 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Objective: To elucidate the transfected effect of albumin ultrasound microbubbles carrying peptide nucleic acids (PNAs) against c-myc gene to the vascular walls and their effect on the intimal proliferation induced by vascular denudation.
Methods: A rabbit iliac artery intimal proliferation model was constructed and PNA against c-myc mRNA was designed and synthesized and was added to albumin solution before ultrasound microbubbles were prepared and encapsulated in matrix of albumin. The ultrasound microbubbles carrying PNA were transfected to intima under ultrasound exposure. The transfected effect was identified by a histochemical method and the expression of c-myc was detected by in situ hybridization. The proliferation of intimal smooth muscle cells was estimated by the expression of proliferative cell nuclear antigen (PCNA) of them. The intimal area and thickness were judged morphologically for intimal hyperplasia.
Results: The ultrasound microbubbles with PNA were successfully prepared and c-myc PNA was transfected to vascular intimal cells. The expression of c-myc and PCNA by intimal vascular smooth muscle cells (vSMCs) was inhibited significantly and the intimal thickness and area were reduced remarkably.
Conclusion: Transfection of c-myc PNA could inhibit proliferartion of vSMCs and intima in the rabbit iliac artery intimal proliferation model and the targeted transfection of albumin ultrasound microbubbles carrying PNA offers a feasible way to facilitate its access to specific cells in vivo and produce bioavailability.
Introduction
Studies on suppression of antisense oligonucleotides on the expression of some oncogenes for the proliferation of vascular smooth muscle cells (vSMCs) progress restrictively because of nuclease digestion with short lifetime and difficulty to enter target cells (Lee et al., Citation2013). Researchers try to modify their molecular structure of these cells to increase their ability to resist nuclease (Grijalvo et al., Citation2014). Peptide nucleic acid (PNA) is a nucleic acid (DNA or RNA) analog in which the sugar phosphate backbone of natural nucleic acid has been replaced by a synthetic peptide backbone formed from N-(2-amino-ethyl)-glycine units and is capable of sequence specific recognition of DNA and RNA obeying the Watson–Crick hydrogen-bonding scheme. It is uncharged and there is no electrostatic repulsion with DNA/RNA and binds complementary DNA and RNA with high affinity and specificity. It is resistant to nuclease and protease and can be connected to the ligand and cotransfected into cells with low cytotoxicity (Nielsen, Citation2010; Bonifazi et al., Citation2012). PNA is mainly used for antisense inhibition of gene expression in the field of drug research and DNA molecular recognition and manipulation (Bai et al., Citation2012). It can be widely used for molecular hybridization, in situ hybridization, mutation analysis detecting pathogen or inherited disease and anti-cancer, anti-viral antisense nucleic acid research and application (Lamla et al., Citation2010; Stender et al., Citation2014). Especially, PNA can replace oligonucleotide to prepare gene array with better stability and specificity and is made of PNA beacon to real-time monitor intracellular RNA expression (Choi et al., Citation2011; An et al., Citation2012; Kam et al., Citation2012). With the deepening of basic research and occurrence of new technologies, PNA will show unparalleled superior performance and broader application prospects. Although antisense PNA has good specificity and stability and is capable of inhibiting or down regulating the expression of target genes in gene replication, transcription and translation processes and thus cure disease, the present problem is the poor cellular uptake of PNA (Gambari, Citation2014). The key point is how to increase intake of antisense PNA by tissues and cells (especially nucleus) and its inhibitory activity.
