?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Curcumin (CUR), a nontoxic polyphenol derived from the rhizome of turmeric (Curcuma longa), has been recognized as an anti-cancer and chemo-preventative agent. However, its clinical application for cancer treatment has been greatly limited due to its poor water-solubility and low bioavailability. To tackle this problem, Pluronic F68–CUR (F68–CUR) conjugate micelles, which are amphiphilic copolymers, were designed and synthesized in this study. These highly stable micelles with CUR concentrated in the core were formulated using the solvent evaporation method and were confirmed by Fourier transform infrared spectroscopy and nuclear magnetic resonance. Physicochemical characterization of F68–CUR conjugate micelles revealed that high drug loading content (DL%; 0.248 mg CUR/1 mg F68) was achieved, and the average particle size of micelles was 115.2 ± 3.0 nm. Compared with free CUR, a significantly higher cytotoxicity against human breast cancer cell line MDA-MB-231 was observed in F68–CUR conjugate micelles. The IC50 value of F68–CUR conjugate micelles was 1.95-fold lower than that of free CUR, indicating that the anti-cancer activity of CUR was significantly improved in the micelles. Furthermore, apoptotic studies demonstrated that F68–CUR conjugate micelles induced more cell apoptosis than that of free CUR. Taken together, these results demonstrate that F68–CUR conjugate micelles are promising to improve the clinical effectiveness of CUR in cancer treatment.
Introduction
Curcumin (CUR), the main active component derived from the root of spice turmeric (Curcuma longa), is a promising therapeutic agent with a wide range of biological activities, including anti-cancer, anti-inflammatory, anti-angiogenic, anti-microbial, chemosensitization and radiosensitization effects (Gururaj et al., Citation2002; Chen et al., Citation2007; Sandur et al., Citation2007; Agrawal & Mishra, Citation2010; Basniwal et al., Citation2011). The therapeutic effects of CUR on cancer include pro-apoptosis, cell cycle arrest, anti-angiogenesis and suppression of tumor transformation and metastasis (Shishodia et al., Citation2007). These anti-cancer properties of CUR have been demonstrated in various types of cancers, including lung, breast, colon, hepatocellular, melanoma and prostate cancer (Wang et al., Citation2009; Ide, Citation2012; Du et al., Citation2013; Anitha et al., Citation2014; Guo et al., Citation2014; Zhang et al., 2014c). However, the clinical application of CUR to treat various cancers is hindered (Anand et al., Citation2007) because CUR has extremely poor water solubility and low bioavailability due to the rapid metabolism and clearance in humans (Pan et al., Citation1999; Tonnesen et al., Citation2002; Yang et al., Citation2007; Salem et al., Citation2014). To improve stability, water solubility and bioactivity of CUR, a variety of CUR formulations, including nanosuspension (Wei et al., Citation2013), polymeric nanoparticles (Rejinold et al., Citation2011) and nanospheres (Arunraj et al., Citation2014) have been developed. To achieve the enhanced permeability and retention (EPR) effect, CUR has been conjugated with poly(ethylene glycol) (Safavy et al., Citation2007), oligo(ethylene glycol) (Tang et al., Citation2010), methoxy-poly(ethylene glycol)–poly(lactic acid) copolymer (Yang et al., Citation2012) and alignate (Dey & Sreenivasan, Citation2014) to form micelles. However, it is still a big challenge to overcome the low drug loading (DL%) and low transportation rate of polymeric micelles (Tian & Mao, Citation2012).
