Abstract
The conjugation of tunable peptides or materials with nanocarriers represents a promising approach for drug delivery to tumor cells. In this study, we report the development of a novel liposomal carrier system that exploits the cell surface binding synergism between photo-sensitive peptides (PSPs) and targeting ligands. The positive charges of the lysine residues on the cell-penetrating peptides (CPPs) were temporarily caged by the photolabile-protective groups (PG), thereby forming a PSP. Furthermore, this PSP enhances specific uptake into cancer cells after rapidly uncaging the PG via near-infrared (NIR) light illumination. In the circulatory system, the cell penetrability of PSP was hindered. In contrast, the asparagine–glycine–arginine (NGR) peptide moieties, selectively bind to CD13-positive tumors, were attached to the nanocarrier to facilitate the active accumulation of this liposomal carrier in tumor tissue. The dual-modified liposomes (PSP/NGR-L) were prepared by emulsification method, and the concentrations of DSPE-PEG2000-psCPP and DSPE-PEG5000-NGR in the liposomes were chosen to be 4% and 1% (molar ratio), respectively. The mean particle size of the PSP/NGR-L was about 95 nm, and the drug entrapment efficiency was more than 90%. Cellular uptake results demonstrated that the proposed PSP/NGR-L had an enhancement of cancer cell recognition and specific uptake. Furthermore, the PSP/NGR-L demonstrated a stronger antitumor efficacy in the HT-1080 tumor model in nude mice with the aid of NIR illumination.
Introduction
Many current chemotherapeutic drugs often cause severe side effects because they are similarly cytotoxic to both cancerous and healthy cells. To overcome this challenge, several different types of nanocarriers (e.g., liposomes) have been developed (Matsumura et al., Citation1986; Fazil et al., Citation2015). Liposomes may accumulate in the tumor tissue via a passive targeting mechanism defined as enhanced permeability and retention (EPR) effect (Fang et al., Citation2011). However, in some cases, the EPR effect may not be sufficient to direct the tissue distribution of drugs due to their non-specific tissue-binding characteristics (Kesharwani et al., Citation2012). Moreover, the encapsulated drugs in the liposomes may have difficulty to escape from the interior of the carrier to exert their activity in the target cells sometimes. Thus, a desirable delivery platform is required that displays both tumor specificity and efficient cellular entry.
To achieve such goal, a plethora of stimulus-sensitive nanostructures, which respond to changes in environmental conditions, such as light (Kocer et al., Citation2005), temperature (Bartlett et al., Citation2013), pH (Kale & Torchilin, Citation2007; Sood et al., Citation2014), and enzymatic activity (Shi et al., Citation2012), have been designed and received extensive attention for their unique advantages for target delivery.
Among the aforementioned stimulus, the endogenous stimulus (enzyme or pH) may suffer from some disadvantages, primarily due to the large inter-individual variability in the expression level of these enzymes or the intratumoral pH (Krate et al., Citation2007). Therefore, a general exterior physical stimulus that is independent of the characteristics of the extracellular tumor microenvironment would be favorable. Of these outer triggers, photo-irradiation is quite advantageous due to its non-invasive nature, desirable modulability, and high spatial resolution (Forman et al., Citation2007). However, one disadvantage of photo-irradiation is that the externally applied trigger is restricted to relative superficial tissues, although deep-seated tissues may be reached with the aid of laparoscopy.
To date, a variety of photo-responsive modalities have been utilized, such as switchable cis–trans isomerization (Wan et al., Citation2013), reversible photo-dimerization (Lu et al., Citation2008), and cleavable covalent linking (Mal et al., Citation2003). Compared with the former two approaches, the presence of a specific cleavable linkage between a photolabile-protective group (PG) and the pharmacological agent provides excellent control of the on/off switch via complete rupture. Recently, the irreversible photoactivation of many promising caged biomolecules have been described, including cell-penetrating peptides (CPPs) (Shamay et al., Citation2011), amino acids (Dadon et al., Citation2010), proteins (Hiraoka & Hamachi, Citation2003), and nucleic acids (Shirakawa et al., Citation2000). Such photo-uncaging has been applied in vitro for payload release from nanocarriers upon ultraviolet (UV) light illumination. In one example, we recently reported a nanostructured lipid carrier (NLC) modified with photoresponsive CPP (pCPP-NLC) (Yang et al., 2014a). The cationic residues of CPP were temporarily masked with the UV one-photon excitation (1PE) responsive group to minimize non-specific cellular uptake. Upon UV light illumination, the 1PE group was cleaved, the CPP regained its penetration activity and thus facilitated rapid intracellular delivery of NLC into cells. However, due to its low penetrability and high irritation for tissues, the utilization of UV light for adjustable payload release in vivo has been limited.
Compared with the UV light, near-infrared (NIR) light can deeply penetrate tissues (>10 cm) and is less damaging to cells. With respect to the development of a PG which can be cleaved via NIR two-photon absorption, multiple studies confirmed that the 4,5-dimethoxy-2-nitrobenzyl group is easily cleaved by NIR radiation (a wavelength of 740 nm), and it was recently reported to be applied as an NIR 2PE-excitation-responsive caged compound to control the function of living cells (Zhao et al., Citation2006; Dakin & Li, Citation2007; Neveu et al., Citation2008). Inspired by these studies, in this work, we attempted to build a “NIR light off-on’”switch to control the penetrability of CPP based on the 4,5-dimethoxy-2-nitrobenzyl group.