Substantial progress has been made in the design of new technologies to improve cellular uptake of PNA. Cell-penetrating peptides (CPPs) are part of the most promising strategy. They are 30 short residue synthetic peptides and are grouped into two major classes: the first, requiring chemical linkage with the therapeutic cargos for cellular internalization and the second, involving formation of stable, non-covalent complexes with therapeutic cargos. They can trigger the movement of cargos across the cell membrane into the cytoplasm of cells and improve their intracellular routing, thereby facilitating interactions with the target. The cargos include PNA, plasmid DNA, oligonucleotide, siRNA, proteins, peptides as well as liposomes. Several CPPs have been successfully applied for the delivery of PNA and present several advantages, including rapid delivery of cargoes into cells with very high efficiency and stability in physiological buffers (Heitz et al., Citation2009). However, endosomal entrapment is a major bottleneck in CPP-mediated delivery and cargos that remain entrapped within endosomes cannot display biological activity since they cannot reach their cytosolic targets (Erazo-Oliveras et al., Citation2012). Some researchers tried to chemically modify the PNA by conjugating a lipid domain or photosensitizers or polymer nanoparticle to raise the antisense activity (Shiraishi & Nielsen, Citation2011a,Citationb). Nonetheless, the targeting of PNA to the particular cell or tissue has not been resolved.
With the development of ultrasound contrast technology and manufacture of microbubble contrast agent which can carry gene, ultrasound has been used for therapy as well as diagnosis (Kusumanto et al., Citation2007; Domenici et al., Citation2013; Yuan et al., Citation2013). Contrast agent microbubbles combined with ultrasound-mediated targeted transfection of gene is a new, non-invasive, effective and convenient technique and can be used in the treatment of tumor or cardiovascular disease (Gong et al., Citation2014; Liao et al., Citation2014; Sun et al., Citation2014). Ultrasound-triggered microbubble destruction is considered as a method of target gene therapy for specific organ. The fundamental principle is: (1) The acoustic contrast agent is a good carrier of gene. Compared with viral vector, contrast agent microbubbles have a greater capacity and can carry antisense oligonucleotide, any DNA fragment and the whole chromosome. (2) Contrast agent reduces the ultrasonic cavitation threshold. Ultrasonic irradiation can destroy contrast agent microbubbles in a particular space (focus area) and specific time and produces cavitation and phonochemistry effects, which widen the intercellular space of surrounding target cells (including vascular endothelial cells and histiocytes) and increases membrane permeability and forms a transient hole on the cell surface (sonoporation). The shock wave generated by rupture of microbubbles promotes the gene released from microbubbles into the targeted cells through broken capillaries and intercellular space of endotheliocyte (Zhao & Lu, Citation2007; Sirsi & Borden, Citation2012). Ultrasound microbubbles prepared with albumin have the characteristics of hypotoxicity, low immunogenicity and low invasiveness. The tumor, myocardium, blood vessels and skeletal muscle and other target tissues have been studied using ultrasound-triggered microbubble destruction technology. Ultrasound exposure (USE) enhances transgene expression in vascular cells by up to 10-fold after naked DNA transfection and performing USE in the presence of microbubble contrast agents is associated with approximately 3000-fold increments in transgene expression (Lawrie et al., Citation2000; Lao & Xiu, Citation2007).
c-myc is an immediate early gene induced by multiple cells and growth factors and plays a crucial role in cell growth and proliferation. The activation and increased expression of it is related with induction, proliferation and migration to the intima of vSMCs land intima hyperplasia (Khanna, Citation2004). Therefore, it is reasonable that inhibiting the expression of c-myc could prevent SMC proliferation and intima hyperplasia.
In this study, a rabbit iliac artery intimal proliferation model was constructed. PNA against c-myc mRNA was designed and synthesized. Albumin was used to prepare ultrasound microbubbles. Quantitative PNA was added to albumin solution before ultrasound microbubbles were prepared and encapsulated in matrix of albumin. The ultrasound microbubbles carrying PNA were transfected to intima under ultrasound exposure. The targeted transfection effect and influence on the proliferation of smooth muscle cells and intima hyperplasia were observed.
Materials and methods
Animals
Thirty New Zealand rabbits, male, 2.3–2.5 kg in weight were used in the study and provided by Southern Medical University Animal Center, Guangzhou, Guangdong Province, China. The animal studies were performed after receiving approval of the Institutional Animal Care and Use Committee of Shenzhen Cardiovascular Hospital.