In recent years, the prodrug delivery system and micellar formulation have been applied in cancer therapy. The prodrug delivery strategies have the ability to overcome the obstacles, such as low water solubility, poor stability, nonselective distribution and severe side effects, for clinical use of hydrophobic drugs (Bildstein et al., Citation2011). Micelles formed by amphiphilic polymers have been successfully used in cancer therapy with desirable benefits, including reduction of rapid renal clearance of drugs, enhancement of permeation and inhibition of multidrug resistance (Tian & Mao, Citation2012; Zhang et al., Citation2014a; Zhang et al., Citation2014b). Recently, polymer–drug conjugate micelles, a novel drug delivery system by combining the prodrug and micellar technologies, have been developed to improve drug water solubility and avoid burst drug release (Hu & Jing, Citation2009; Ediriwickrema & Saltzman, Citation2015). These micelles are made of nontoxic, biodegradable and nonimmunogenic backbones with the EPR effect and property of active tumor targeting (Hu & Jing, Citation2009). In these micelles, drugs are conjugated with polymers to form a hydrophobic core, and the hydrophilic part of polymers encircles the core to make an outer shell with high water solubility (Yokoyama et al., Citation1992). Pluronic block copolymers are a widely used backbone to form micelles that have a core of poly(propylene oxide) (PPO) and a corona of hydrated poly(ethylene oxide) (PEO). These micelles have been utilized to deliver hydrophobic drugs with improved bioavailability and efficacy (Batrakova & Kabanov, Citation2008; Ediriwickrema & Saltzman, Citation2015). Pluronic block copolymers can also improve oral absorption of drugs, further indicating that they are a promising carrier for drug delivery (Kabanov et al., Citation2002). Pluronic F68 (F68; product name: Poloxamer 188) is a Pluronic block copolymer that has been approved by the US Food and Drug Administration for intravenous injection and has been used as an emulsifier in pharmaceutical formulations (Mei et al., Citation2009).
In this study, CUR was conjugated to F68 via an ester linkage. The resulting F68–CUR conjugate had higher DL% and greatly enhanced the stability and water solubility of CUR. It is postulated that F68–CUR stabilized CUR in aqueous medium by self-assembling to micelles with hydrophobic CUR in the core and hydrophilic part in the outer shell. Formation of F68–CUR was confirmed by nuclear magnetic resonance (1H NMR) spectroscopy and Fourier transform infrared spectroscopy (FTIR). The characteristics of F68–CUR conjugate micelles were further assessed by transmission electron microscopy (TEM) and dynamic light scattering (DLS). In addition, we examined the cytotoxicity and pro-apoptotic effect of F68–CUR conjugate micelles on human breast cancer cells MDA-MB-231 by using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenylterazolium bromide (MTT) assay, Hoechst 33342 staining, mitochondrial membrane potential (MMP) analysis, and Annexin V/PI staining assay. We found that F68–CUR conjugate micelles have an ideal particle size, good stability and high DL%. They also greatly induce apoptosis in MDA-MB-231 cells.
Materials and methods
Materials and cell culture
Poloxamer 188 (F68) was purchased from BASF SE (Ludwigshafen, Germany). Succinic anhydride was purchased from AI Chemical Reagent (Chengdu, China). 4-Dimethylaminopyridine (DMAP) and dicyclohexylcarbodiimide (DCC) were purchased from GL Biochem (Shanghai, China). d-α-tocopherol polyethylene glycol 1000 succinate (TPGS) and CUR were purchased from Aladdin Industrial Corporation (Shanghai, China). All chemicals were of analytical grade.
Human breast cancer cells MDA-MB-231 (American Type Culture Collection, USA) were cultured in Dulbecco’s modified Eagle medium/nutrient mixture F-12 ham (Hyclone, Logan, UT) containing 10% (v/v) fetal bovine serum (Invitogen, Burlington, CA) at 37 °C in a 5% CO2 atmosphere. Cells were treated with F68–CUR conjugate micelles, CUR, or blank F68 micelles. Untreated cells were used as control.
Synthesis of F68–CUR
F68–CUR was synthesized by esterification with two main steps: transform the hydroxyl group in F68 to carboxyl group and conjugate F68 and CUR. F68 (0.05 mM), succinic anhydride (0.11 mM), DCC (0.11 mM) and DMAP (0.11 mM) were dissolved in 20 mL dichloromethane under nitrogen and reacted for 24 h with stirring. The reaction product was concentrated by rotary evaporation and precipitated in ice-cold diethyl ether (4 °C) for 24 h. The precipitate F68-COOH was vacuum-dried and collected by centrifugation at 4000 rpm. F68-COOH (0.05 mM), CUR (0.11 mM), DCC (0.11 mM) and DMAP (0.11 mM) were dissolved in 20 mL dimethyl sulfoxide (DMSO) under nitrogen and reacted for 24 h with stirring. The resulting product F68–CUR was purified by filtration. Unbound entities and DMSO were removed by using a dialysis bag with molecular weight cut-off (MWCO) at 3.5 kDa in DMSO and Milli-Q water, respectively, for 48 h. F68–CUR was obtained by lyophilization and stored at 4 °C.