Although combining EPR effect and photo-triggering release may achieve a desirable modulability and high spatial resolution to some extent, nanocarrier modified with active targeting ligands is still required to enhance its accumulation in the tumor site and hence elevate targeting drug delivery efficiency. This is because the EPR effect may not be able to direct sufficient nanocarriers to the tumor site sometimes. To address such problem, targeting ligands were used to guide more drug carriers to the target sites (Wang et al., Citation2015). CD13 is a widely used tumor vascular targets (Lorenzo et al., Citation2010), and asparagine–glycine–arginine (NGR) peptide motif is specially target to the tumor vascular antigen CD13. Drugs and drug-loaded nanocarrier modified with NGRs can improve their biodistribution and therapeutic effect in tumor sites (Garde et al., Citation2007). Therefore, NGR ligands could be used to modify the surface of nanocarrier to further improve the targeting ability of the nanocarrier to tumor cells highly expressed CD13 in this study.
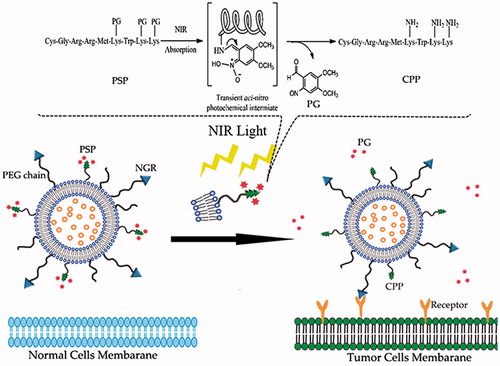
In this paper, a rational strategy was employed to take advantage of synergistic effect of photo-sensitive peptide (PSP) and NGR, to generate a more selective and efficient system for drug delivery to tumor cells. A schematic of the dual-modified liposomal system modified with PSP and NGR is shown in . The PSP includes two parts: CPP (CGRRMKWKK) and PG (1-(bromomethyl)-4,5-dimethoxy-2-nitrobenzene). CPP, derived from penetratin, is a novel CPP that enhances the delivery of molecules across biological barriers to achieve intracellular access (Fischer et al., Citation2000). The positive charges of the lysine residues on the CPP were temporarily masked by PGs, thus forming the PSP. The PSP enhances specific cellular uptake via selective removal of the PGs at the targeted site, which is illuminated by NIR light. In the circulation, the penetration effect of the CPP is hindered, but upon arriving at the targeted tissue, the uncaging of the PSP is triggered by NIR light, causing the non-functional PSP to be converted to an activated CPP and aid the cell penetration of the prepared liposomes.
Figure 1. Schematic illustration of the PSP and NGR co-modified liposomal drug delivery system (PSP/NRG-L). The dual-modified liposomes are retained in the tumor site by the NGR ligand due to the active targeting effect. In the dark, the PSP/NRG-L is inactive, as it cannot penetrate the tumor cell membrane. However, upon illumination using NIR light at the tumor site, the photolabile-protective group is released, the interaction of the liposomes with the cell membrane is restored and the activated liposomes rapidly enter the cells.
Experimental materials
1,2-Distearoyl-sn-glycero-3-phosphoethanolamine-N-methoxy(polyethyleneglycol) (ammonium salt) (DSPE-mPEG2000) and 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-maleimide(polyethylene glycol) (DSPE-PEG2000-MAL or DSPE-PEG5000-MAL) were purchased from Avanti Polar Lipids Inc. (Alabaster, AL). The NGR (CYGGRGNG) and CPP peptide motifs (CGRRMKWKK) were custom-synthesized by Shanghai GL Biochem Co., Ltd. (Shanghai, China). Egg lecithin (EPC) and cholesterol (Chol) were purchased from Lipoid GmbH (Mannheim, Germany). Vinorelbine bitartrate (VB) was provided by Min Sheng Pharmaceutical Co. (Zhejiang, China). Navelbine® (vinorelbine bitartrate injection) was purchased from Pierre Fabre Medicament (Boulogne, France). All chemicals were of reagent grade and were obtained from Sigma-Aldrich (St. Louis, MO), unless otherwise stated.
Human fibrosarcoma cells (HT-1080 cells) and human breast adenocarcinoma cells (MCF-7 cells) were used to evaluate the targeting effect of the constructed liposomes, as these two cell lines have previously been used as CD13-positive (CD13+) and CD13-negative (CD13−) cell models, respectively (Negussie et al., Citation2010). HT-1080 cells and MCF-7 cells purchased from the Cell Resource Centre of IBMS (Beijing, China) were maintained in culture medium consisting of modified eagle’s medium (MEM) and Dulbecco’s modified eagle’s medium (DMEM) supplemented with 10% FBS, 100 IU/mL penicillin, and 100 mg/mL streptomycin. The cells were maintained in a 37 °C humidified incubator in a 5% CO2 atmosphere.
Female BALB/c nude mice (weighing 18–20 g) were purchased from Vital River Laboratories (Beijing, China). All animals were handled according to the code of ethics in research, training and testing of drugs as stated by the Animal Care and Use Ethics Committee of the Academy of Military Medical Sciences.
Methods
Synthesis of the PSP
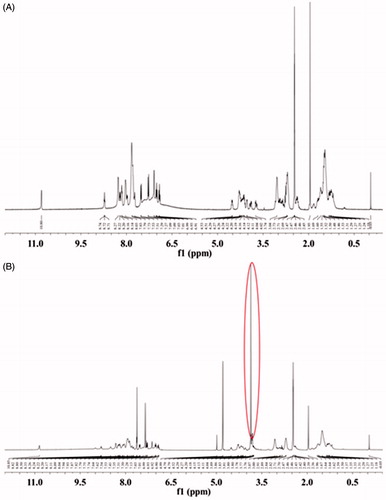
The PSP (primary sequence CGRRMKPGWKPGKPG) was synthesized as described previously (Shigenaga et al., Citation2010), with slight modifications. Briefly, the CPP was synthesized to include 3 Lys side chains orthogonally protected by the 4-methyltrityl (Mtt) group. After synthesis of the fully protected peptide, the Mtt groups were selectively removed by 10 repeated washes with 1% trifluoroacetic acid (TFA) in dry dichloromethane (DCM) for 5 min per wash. The free ɛ-amine of the Lys side chain was reacted with 4,5-dimethoxy-2-nitrobenzene chloroformate, using N-hydroxybenzotriazole (HOBT) as a coupling reagent and DIPEA base in dry DCM containing 1 M LiCl for 4 h and then again for 12 h. The psCPP, bound to the 4,5-dimethoxy-2-nitrobenzyl group at the desired positions, was obtained after cleavage and global deprotection using the common cleavage mixture (95% TFA). The production of the final PSP was confirmed via 1H NMR spectroscopy. CPP and PSP were solubilized in DMSO-D6 and analyzed in a 400 MHz spectrometer (JNM-ECA-400, JEOL Ltd., Tokyo, Japan).