Main reagents and instrument
The oligo-PNA against c-myc mRNA was synthesized by AoKe Biotech Co. Ltd, Beijing, China. Mouse anti-α-actin and anti-PCNA monoclonal antibodies, immunohistochemical reagents were purchased from MaiXin Biotech Co. Ltd, Fuzhou, Fujian Province, China. The probe for c-myc mRNA was synthesized by Applied Biosystems Inc, Foster City, CA. DIG Oligonucleotide Tailing Kit was purchased from Roche, Mannheim, Germany. Bovine serum albumin (BSA) was purchased from XiangBo Biotech, Shenzhen, Guangdong Province, Guangdong Province, China. HRP-Streptavidin, trueblueTM and Orcein were purchased from JingMei Biotech, Shenzhen, China. Perfluoropropane (Halocarbon-218) was supplied by JieRui Co. Ltd, Fushan, Guangdong Province, China. Zeta potential analyzer was produced by Brookhaven Instruments Corporation (Holtsville, NY). Diagnostic ultrasonic generator (PHILIPS-iE33) was a product of PHILIPS, Co Ltd, Bothell, WA. Therapeutic ultrasound Unit (US-700) was made by ITO Co. Ltd, Tokyo, Japan.
Design of oligo-PNA against c-myc mRNA
The cDNA sequences of c-myc proto-oncogene for virus, mouse, rat, rabbit, goat, cat, dog, pig and homo sapien were compared and found to have same base sequence from 133 to 177 position. The oligo-PNA sequence against c-myc mRNA was designed according to the principle of nucleic acid probe and its 5′N-terminal was marked with a biotin molecule.
136
5′-AGC GAG GAT ATC TGG AAG AAA TTC GAG-3′
162
The preparation of ultrasound microbubbles carrying PNA
The whole procedure was carried out under sterile condition. Ten milliliters of BSA (5%, w/v) with sucrose (final concentration: 10%, w/v) were prepared in 50 mL plastic centrifuge tubes and thoroughly mixed with 2 mg PNA and saturated with oxygen and perfluoropropane (flow rate: 6 mL/min) by turns for 10 min (Du et al., Citation2001) and dispersed with ultrasonic generator (180 W, 20 kHz, for 1 min). The microbubbles concentration was adjusted to 0.8–1.8 × 109/mL and PNA content was 0.2 mg/mL. The prepared microbubbles were stored at 4 °C for further use. Some of the microbubbles were taken for morphological observation, count, particle size and surface zeta potential.
Vascular model and ultrasound-targeted transfection
Thirty rabbits were randomly divided into two groups: experimental group (n = 14) and control group (n = 12). The models of vSMCs proliferation and intimal hyperplasia were constructed as follows: All surgical procedures were carried out on the rabbits under intravenous anesthesia (25 mg/kg pentobarbital sodium). The superficial femoral artery was separated. The artery was retrogradely inserted with balloon catheter (diameter of 2.5 mm) to abdominal aorta and the saccule was inflated with a pressure pump (5–8 atm). Then the balloon catheter was slowly pulled back to the arterious incision and the saccule was evacuated. The above process was repeated three times. In the experimental group, the liver was chosen as ultrasound visualization contrast organ and was observed with 2D diagnostic ultrasonic generator soon after intravenously injecting 5 mL of ultrasound microbubbles (containing 1 mg PNA) and a strong resonance of the ultrasound signal was seen. After that, a therapeutic ultrasound treatment (1 MHz, 1.5 w/cm2, 6 min, according to Ling report (Citation2002) with some improvement) was given, followed by a normal liver resonance. In control group, physiologic saline injection with the ultrasound treatment was given after the surgical operation. In addition, each of 5 mL microbubbles without PNA (n = 2) and PNA solution without microbubbles (n = 2) was given for experiment-related controls.