Characterization of F68–CUR
To confirm successful synthesis of F68–CUR (Zhang et al., Citation2014a), 1H NMR spectroscopy and FTIR were used to analyze the structure. F68–CUR was detected by 1H NMR spectroscopy (Bruker, Karlsruhe, Germany) at 400 MHz with the solvent CDCl3. F68 or F68–CUR was mixed with KBr and scanned by using a NEXUS 670 FT-IR spectrophotometer (Nicolet, Hudson, NH) with a spectra range of 400–4000 cm−1.
Preparation of F68–CUR conjugate micelles
F68–CUR conjugate micelles were prepared by using the solvent evaporation method as previously reported (Huang & Luo, Citation2014). The surfactant TPGS was used to improve the morphology and stability of micelles. F68–CUR (10 mg) was dissolved in methane, added dropwise into phosphate buffered saline (PBS) containing 0.03% TPGS and stirred until methane was completely evaporated. Undissolved materials were removed by filtration (0.45 μm Millipore), and F68–CUR conjugate micelles were obtained. Blank F68 micelles were prepared by using the same method.
Characterization of F68–CUR conjugate micelles
F68–CUR conjugate micelles were evaluated for particle size, morphology and DL%. Particle size was determined by DLS at 25 °C with a ZetasizerNano ZSP system (Malvern Instruments, Worcestershire, UK). To observe the morphology of micelles, they were transferred to the carbon-coated copper grids and examined by a high resolution TEM (HRTEM, Tecnai G20, FEI Company, Hillsboro, OR) in the condition of 200 kV. Waters e2695 HPLC with a C18 reverse-phase liquid chromatography column (250 mm ×4.6 mm) was used to detect the CUR concentration in the F68–CUR conjugate micelles at a flow velocity of 1 mL/min and maximum absorption wavelength at 425 nm. The mobile phase was 0.25% acetic acid solution/acetonitrile (40/60, v/v). To break the chemical bond between F68 and CUR, F68–CUR conjugate micelles were mixed with acetonitrile and 10 μL hydrochloric acid. The DL% was obtained by using the following equation (Zheng et al., Citation2009):
The stability of micelles
To study the stability of F68–CUR conjugate micelles, the long-term changes in particle size of micelles were measured by DLS. In brief, F68–CUR conjugate micelles were diluted in PBS to the concentration of 0.5 mg/mL and kept at 4 or 37 °C for seven days (Liu et al., Citation2005). At pre-determined time-points, 1 mL of the solution was taken out for DLS measurement.
In vitro drug release
The dialysis method was used to study the drug release behavior of F68–CUR conjugate micelles. Ethanol was used to dissolve CUR due to its poor water solubility (Zhu, 2015). Thus, CUR dissolved in ethanol was used as control. The release medium was 40 mL PBS containing 1% Tween 80. CUR solution (0.2 mg/mL; 2 mL) or F68–CUR conjugate micelles were added to the dialysis bags with MWCO at 3.5 kDa. The bags were placed in 40 mL release medium and incubated in a shaker at 100 rpm. At pre-determined time-points, 1 mL release medium was taken out, and 1 mL fresh release medium was refilled. The collected release medium was diluted with an equal volume of acetonitrile and centrifuged at 10 000 rpm. HPLC was used to detect the CUR concentrations in the collected release medium. The cumulative drug release of CUR was calculated by the following equations (Zhao & Liu, Citation2014):
Mi represents the drug release amount at the time of i. Ci (mg/mL) represents the CUR concentration in the collected release medium at the time of i.
represents the total amount of CUR in all the collected release medium before the time of j. MA is the initial amount of CUR in the dialysis bag.
Cell viability study
The in vitro cytotoxicity of F68–CUR conjugate micelles on MDA-MB-231 was assessed by the MTT assay (Wang et al., Citation2013). Cells were seeded in a 96-well plate at a density of 5 × 103 cells/well, incubated for 24 h, and treated with F68–CUR conjugate micelles, CUR, or blank F68 micelles at different concentrations ranged from 1 to 8 μg/mL. After incubation for 24 h, the medium was replaced with 20 μL MTT solution (5 μg/mL, Genview) and incubated for another 4 h. DMSO (150 μL) was added to each well to dissolve the formazan crystals. The absorbance at 570 nm, representing cell viability, was measured by a microplate reader (Thermo Labsystems Co. Ltd., Shanghai, China).