NIR-induced uncaging of the PSP
A solution containing the PSP (400 μM) was prepared in MOPS buffer at pH 7.4 and was illuminated using a NIR pulse laser (λ = 740 nm, 3.48 × 1012 photon s−1). Aliquots (50 μL) were removed at various time points and immediately frozen in the dark until analysis via reverse-phase HPLC (Agilent 1211, Agilent Technologies, Santa Clara, CA) using a linear gradient (1–60% acetonitrile in water containing 0.1% TFA over 33 min).
Synthesis of functional conjugates
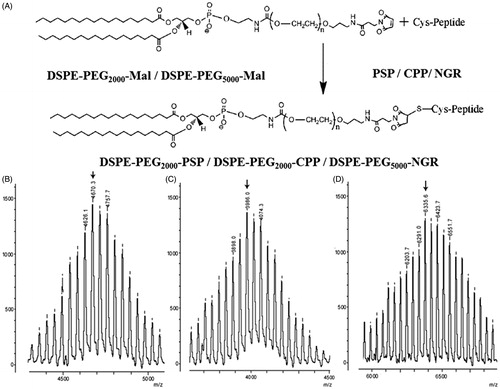
The DSPE-PEG2000-PSP, DSPE-PEG2000-CPP and DSPE-PEG5000-NGR were synthesized as previously described by Gao et al. (Citation2013), with slight modifications. Briefly, the PSP, CPP, or NGR were conjugated with DSPE-PEG2000-MAL or DSPE-PEG5000-MAL (1:1 molar ratio) in DMF containing triethylamine (TEA, 5 eq) at room temperature (20–25 °C). After 48 h of stirring under nitrogen protection, the resulting solution was incubated in l-cysteine (at a concentration 10-fold greater than the molar ratio of the maleimide residues) for 4 h to cap the non-reacted maleimide group. The reaction mixture was dialyzed (molecular weight cutoff (MWCO) of 25 kDa) in distilled water for 48 h to remove the DMF and excess peptides. The final solution was lyophilized and stored at −20 °C for further use. The conjugations were confirmed by determining the molecular weight of the resulting DSPE-PEG2000-PSP, DSPE-PEG2000-CPP, and DSPE-PEG5000-NGR via MALDI-TOF MS.
Preparation of liposomes
To prepare the normal liposomes (N-L) and NGR-modified liposomes (NGR-L), a lipid composition (molar ratio) of EPC:Chol:DSPE-mPEG2000 = 20:10:2 (N-L) and EPC:Chol: DSPE-mPEG2000: DSPE-PEG5000-NGR = 20:10:1:1 (NGR-L) was used, according to a previous report with some modifications (Zhang et al., Citation2011). Briefly, a thin lipid film was formed in a round-bottom flask by dissolving the lipids in chloroform and removing the solvent in a rotary evaporator for 30 min at 40 °C. The film was dried to remove the residual organic solvent. After continued vacuum drying for 24 h at room temperature, hydration of the lipid film was performed using citric acid–sodium citrate buffer (300 mM, pH 4) at 60 °C. During the hydration procedure, a rotator was used (150 rpm) to revolve the round-bottom flask and to facilitate suspension of the lipid film in the hydrating medium. To control the size, the lipid dispersion was extruded 11 times through 100-nm polycarbonate membrane filters using a mini extruder (Avanti, Tonawanda, NY) at 40 °C.
To prepare the CPP-modified liposomes (CPP-L) and PSP/NGR-co-modified liposomes (PSP/NGR-L), the post-insertion technique was used. Briefly, a lipid film of DSPE-PEG2000-PSP or DSPE-PEG2000-CPP was prepared by rotary evaporation and further dried under vacuum for 1 h. The dried lipid film was subsequently hydrated with citric acid–sodium citrate buffer (300 mM, pH 4) to form micelles and incubated for 15 min at 60 °C. For the PSP/NGR-L preparations, 0.25 mL of micelle solution (DSPE-PEG2000-PSP micelles) was added to 1 mL of preformed NGR-L at the required molar ratio (4% DSPE-PEG-peptides of total lipid) and incubated for 4 h at 60 °C in a water bath. For the CPP-L preparation, micelles of DSPE-PEG2000-CPP were incubated with preformed N-L at the above molar ratio and conditions.
All the obtained liposomes (N-L, NGR-L, CPP-L and PSP/NGR-L) were then eluted through a dextran gel (G50) column, using phosphate-buffered saline (200 mM, pH 6.4) as the eluent. As a result, liposomes with a pH gradient between the interior and the extrinsic phase were generated. Next, liposomes containing VB were prepared using the pH gradient method. The VB solution was directly added to each blank liposome suspension and incubated for approximately 30 min.