Specimen preparation and patho-examination
All observed animals were sacrificed with 10% potassium chloride (10 mL, quickly intravenously) 1 week after operation (Ji et al., Citation2001) in accordance with institutional guidelines and the iliac arteries were examined pathologically. Intimal hyperplasia of denuded arteries was judged morphometrically. The thickness and area of the proliferative intima for arterious cross-section were directly measured by multifunction color patho-image analysis system. To identify the intimal smooth muscle cells (SMCs) and their proliferation, two monoclonal antibodies to SMC α-actin (1:200 dilution) and to PCNA (1:100 dilution) were used with a labeled streptavidin–biotin method. The non-specific background level of immunostaining was determined in two ways: (1) negative-matched specimens by replacing SMC α-actin antibody or PCNA antibody with PBS without an observed positive reaction; and (2) tunica media SMCs of rabbit normal artery (α-actin-positive) and human poorly differentiated pulmonary adenocarcinoma (PCNA-positive) were chosen as positive-matched specimens. For quantification, 200 intimal SMCs were analyzed and the number of PCNA-positive cells was calculated. For transfection effect, biotin-labeled PNA transfected to denuded arteries was detected with following method: Paraffin-imbedded sections were deparaffinaged by xylene, hydrated in graded ethanol and washed with PBS solution and incubated with diluented HRP-Streptavidin at 37 °C. After washing with 1 × biotin wash solution, the sections were stained with TrueblueTM. The cell nuclei were stained with Orcein. The bluish green products localized in cytoplasm or nucleus were considered as positively reactive cells. For transfection efficiency, 200 intimal cells were analyzed and the percentage of positive cells was calculated. The expression of c-myc was detected with in situ hybridization. The probe for c-myc mRNA was designed according to cDNA sequence homology of different species as follows: 5′-CCCAGC GAG GAT ATC T GG AAG AAA T T C GAGCTG CTG CCC ACC CCG-3′. DIG Oligonucleotide Tailing Kit was used for labeling the probe following the instructions. The quality of the labeled probe was characterized by using the DNA dot blot technique because of its specificity and sensitivity. In situ hybridization was carried out on cross-paraffin sections according to a previously published method (Ji et al., Citation2001). The purple blue products localized in the cytoplasm were considered as positive. Positive SMCs in a 0.25 × 4 mm2 intimal area under high magnification (×400) were counted.
Statistical analysis
Statistical evaluation was done using SPSS 11.0 was a product of IBM Corporation, Armonk, NY. The results were expressed as ± s. The interclass difference used variance analysis. Means were considered significantly different when p < 0.05.
Results
Physical features of ultrasound microbubbles
The prepared microbubbles were spherosome and cystic with very good dispersity and their size were 2–5 μm in diameter (). They were heat-resistant (40 °C, 30 min) and can be stored at 4 °C for 2 weeks without morphological change (fusion or rupture) and the count was not significantly different from neo-prepared microbubbles. The microbubble count was 0.8–1.8 × 109/mL.
Figure 1. (a) The size of prepared microbubbles were 2–5 μm in diameter with very good dispersity. HE 1000×. (b) HRP-Streptavidin and TrueblueTM stain showed positive intimal cells of transfection with antisense oligo-PNA against c-myc. 400×. (c) The intimal thickening with abundant proliferative cells one week after vascular injury. Elastic fiber staining. 40×. (d) Immunohistochemical stain shows majority of cells were positive with the monoclonal antibody SMC actin. 200×. (e) Immunohistochemical stain shows some neointimal cells were positive with antibody PCNA. 200×.