Hoechst 33342 staining for nuclear morphology
MDA-MB-231 cells were seeded in 24-well plates at 1 × 104 cells/well and treated with CUR or F68–CUR conjugate micelles at an equivalent CUR concentration of 8 μg/mL for 24 h. Cells were washed, fixed and stained with PBS, paraformaldehyde and Hoechst 33342, respectively. A laser scan confocal microscope (ZEISS LSM700, Waldorf, Germany) with 20× magnification was used to observe nuclear morphological changes in stained cells.
Detection of early apoptotic cells by MMP measurement
MMP was determined by a flow cytometer (FCM, FACSC alibur-sort4-BD, Franklin Lakes, NJ) using the JC-1 apoptosis detection kit (Beyotime Institute of Biotechnology, Shanghai, China). CUR and F68–CUR conjugate micelles at an equivalent CUR concentration of 8 μg/mL were added to six-well plates seeded with 5 × 105 cells/well. Cells were cultivated for 24 h, suspended in incubation buffer containing 0.2% JC-1, incubated at 37 °C in a 5% CO2 atmosphere for 20 min, washed by incubation buffer, and immediately detected by FCM.
Detection of apoptotic cells by the Annexin V/PI staining assay
Apoptotic cells were detected by the Annexin V-FITC apoptosis detection kit (Beyotime Institute of Biotechnology). MDA-MB-231 cells were exposed to F68–CUR conjugate micelles or CUR at different concentrations ranged from 1 to 8 μg/mL for 24 h. Cells were collected and suspended in binding buffer. Annexin V-FITC and propidium iodide (PI) were added to the cell suspension and incubated in dark at room temperature for 15 min. Cells were analyzed by FCM within 1 h (He et al., Citation2013). Annexin V-FITC+/PI− cells were considered as early apoptotic cells, and Annexin V-FITC+/PI+ cells were regarded as late apoptotic cells.
Data analysis
All data were expressed as mean ± SD. Analyses were performed using the SPSS 16.0 statistical software (SPSS Inc., Chicago, IL). Comparisons between groups were analyzed by one-way ANOVA. A p value less than 0.05 was considered statistically significant.
Results and discussion
Synthesis and characterization of F68–CUR
The hydroxyl groups at the end of F68 (PEO) were carboxylated and reacted with the phenolic hydroxyl group of CUR to form F68–CUR. In water, F68–CUR can self-assemble to micelles with a hydrophobic core and a hydrophilic shell (Song et al., Citation2014). shows the synthetic route of F68–CUR. The structure of F68–CUR was validated by 1H NMR (). A strong peak at 3.6 ppm was identified as the –CH2 group in PEO, and the peaks at 1.13, 3.40 and 3.54 ppm belonged to the –CH3, –CH, –CH2 of PPO in F68, respectively (). Moreover, the peaks appeared at 7.6 and 6.5 ppm, 3.95 ppm and 6.93 ppm were attributed to the hydrogen atom of olefin in the benzene ring, the methoxy group in CUR and the phenolic hydroxyl group in CUR, respectively (). Peaks at 7.13, 7.04 and 6.93 ppm belonged to the benzene ring in CUR ().
Figure 1. The synthesis of F68–CUR. Abbreviations: F68, Pluronic F68; CUR, curcumin; PEO, poly(ethylene oxide); PPO, poly(propylene oxide).
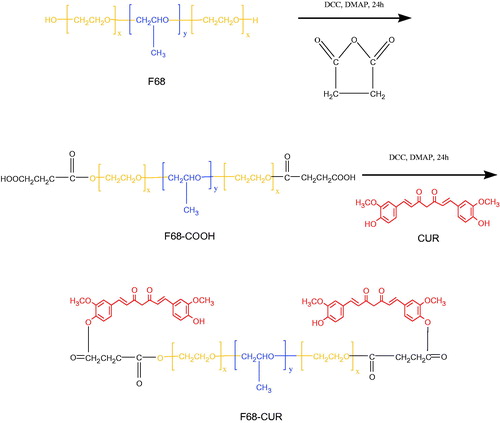
Figure 2. The 1H NMR spectrum of F68 (A) and F68–CUR (B). Abbreviations: F68, Pluronic F68; CUR, curcumin.