Characterization of the liposomes
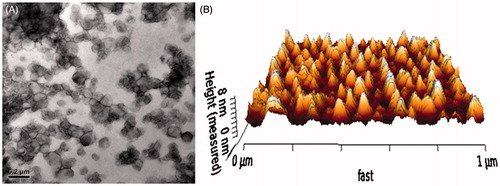
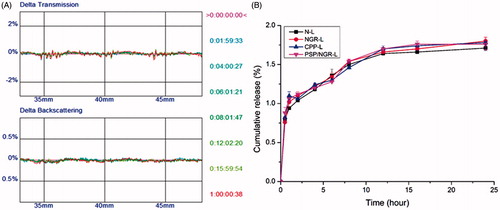
The mean diameter and the zeta-potential of each formulation were determined in three serial measurements using a Malvern Zetasizer Nano ZS90 instrument (Malvern Instruments Ltd., Malvern, UK). The morphology of PSP/NGR-L was observed via transmission electron microscopy (TEM, HITACHI, H-7650, Osaka, Japan) and atomic force microscopy (AFM, NanoWizarc, JPK Ltd., Deutschland, Germany). The stability of PSP/NGR-L in serum (10% FBS) was determined by evaluating the variations of particle size and size distribution using a Turbiscan Lab® Expert analyzer (Formulaction, L'Union, France).
The VB concentration was determined via an HPLC (Agilent 1211, Agilent Technologies, Santa Clara, CA). An Akasil C18 column (4.6 mm × 250 mm, 5 μm, Agela Technologies, Newark, DE) was used at 40 °C. The mobile phase was a mixture of methanol solution containing 0.2% decane sulfonic sodium/0.05 M natrium biphosphoricum water solution (80/20, v/v), at a flow rate of 1 mL/min. The sample injection volumes were 20 μL, and VB detection was performed using a UV detector at a wavelength of 267 nm.
To determine the encapsulation efficiency of the liposomes, the final liposomes were passed through a Sephadex G50 column (Roche, Indianapolis, IN) to remove the free VB. Next, the filtered liposomes were lysed using 10% Triton X-100 (v/v), and the VB concentration was measured via an HPLC as described above. In addition, the same amount of liposomes was treated in the same manner except that they were passed through a Sephadex G50 column (Roche, Indianapolis, IN) to obtain the total concentration of VB. The encapsulation efficiency was estimated by the following formula:
Encapsulation efficiency % = [VB concentration in the filtered liposomes/VB concentration in the un-filtered liposomes] × 100%
In vitro drug release assay
Dialysis was performed to study the in vitro release of VB from the various VB-loaded liposomes. The release medium was diluted in serum (10% FBS in PBS). In brief, the liposomal VB sample (1 mL) was diluted in the medium, then transferred to a dialysis bag (molecular weight cutoff of 12 000–14 000 Da) and dialyzed against 40 mL of the medium with continuous gentle stirring for 24 h at 37 °C. At various time points, 800 μL aliquots were withdrawn from the conical flask for drug analysis, and an equal volume of the medium was added. The leakage of VB was determined via an HPLC as previously described.
Analysis of cellular uptake
Flow cytometry
Approximately 2 × 105 HT-1080 or MCF-7 cells per well were seeded on 6-well plates. After the attachment period, the cells were rinsed with PBS and incubated in different liposomal formulations containing 6-coumarin (COU) with or without pretreatment with NIR light (λ = 740 nm, 3.48 × 1012 photons s−1) for 30 min. The concentration of COU was 40 μg/mL. After incubation in the specified formulation for 4 h at 37 °C, the cells were trypsinized, washed twice with cold PBS, and then immediately analyzed via flow cytometry (BD Biosciences, San Jose, CA).
Confocal laser scanning microscopy (CLSM)
Following the culture of HT-1080 or MCF-7 cells (2 × 105 cells per well) for 24 h on a Petri dish, a free or conjugated liposomal COU (with or without pretreatment for 30 min with NIR light, λ = 740 nm, 3.48 × 1012 photons s−1) was introduced. The final concentration of each COU was 40 μg/mL. Following treatment for 4 h, the medium was removed and discarded, and the cells were washed with cold PBS (0.1 M, pH 7.4) three times, followed by fixation using 4% paraformaldehyde for 20 min. Nuclear staining was performed using Hoechst 33258 for 10 min at ambient temperature. The fluorescent images of the cells were analyzed via CLSM (UltraVIEW Vox, PerkinElmer, San Jose, CA).
In vitro cytotoxicity assay
The cytotoxicity of Navelbine®, various VB-loaded liposomes and free PSP to HT-1080 and MCF-7 cells were measured via the MTT assay. The cells were seeded on a 96-well plate at a density of approximately 4000 cells/well. After incubation for 24 h (37 °C, 5% CO2), the cells were treated with various VB-loaded formulations, PSP or Navelbine® at a range of concentrations and were then exposed to NIR light (λ = 740 nm, 3.48 × 1012 photon s−1, time = 30 min) or maintained in the dark. After the cells were further incubated in the dark for 72 h, 20 μL of MTT solution (5 mg/mL in PBS) was added to each well, and the plates were incubated for another 4 h. Next, the MTT-containing medium in each well was replaced with 200 μL of dimethyl sulfoxide (DMSO), and the mixture was shaken at room temperature to dissolve the reacted dye. The absorbance of each well was measured using a microplate reader (Model 680, BIO-RAD, Hercules, CA) at a wavelength of 570 nm. All samples were evaluated in sextuplicate.
In vivo antitumor efficacy
To assess the antitumor efficacy, a xenograft tumor model was produced via subcutaneous injection of HT-1080 cells as described in our previous report (Yang et al., 2014b). The HT-1080 tumor-xenografted mice were intravenously injected via tail vein with 5% glucose (control), 10 mg/kg of Navelbine®, or various liposomal formulations carrying VB on the 6th, 9th, 12th, and 15th days. Four hours after administration, the group treated with PSP/NGR-L was irradiated with or without an NIR laser (λ = 740 nm, 3.48 × 1012 photon s−1, t = 30 min). The body weight and tumor size were measured every 3 d. The estimated tumor volume was calculated using the formula: volume (mm3) = (length × width2)/2. On the 24th day of tumor inoculation, the animals were sacrificed, and the tumors were weighed and photographed.