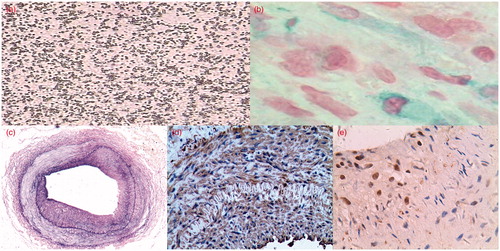
The effect of targeting transfection of PNA
There were no PNA-positive cells found in arterious walls in control group and experiment-related control groups. In the experimental group, PNA-positive cells were mainly distributed in the proliferative intima and a few were found in the tunica media. Two hundred intimal cells were calculated for PNA-positive cells. The average positive count was 67.25 ± 25.10 with 33.63% of transfection rate ().
Pathology, immunohistochemistry and in situ hybridization
One week after injury, the absence of endothelial cells, intimal thickening with abundant proliferative cells and thrombosis could be seen and lumen was narrowed (). Immunohistochemical stain showed that the majority of cells were positive with the monoclonal antibody SMC actin, indicating that they were SMCs (); some cells were positive with antibody PCNA, indicating the proliferative cells (). The intimal thickness, area and the count of c-myc mRNA and PCNA positive cells were shown in . Compared with control group, the intimal thickness, area, PCNA-positive and c-myc mRNA-positive cell count in experimental groups were significantly decreased, indicating that the designed antisense PNA could inhibit arterious intimal SMC proliferation and intimal thickening after de-endothelialization.
Table 1. Intimal thickness, area, PCNA expression and c-myc mRNA 1 week after vascular injury (x ± s).
Discussion
Structurally, albumin microbubbles are comprised of two principal components – an encapsulating shell and an inner gas core. This configuration enables microbubbles to be loaded with drugs or genes for additional therapeutic effect. Application of sufficient ultrasound energy can release this payload, resulting in site-specific delivery. Extensive pre-clinical studies illustrate that combining microbubbles and ultrasound can result in enhanced drug delivery or gene expression at spatially selective sites (Martin & Dayton, Citation2013). In the past, the gene or plasmid was connected with microbubbles after they were prepared and was located on the surface of microbubbles (Ji et al., Citation2012). The combined stability and DNA content is difficult to estimate. The quantity and effect of targeted transfection of gene is difficult to control (Li et al., Citation2007). PNA belongs to peptide structurally and its physical traits are similar to albumin, it is uncharged and water-soluble and there is no physical and chemical effect with albumin. In this experiment, PNA was added to albumin solution before ultrasound microbubbles were prepared. The prepared microbubbles with PNA were spherosome and cystic with very good dispersity and their size were 2–5 μm in diameter, which fit the demand of the experiment. The ultrasound microbubbles were injected intravenously and they fractured instantaneously when they passed through ultrasound exposure region of the damaged blood vessel and promoted release of PNA from encapsulating shell and entering vascular wall (especially the actively proliferative SMC of intima). The antisense PNA against c-myc was targeted transfected successfully at the local vascular wall.
In vitro and in vivo research demonstrated that c-myc is closely correlated with progression of atherosclerosis, restenosis after balloon dilatation or stenting, hypertensive disease and obstructive vascular disease (Wang et al., Citation2011). In a rat experimental study, there was no or tenuous expression of c-myc in normal artery wall without injury. There were two expression peak times of c-myc at the artery wall after balloon injury. The first peak time occurred at 4–6 h and the second occurred at the seventh day after injury, just in accordance with the crest-time of hyperplasia of SMC and intimal thickening (Liang et al., Citation2007). In this study, the designed antisense PNA against c-myc carried by ultrasound microbubbles could not only increase in cellular uptake of PNA, but also make up with the capacity of targeting transfection under ultrasound field, following the reduction of SMCs proliferation and intima hyperplasia. This provides the experimental basis for transgene to prevent multiple cardiovascular diseases. The targeted transfection technique of albumin ultrasound microbubbles carrying PNA offers another feasible way to promote PNA entering the specific cells and educe effect.
