 29%, whereas, NPs with PVA + RL 100 and only RL 100 was higher % EE.
The F1 and F2 had higher entrapment efficiency and drug content. The gel formed through gellan gum was immediate and it remained for extended period of time due to the formation of double helix structure of covalent bond. The clarity of the formulation was assessed visually and was found to be clear. The release profile followed zero order kinetics and diffusion controlled release mechanism for 12 h as the polymer Eudragit has property to show timed release (). Overall result of the study indicate that such gel forming system loaded with nanoparticles is an excellent drug carrier for prolonged ophthalmic delivery of moxifloxacin.
Stability study
Stability data recorded over a month period under elevated temperature conditions and showed no changes in the results whatsoever which indicated the formulation to be stable.
Histopathological study
Histopathological study of eye was conducted by exposing eye to the formulation and no irritation was observed in the image of inverted microscope. Results are shown in . Live rabbits eyes were not used for this study because Indian rules are laid down by CPCSEA, which make difficult to do the study.
Conclusion
The formulation of moxifloxacin was found liquid at the formulated pH and formed gel in the presence of mono or divalent cations. The gel formed in situ showed sustained drug release over a period of 10–12 h. The formulations were less viscous before instillation and formed strong gel after instilling it into cul-de-sac. It is thus concluded that by adopting a systematic formulation approach, an optimum point can be reached in the shortest time with minimum efforts to achieve desirable rheological and in vitro release property for in situ gel forming system. Therefore, the combined approach of nanoparticles with in situ gel formation can be used for ophthalmic drug delivery. The optimized formulation of nanoparticles loaded in situ gel was stable, provided sustained release of drug over a period of 12 h so it minimize the frequency of administration. Thus optimized formulation enhanced the patient compliance.
Acknowledgements
The authors express their thanks to Principal, Parul Institute of Pharmacy, Vadodara for providing all materials and necessary facilities to work.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
References
- Andreas Z, Jorg K. (1995). Microspheres and nano particles used in ocular delivery systems. Adv Drug Del Rev 16:61–70
- Balasubramanium J, Kant S, Pandit J. (2003). In vitro and in vivo evaluation of the Gelrite® gellan gum-based ocular delivery system for indomethacin. Acta Pharma 53:251–61. https://doi.org/http://www.ncbi.nlm.nih.gov/pubmed/14769232
- Dengning X. (2010). Preparation of stable nitrendipine nanosuspensions using the precipitation–ultrasonication method for enhancement of dissolution and oral bioavailability. Eur J Pharm 40:325–34
- Guana J. (2011). Optimized preparation of levofloxacin-loaded chitosan nanoparticles by ionotropic gelation. Phy Proc 22:163–9
- Hany MA, Peter Y, Nicholas B. (2009). Preparation of hydrocortisone nanosuspension through a bottom-up nanoprecipitation technique using microfluidic reactors. Int J Pharm 375:107–13
- Hongyi Q. (2007). Development of a poloxamer analogs/carbopol-based in situ gelling and mucoadhesive ophthalmic delivery system for puerarin. Int J Pharm 337:178–87
- Ibrahim MM, Abd-Elgawad AE, Soliman OA, Jablonski MM. (2013a). Nanoparticle-based topical ophthalmic formulations for sustained celecoxib release. J Pharm Sci 102:1036–53
- Ibrahim MM, Abd-Elgawad AE, Soliman OA, Jablonski MM. (2013b). Novel topical ophthalmic formulations for management of glaucoma. Pharm Res 30:2818–31
- Johan C, Katarina E, Roger P, Katarina J. (1998). Rheological evaluation of Gelrite in situ gels for ophthalmic use. Eur J Pharm Sci 6:113–19
- Kassem MA. (2007). Nanosuspension as an ophthalmic delivery system for certain glucocorticoid drugs. Int J Pharm 340:126–33
- Katiyar S, Pandit J, Mondal RS, et al. (2014). In situ gelling dorzolamide loaded chitosan nanoparticles for the treatment of glaucoma. Carbohydr Polym 102:117–24
- Koichi B. (2011). A method for enhancing the ocular penetration of eye drops using nanoparticles of hyrolyzable dye. J Control Release 153:278–87
- Kortejarvi H, Yliperttula M, Dressman JB, et al. (2005). Bio waiver monographs for immediate release solid oral dosage forms: ranitidine hydrochloride. J Pharm Sci 94:1617–25
- Lou J, Hu W, Tian R, et al. (2014). Optimization and evaluation of a thermo responsive ophthalmic in situ gel containing curcumin loaded albumin nanoparticles. Int J Nanomed 9:2517–25
- Mehrdad H, Amir A, Pedram R. (2008). Hydrogel nanoparticles in drug delivery. Adv Drug Del Rev 60:1638–50
- Nagarwal R, Kumar R, Dhanwant M, Pandit JK. (2011). Modified PLA nano in situ gel: a potential ophthalmic drug delivery system. Colloids Surf B Biointerfaces 86:28–35
- Niederer RL, McGhee NJ. (2010). Clinical in vivo confocal microscopy of the human cornea in health and disease. Prog Retinal Eye Res 29:30–58
- O’Brien TP. (2012). Besifloxacin ophthalmic suspension, 0.6%: a novel topical fluoroquinolone for bacterial conjunctivitis. Adv Ther 29:473–90
- Paulsson M, Hagerstrom H, Edsman K. (1999). Rheological studies of the gelation of deacetylated gellan gum (Gelrite) in physiological conditions. Eur J Pharm Sci 9:99–105
- Rathapon A, Suthira T, Asira F, Sukitaya V. (2011). Optimization and evaluation of thermo responsive diclofenac sodium ophthalmic in situ gels. Int J Pharm 411:128–35
- Rosario P. (2002). Eudragit RS100 nano-suspensions for the ophthalmic controlled delivery of ibuprofen. Eur J Pharm Sci 16:53–61
- Singh J, Chhabra G, Pathak K. (2014). Development of acetazolamide-loaded, pH-triggered polymeric nanoparticulate in situ gel for sustained ocular delivery: in vitro. ex vivo evaluation and pharmacodynamic study. Drug Dev Ind Pharm 40:1223–32
- Taís G. (2010). A poloxamer/chitosan in situ forming gel with prolonged retention time for ocular delivery. Eur J Pharm Biopharm 75:186–93
- Tayel SA, El-Nabarawi MA, Tadros MI, Abd-Elsalam WH. (2013). Promising ion-sensitive in situ ocular nanoemulsion gels of terbinafine hydrochloride: design, in vitro characterization and in vivo estimation of the ocular irritation and drug pharmacokinetics in the aqueous humor of rabbits. Int J Pharm 443:293–305
- Wavikar PR, Vavia PR. (2014). Rivastigmine-loaded in situ gelling nanostructured lipid carriers for nose to brain delivery. J Liposome Res 9:1–9
- Xiangrong S. (2008). Dual agents loaded PLGA nanoparticles: systematic study of particle size and drug entrapment efficiency. Eur J Pharm Biopharm 69:445–3