Statistical analysis
The data are presented as the means ± standard deviation (SD). The difference between any two groups was determined via ANOVA. p < 0.05 was considered to be statistically significant.
Results and discussion
Synthesis, identification and NIR-induced uncaging of PSP
To achieve conditional, light-regulated cell penetration, the positive Lys residues on the minimal sequence of the penetratin-based CPP required for translocation (CGRRMKWKK) were masked with PGs (4,5-dimethoxy-2-nitrobenzene chloroformate) to minimize non-specific cellular uptake. CPP was modified with excess of PGs to form the PSP, CGRRMKPGWKPGKPG. PSP was verified by 1H NMR. The reference peak of tetramethylsilane (TMS) was found at δ = 0.00 ppm. Compared with the CPP spectrum (), the characteristic 1H NMR peaks of methoxy groups (CH3O) at δ = 3.83 and δ = 3.76 ppm were appeared in the spectrum of PSP (). It indicated that the two-photon excitation-responsive protective groups had reacted with the primary amine groups of CPP and further indicated the successful construction of PSP.
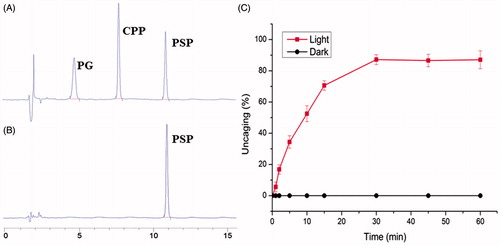
The NIR light-induced uncaging of PSP, used in the dual-modified liposomes (PSP/NGR-L), is the primary step in this study. The rate of PSP uncaging was characterized by exposing this formulation in buffer to NIR light. Aliquots were removed at various time points, and the molar fractions of caged and free CPP were calculated based on the HPLC chromatograms. Consequently, two newly formed peaks appeared in the HPLC chromatograph at approximately 7.9 min (the same retention time as the CPP) and 4.7 min (), which is different from the tested PSP sample (approximately 11.1 min) (). The results indicated the occurrence of specific uncaging, as predicted. As illustrated in , the uncaging of PSP was clearly associated with the illumination time. As the duration of illumination increased, PSP uncaging increased, whereas the extent of PSP uncaging remained unchanged in the dark. When the illumination time was 30 min, approximately 87.28 ± 2.87% of the initial intact PSP construct was uncaged, reflecting that there was sufficient CPP available for cell binding and penetration. Thus, the NIR light exposure time in further experiments was set as 30 min.
Synthesis and identification of functional conjugates
The PSP/NGR-L or CPP-L was developed via modification with the synthesized functional material DSPE-PEG2000-PSP, DSPE-PEG2000-CPP, or DSPE-PEG5000-NGR. Here, we synthesized these compounds via the thiolene “click” reaction of the maleimide group with the sulfhydryl group of PSP, CPP, or NGR (). As shown in , the MALDI-TOF MS results confirmed the successful formation of DSPE-PEG2000-PSP, DSPE-PEG2000-CPP, and DSPE-PEG5000-NGR at observed mass–charge ratios of approximately 4670.3 (, marked by an arrow), 3986.0 (, marked by an arrow), and 6335.6 (, marked by an arrow), which were equivalent to the theoretical mass–charge ratios of 4070.0, 3987.0, and 6336.0, respectively.
Preparation and characterization of liposomes
As shown in , the VB encapsulation efficiency of each liposome was more than 90%, and the modifications of NGR, CPP and/or PSP on the surface of the liposomes did not affect the ultimate encapsulation efficiency. The sizes of N-L, NGR-L, CPP-L, and PSP/NGR-L formulations were between approximately 94.26 and 96.05 nm, which was not significantly affected by the NGR, CPP, and/or PSP modification. All these types of liposomes, except for CPP-L, exhibited a slightly negative charge from approximately −3.34 mV to −6.11 mV. The positive potential of CPP-L (3.11 mV) was provided by the CPP, which contains positively charged head groups. In addition, TEM and AFM images of PSP/NGR-L demonstrated that the particle sizes were similar to those determined using a laser particle analyzer (90–100 nm) ().
Table 1. Characteristics of the liposomes.
The stability of the liposomal particles under physiological conditions is a prerequisite for further applications in vivo, and thus 10% FBS was used to mimic the in vivo conditions. PSP/NGR-L was relatively stable in the presence of 10% FBS, even after 24 h (), which may be due to the steric stabilization of PEG. The stability results based on the Turbiscan Lab® Expert (Formulaction, L'Union, France) measurements were also supported by the in vitro release assay (), which indicated that conjugation of the peptides with N-L did not increase VB leakage during 24 h. Moreover, the delayed drug release of these liposomes would be beneficial for the prevention of rapid leakage during the process of drug delivery, and for the accumulation of drugs in the cancer tissue.
Analysis of cellular uptake via flow cytometry and CLSM
In this study, we evaluated the synergistic effect of the two ligands and confirmed the targeting specificity of the dual-modified liposomes via cellular uptake tests. HT-1080 cells (CD13+) and MCF-7 cells (CD13−) have been widely used and examined for investigations of the tumor vasculature-specific ligand NGR (Negussie et al., Citation2010). Thus, HT-1080 cells and MCF-7 cells were used to evaluate the in vitro targeting properties of the designed peptides to tumor endothelial cells, with or without pretreatment of NIR illumination. The cellular uptake of each liposome formulation was investigated via flow cytometry and CLSM analysis, using an encapsulated COU as a fluorescent marker.