Ultrasound-triggered targeted transfection of c-myc PNA, in spite of some advantages, is a new technique in experimental situations. Some problems should be settled before applying it clinically. These include: (1) The cavitation of ultrasound microbubbles may damage cells and tissues while promoting substances into them, including blood capillary rupture, and bleeding, staining substance spills; (2) to optimize the ultrasound exposure time and sound pressure to educe maximum therapeutic effect with minimum side effect; (3) The injection dose of microbubbles carrying PNA should be considered for better transfection effectiveness; (4) Ultrasonic treatment may change the biological activity of the drugs or genes. (5) Superiority of this method carrying PNA should be compared with other foregone methods such as CPPs. (6) How firmly the PNA was bound should be determined, the integrity of the PNA after sonication and stability of PNA should be tested. All of these issues should be thoroughly investigated.
Conclusion
The ultrasound microbubbles with PNA were successfully prepared and c-myc PNA was transfected to vascular intimal cells, which could inhibit proliferation of vSMCs and intima in the rabbit iliac artery intimal proliferation model. Our study offers a feasible way to facilitate the access of PNA to specific cells in vivo and produce bioavailability.
Declaration of interest
The authors report no declarations of interest. This work was supported by Shenzhen Science and Technology Innovation Council (No. JCYJ20130329161430888).
References
- An DJ, Jeong W, Jeoung HY, et al. (2012). Peptide nucleic acid-based (PNA) array for the antigenic discrimination of canine parvovirus. Res Vet Sci 93:515–19
- Bai H, You Y, Yan H, et al. (2012). Antisense inhibition of gene expression and growth in gram-negative bacteria by cell-penetrating peptide conjugates of peptide nucleic acids targeted to rpoD gene. Biomaterials 33:659–67
- Bonifazi D, Carloni LE, Corvaglia V, Delforge A. (2012). Peptide nucleic acids in materials science. Artif DNA PNA XNA 3:112–22
- Choi YJ, Kim HS, Lee SH, et al. (2011). Evaluation of peptide nucleic acid array for the detection of hepatitis B virus mutations associated with antiviral resistance. Arch Virol 156:1517–24
- Domenici F, Giliberti C, Bedini A, et al. (2013). Ultrasound well below the intensity threshold of cavitation can promote efficient uptake of small drug model molecules in fibroblast cells. Drug Deliv 20:285–95
- Du YF, Wan MX, Zhao WM. (2001). Study on the preparation of a new sugar albumin microbubble ultrasound contrast agent. Acta Pharm Sinc 36:859–62
- Erazo-Oliveras A, Muthukrishnan N, Baker R, et al. (2012). Improving the endosomal escape of cell-penetrating peptides and their cargos: strategies and challenges. Pharmaceuticals 5:1177–209
- Gambari R. (2014). Peptide nucleic acids: a review on recent patents and technology transfer. Expert Opin Ther Pat 24:267–94
- Gong Y, Wang Z, Dong G, et al. (2014). Low-intensity focused ultrasound mediated localized drug delivery for liver tumors in rabbits. Drug Deliv. [Epub ahead of print]
- Grijalvo S, Aviñó A, Eritja R. (2014). Oligonucleotide delivery: a patent review (2010–2013). Expert Opin Ther Pat 24:801–19
- Heitz F, Morris MC, Divita G. (2009). Twenty years of cell-penetrating peptides: from molecular mechanisms to therapeutics. Br J Pharmacol 157:195–206
- Ji J, Ji S-Y, Yang J-A, et al. (2012). Ultrasound-targeted transfection of tissue-type plasminogen activator gene carried by albumin nanoparticles to dog myocardium to prevent thrombosis after heart mechanical valve replacement. Int J Nanomed 7:2911–19