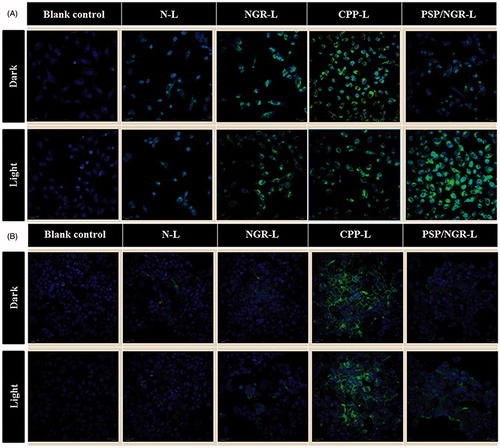
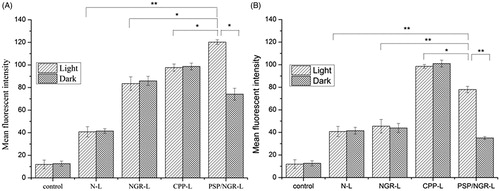
For HT-1080 cells (CD13+), as shown in , a significantly increased mean fluorescence intensity was detected in the cells treated with NGR-L and PSP/NGR-L compared with those treated with N-L, suggesting the role of NGR. In addition, among all the liposomal formulations pretreated with NIR light, PSP/NGR-L displayed the greatest improvement in the cellular uptake of COU. However, the internalization of PSP/NGR-L in the dark was less than that of CPP-L or NGR-L. Taken together, these results indicated that when PSP was cleaved by NIR light and activated to form the CPP, the cellular uptake of PSP/NGR-L was enhanced due to the CPP-mediated cell penetration and receptor-mediated endocytosis. Thus, the synergistic effect of the two ligands on cellular uptake was clear. For MCF-7 cells (CD13−), as shown in , without NIR light illumination, the greatest uptake of COU occurred due to CPP-L treatment, indicating CPP-mediated translocation into cells. In addition, the uptake efficiency of PSP/NGR-L and NGR-L was not ideal. The mean fluorescence intensity of these compounds declined to a level similar to that of N-L. Following pretreatment with NIR light illumination, only PSP/NGR-L displayed a significant improvement in cellular uptake, suggesting the activation of PSP by NIR light. However, N-L, NGR-L and CPP-L were not affected by pretreatment with NIR light. As shown in , the amount of cellular uptake of PSP/NGR-L in MCF-7 cells was much less than in HT-1080 cells, which express substantial CD13 on the cell surface, and the synergistic effect of PSP/NGR-L on cellular uptake varied from cell to cell. When the expression level of CD13 on the cell surface was low (such as in MCF-7 cells), PSP/NGR-L did not efficiently recognize and bind to the target cells via the NGR motif, and thus, the cellular uptake of PSP/NGR-L was primarily mediated by PSP following illumination. These results indicated that only when the expression of CD13 on the cell surface reached a specific level did the synergistic effect between the PSP and NGR motifs emerge (Kibria et al., Citation2011).
Figure 7. Flow cytometry measurement of liposome uptake by CD13+ HT-1080 cells (A) and CD13− MCF-7 cells (B). The data are presented as the means ± SD (n = 3). *p < 0.05; **p < 0.01.
Consistent with the above findings, CLSM analysis also confirmed the significant synergistic effect of PSP/NGR-L on cellular uptake in HT-1080 cells. This finding was clearer than that in MCF-7 cells, thereby strengthening the hypothesis that PSP/NGR-L exhibits increased cellular uptake efficiency and target specificity in vitro. For HT-1080 cells, as shown in , for the groups not pretreated with NIR light, some of the liposomes (NGR-L, CPP-L, and PSP/NGR-L) displayed increased intracellular distribution compared with N-L, which could stem from the effects of their NGR or CPP ligands. However, among all the examined formulations, PSP/NGR-L pretreated with NIR light displayed the highest intracellular fluorescence intensity. Cancer cell recognition using dual-modification was greatly enhanced compared with that of mono-modification and non-modification liposomes. For MCF-7 cells, as shown in , following NIR light illumination, the cellular uptake of PSP/NGR-L was increased but was not clearly increased as in HT-1080 cells. These results corresponded with the results using the CD13− MCF-7 cells and CD13+ HT-1080 cells, revealed that the low level of CD13 expression resulted in reduced internalization.
Taken together, the cellular uptake results strongly supported our hypothesis that the synergistic effect of these two ligands can play a key role in the enhancement of cancer cell recognition and uptake, and the reduction of non-specific cellular uptake.
In vitro cytotoxicity assay
As shown in , the PSP displayed very little toxicity to HT-1080 and MCF-7 cells after 30 min of NIR light illumination or not, which revealed that the PSPs were relatively safe. This finding also indicated that the PG and its decomposition products exerted no significant effect on cell viability. With the increased concentration of VB, the anti-proliferative activity of Navelbine® significantly increased in both HT-1080 and MCF-7 cells, but NGR-L and PSP/NGR-L displayed stronger anti-proliferative activities in HT-1080 cells than MCF-7 cells. In particular, NGR-L and PSP/NGR-L displayed stronger anti-proliferative effects on HT-1080 cells than MCF-7 cells, which were correlated with the expression level of CD13, despite the finding that CPP-L and Navelbine® displayed nearly equivalent anti-proliferative effects on both HT-1080 and MCF-7 cells.
Figure 9. The cytotoxicity of VB-loaded liposomes, Navelbine® and PSP. The data are presented as the means ± SD (n = 6). (A) p < 0.05 versus N-L; (B) p < 0.05 versus NGR-L; (C) p < 0.05 versus CPP-L; (D) p < 0.05 versus Navelbine®.
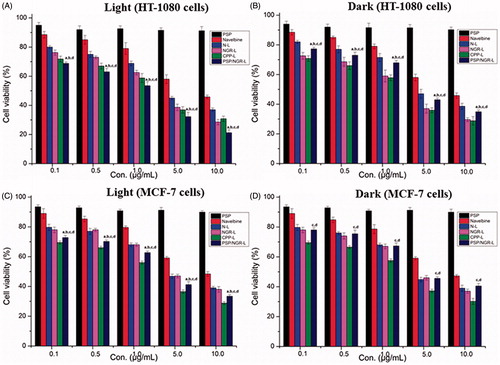
These results demonstrated that the PSP/NGR-L-mediated anti-proliferative activities depended on both the level of CD13 expression and the presence of NIR light. At the same concentration, PSP/NGR-L formulation displayed an increased anti-proliferative effect on HT-1080 and MCF-7 cells following a NIR light illumination. This result clearly showed that removal of PG by NIR light facilitated the restoration of CPP activity in the cells examined. Moreover, with respect to the different cell lines, the anti-proliferative effects were stronger on HT-1080 cells than MCF-7 cells, independent of the presence of NIR light.
In vivo antitumor efficacy
To determine whether the proposal dual-modified liposomal system displays antitumor activity in vivo, the effects of these liposomes on tumor growth inhibition in animals were investigated. As shown in , the tumor volume of mice administered 5% glucose as a control rapidly increased over 24 d, whereas the tumor volume of mice administered Navelbine® or various liposomal formulations was significantly inhibited. After administration, the maximal reduction in tumor growth was noted in the group treated with VB-loaded PSP/NGR-L (with NIR). This was well correlated with the above-mentioned in vitro data. Compared with N-L, encapsulating VB into NGR-L retarded tumor progression greatly. This result revealed that the incorporation of NGRs onto the carrier could enhance the antitumor therapeutic effects in vivo. However, CPP-L possessed a lower antitumor therapeutic effect in vivo than other modified liposomes, which could be attributed to CPP’s lack of selectivity.
Figure 10. The antitumor effects of VB-loaded liposomes on HT-1080 tumor-bearing BALB/C mice. The tumor growth curves of mice receiving different VB-loaded liposomes or Navelbine® (n = 6, mean ± SD) (A). Photographs of the tumors at the end of treatment (B). The weights of the dissected tumors at the end of treatment (n = 6, mean ± SD) (C). Body weight changes in the mice during the treatment (D).
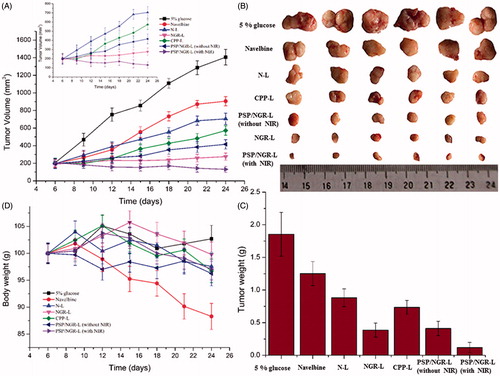
The excised tumors treated with the modified liposomes (NGR-L, CPP-L, and PSP/NGR-L) exhibited a much smaller size and weight, as shown in . This result revealed that the incorporation of either PSP or NGR onto the carrier could enhance the in vivo therapeutic antitumor effects. PSP/NGR-L was more effective in reducing tumor volume than Navelbine® and mono-modified liposomes. The synergistic activity of PSP and NGR was evident in these results. The changes in the body weights of the animals were also recorded as an indication of safety. As shown in , there was no significant change in the body weight of the N-L, NGR-L, CPP-L, PSP/NGR-L (without NIR), and PSP/NGR-L (with NIR) groups during the experimental period. However, greater than 10% weight loss was detected in the Navelbine®-treated group at the end of the experimental period. The weight loss of the Navelbine® group was likely due to effects of non-targeted VB.
These results indicate that the PSP- and NGR-modified liposomal delivery system is more efficient and safe than Navelbine®, which is most likely due to the improvements in the selectivity and efficiency of drug delivery into tumor cells.
Conclusions
In this study, we developed a payload delivery vehicle consisting of modified lipids that displayed enhanced target specificity and delivery efficiency for the tumor, by exploiting the synergistic activities of PSP and NGR. The constructed delivery system, PSP/NGR-L, displayed satisfactory physicochemical properties, such as suitable particle size, high encapsulation efficiency, high stability, and targeting delivery ability. The enhancements in cellular uptake and cytotoxicity of PSP/NGR-L to HT-1080 cells demonstrated the association between NIR light illumination and CD13 receptor expression. Furthermore, PSP/NGR-L could penetrate deeply into multicellular tumor spheroids due to the function of the CPP. Finally, treatment with PSP/NGR-L resulted in stronger antitumor efficacy than mono-modified or non-modified liposomes in the HT-1080 tumor-bearing nude mouse models. Although preliminary, the results of this study demonstrate the tremendous potential of this dual-modified liposome system for the efficient delivery of therapeutic agents for oncotherapy.
Declaration of interest
We are grateful to the financial support from the National Natural Science Foundation of China (Grant nos. 81202466, 81402874 and 81102498) and the Important National Science & Technology Specific Projects (Grant no. 2012ZX09301003-001-009) of China.