- Ji J, Lusheng S, Weihua F, Wenping L. (2001). Interferon-γ inhibits in situ expression of PDGF-β mRNA by smooth muscle cells in injured rabbit arteries after transluminal balloon angioplasty. China Med J 114:139–42
- Kam Y, Rubinstein A, Nissan A, et al. (2012). Detection of endogenous K-ras mRNA in living cells at a single base resolution by a PNA molecular beacon. Mol Pharm 9:685–93
- Khanna A. (2004). Concerted effect of transforming growth factor-beta, cyclin inhibitor p21, and c-myc on smooth muscle cell proliferation. Am J Physiol Heart Circ Physiol 286:H1133–40
- Kusumanto YH, Mulder NH, Dam WA, et al. (2007). Improvement of in vivo transfer of plasmid DNA in muscle: comparison of electroporation versus ultrasound. Drug Deliv 14:273–7
- Lamla M, Seliger H, Kaufmann D. (2010). Differences in uptake, localization, and processing of PNAs modified by COX VIII pre-sequence peptide and by triphenylphoshonium cation into mitochondria of tumor cells. Drug Deliv 17:263–71
- Lao Y, Xiu JC. (2007). Progress of ultrasound microbubble contrast agent with gene and drug targeting therapy. J Med Imaging 17:1359–61
- Lawrie A, Brisken AF, Francis SE, et al. (2000). Microbubble-enhanced ultrasound for vascular gene delivery. Gene Ther 7:2023–7
- Lee RG, Crosby J, Baker BF, et al. (2013). Antisense technology: an emerging platform for cardiovascular disease therapeutics. J Cardiovasc Transl Res 6:969–80
- Li X, He S, Gao Y, Zhang Y. (2007). Study for preparing specific targeted ultrasound contrast agent. Chinese J Ultrasound Med 23:493–5
- Liang CY, Zhou DB, Yu XG, et al. (2007). Expression of c-myc mRNA on early restenosis after carotid endarterectomy. Zhonghua Wai Ke Za Zhi 45:555–7
- Liao AH, Ma WC, Wang CH, Yeh MK. (2014). Penetration depth, concentration and efficiency of transdermal α-arbutin delivery after ultrasound treatment with albumin-shelled microbubbles in mice. Drug Deliv. [Epub ahead of print]
- Ling ZY, Wang Z, Ran HT, et al. (2002). Experiment study on ultrasound-mediated microbubble destruction deliver VEGF gene to ischemic myocardium of rats. Chinese Ultrasound Med 18:502–4
- Martin KH, Dayton PA. (2013). Current status and prospects for microbubbles in ultrasound theranostics. Wiley Interdiscip Rev Nanomed Nanobiotechnol 5:329–45
- Nielsen PE. (2010). Gene targeting and expression modulation by peptide nucleic acids (PNA). Curr Pharm Des 16:3118–23
- Shiraishi T, Nielsen PE. (2011a). Enhanced cellular delivery of cell-penetrating peptide–peptide nucleic acid conjugates by photochemical internalization. Methods Mol Biol 683:391–7
- Shiraishi T, Nielsen PE. (2011b). Improved cellular uptake of antisense peptide nucleic acids by conjugation to a cell-penetrating peptide and a lipid domain. Methods Mol Biol 751:209–21
- Sirsi SR, Borden MA. (2012). Advances in ultrasound mediated gene therapy using microbubble contrast agents. Theranostics 2:1208–22
- Stender H, Williams B, Coull J. (2014). PNA fluorescent in situ hybridization (FISH) for rapid microbiology and cytogenetic analysis. Methods Mol Biol 1050:167–78
- Sun RR, Noble ML, Sun SS, et al. (2014). Development of therapeutic microbubbles for enhancing ultrasound-mediated gene delivery. J Control Release 182:111–20
- Wang J, Liu K, Shen L, et al. (2011). Small interfering RNA to c-myc inhibits vein graft restenosis in a rat vein graft model. J Surg Res 169:e85–91
- Yuan QY, Huang J, Li XJ, et al. (2013). A transendocardial delivery and intracardiac ultrasound irradiation treatment catheter. Drug Deliv 20:252–7
- Zhao YZ, Lu CT. (2007). Recent advances in the applications of ultrasonic microbubbles as gene delivery systems. Yao Xue Xue Bao 42:127–31