References
- Bartlett RL II, Sharma S, Panitch A. (2013). Cell-penetrating peptides released from thermosensitive nanoparticles suppress pro-inflammatory cytokine response by specifically targeting inflamed cartilage explants. Nanomedicine 9:419–27
- Dadon Z, Samiappan M, Safranchik EY, Ashkenasy G. (2010). Light-induced peptide replication controls logic operations in small networks. Chemistry 16:12096–9
- Dakin K, Li WH. (2007). Cell membrane permeable esters of D-myo-inositol 1,4,5-trisphosphate. Cell Calcium 42:291–301
- Fang J, Nakamura H, Maeda H. (2011). The EPR effect: unique features of tumor blood vessels for drug delivery, factors involved, and limitations and augmentation of the effect. Adv Drug Deliv Rev 63:136–51
- Fazil M, Baboota S, Sahni JK, et al. (2015). Bisphosphonates: therapeutics potential and recent advances in drug delivery. Drug Deliv 22:1–9
- Fischer PM, Zhelev NZ, Wang S, et al. (2000). Structure–activity relationship of truncated and substituted analogues of the intracellular delivery vector Penetratin. J Pept Res 55:163–72
- Forman J, Dietrich M, Monroe WT. (2007). Photobiological and thermal effects of photoactivating UVA light doses on cell cultures. Photochem Photobiol Sci 6:649–58
- Garde SV, Forte AJ, Ge M, et al. (2007). Binding and internalization of NGR-peptide-targeted liposomal doxorubicin (TVT-DOX) in CD 13-expressing cells and its antitumor effects. AntiCancer Drugs 18:1189–200
- Gao W, Xiang B, Meng TT, et al. (2013). Chemotherapeutic drug delivery to cancer cells using a combination of folate targeting and tumor microenvironment-sensitive polypeptides. Biomaterials 34:4137–49
- Hiraoka T, Hamachi I. (2003). Caged RNase: photoactivation of the enzyme from perfect off-state by site-specific incorporation of 2-nitrobenzyl moiety. Bioorg Med Chem Lett 13:13–15
- Kale AA, Torchilin VP. (2007). “Smart” drug carriers: PEGylated TATp-modified pH-sensitive liposomes. J Liposome Res 17:197–203
- Kesharwani P, Gajbhiye V, Jain NK. (2012). A review of nanocarriers for the delivery of small interfering RNA. Biomaterials 33:713–50
- Kibria G, Hatakeyama H, Ohga N, et al. (2011). Dual-ligand modification of PEGylated liposomes shows better cell selectivity and efficient gene delivery. J Control Release 153:141–8
- Kocer A, Walko M, Meijberg W, Feringa BL. (2005). A light-actuated nanovalve derived from a channel protein. Science 309:755–8
- Krate F, Abu Ajaj K, Warnecke A. (2007). Anticancer carrier-linked prodrugs in clinical trials. Expert Opin Investig Drugs 16:1037–58
- Lorenzo GC, Buonerba C, Biglietto M, et al. (2010). The therapy of kidney cancer with biomolecular drugs. Cancer Treat Rev 36:S16–S20
- Lu J, Choi E, Tamanoi F, Zink JI. (2008). Light-activated nanoimpeller-controlled drug release in cancer cells. Small 4:421–6
- Neveu P, Aujard L, Benbrahim C, et al. (2008). A caged retinoic acid for one- and two-photon excitation in zebrafish embryos. Angew Chem, Int Ed 47:3744–6
- Mal NK, Fujiwara M, Tanaka Y. (2003). Photocontrolled reversible release of guest molecules from coumarin-modified mesoporous silica. Nature 421:350–3
- Matsumura Y, Maeda H. (1986). A new concept for macromolecular therapeutic in cancer chemotherapy: mechanism of tumoritropic accumulation of proteins and the antitumor agent Smancs. Cancer Res 46:6387–92
- Negussie AH, Miller JL, Reddy G, et al. (2010). Synthesis and in vitro evaluation of cyclic NGR peptide targeted thermally sensitive liposome. J Control Release 143:265–73
- Shamay Y, Adar L, Ashkenasy G, David A. (2011). Light induced drug delivery into cancer cells. Biomaterials 32:1377–86
- Shi NQ, Gao W, Bai X, Qi XR. (2012). Enhancing cellular uptake of activable cell-penetrating peptide-doxorubicin conjugate by enzymatic cleavage. Int J Nanomed 7:1613–21
- Shirakawa I, Chaen S, Bagshaw CR, Sugi H. (2000). Measurement of nucleotide exchange rate constants in single rabbit soleus myofibrils during shortening and lengthening using a fluorescent ATP analog. Biophys J 78:918–26
- Shigenaga A, Yamamoto J, Sumikawa Y, et al. (2010). Development and photo-responsive peptide bond cleavage reaction of two-photon near-infrared excitation-responsive peptide. Tetrahedron Lett 51:2868–71
- Sood N, Bhardwaj A, Mehta S, Mehta A. (2014) Stimuli-responsive hydrogels in drug delivery and tissue engineering. Drug Deliv 21:1–23
- Wan XJ, Liu T, Hu JM, Liu SY. (2013). Photo-degradable, protein-polyelectrolyte complex-coated, mesoporous silica. Macromol Rapid Commun 34:341–7
- Wang Z, Li Z, Zhang D, et al. (2015). Development of etoposide-loaded bovine serum albumin nanosuspensions for parenteral delivery. Drug Deliv 22:79–85
- Yang Y, Yang YF, Xie XY, et al. (2014a). Preparation and characterization of photo-responsive cell-penetrating peptide-mediated nanostructured lipid carrier. J Drug Target 22:891–900
- Yang YF, Yang Y, Xie XY, et al. (2014b). PEGylated liposomes with NGR ligand and heat-activable cell-penetrating peptide-doxorubicin conjugate for tumor-specific therapy. Biomaterials 35:4368–81
- Zhang H, Wang ZY, Gong W, et al. (2011). Development and characteristics of temperature-sensitive liposomes for vinorelbine bitartrate. Int J Pharm 414:56–62
- Zhao J, Gover TD, Muralidharan S, et al. (2006). Caged vanilloid ligands for activation of TRPV1 receptors by 1- and 2-photon excitation. Biochemistry 45:4915–26