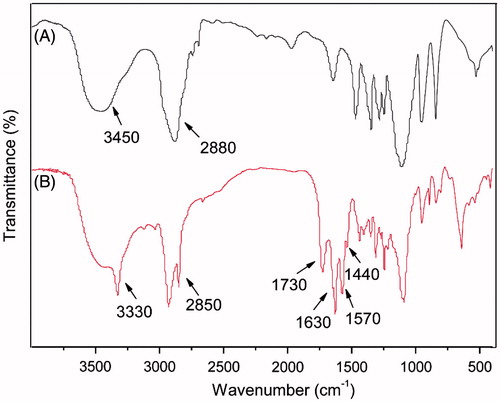
In addition, the F68–CUR structure was confirmed by FTIR (). The stretching vibration peaks at 3450 and 2880 cm−1 were attributed to the –OH and –CH3 groups in F68, respectively (). In the FTIR spectra of F68–CUR, the sharp peak appeared at 3330 cm−1 was the stretching vibration peak of the phenolic hydroxyl group in CUR, and the peak belonged to the –OCH3 group of CUR was shifted to 2850 cm−1 because of the oxygen atom. The peak of the –C = C– group at the edge of the benzene ring in CUR was at 1730 cm−1, and the peak associated with the –C = O group of CUR was at 1630 cm−1. Taken together, these spectra results indicated that F68–CUR was successfully synthesized.
Characterization of F68–CUR conjugate micelles
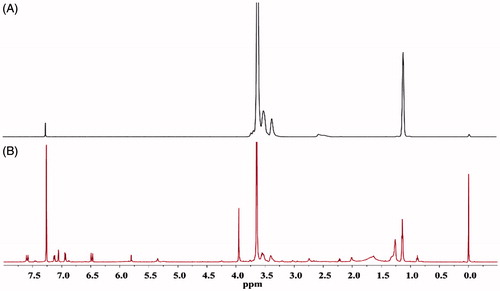
F68 has been used in various nanoformulations as a stabilizer (Ma et al., Citation2013), solubilizer (Saha et al., Citation2013), emulsifier (Shah & Agrawal, Citation2012) and drug carrier (Byagari et al., Citation2014). F68 nanoparticles are stable and enhance the therapeutic effects of the formulated drugs (Byagari et al., Citation2014). To better understand the characteristics of F68–CUR conjugate micelles, their particle size, morphology and DL% were evaluated. The particle size of F68–CUR conjugate micelles was determined by DLS, and their mean diameter was 114 ± 3.5 nm (). It has been reported that polymer micelles with average diameters ranged from 10 to 200 nm have better ability to facilitate drug accumulation in tumors due to the EPR effect (Ke et al., Citation2014). Thus, the conjugation between F68 and CUR stabilizes the micelles by the hydrophobic core and hydrophilic shell structure, and these micelles may have higher accumulation in tumor issues and exert stronger anti-cancer activity compared to free CUR.
Figure 4. DLS analysis revealed the particle size of F68–CUR conjugate micelles with the copolymer concentration at 1 mg/mL (A). The TEM imaged the morphology of F68–CUR conjugate micelles (B).
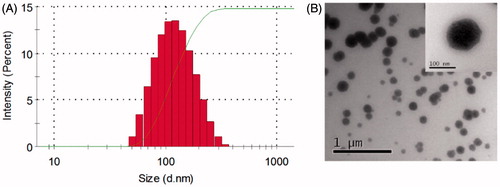
The TEM images revealed that F68–CUR conjugate micelles were uniform in particle size and spherical with smooth edges (), indicating that they are intact and fully enclose the hydrophobic drug CUR. Due to the dehydration effect caused by the sample preparation steps in TEM analysis, the diameter of the micelles in the TEM image was slightly smaller than that of the micelles measured by DLS. Moreover, the HPLC results showed that the DL% of F68–CUR conjugate micelles was 24.80%. Interestingly, the amount of loaded CUR in the micelles was higher than the amount of CUR grafted to F68. This may be due to the encapsulation of CUR in F68–CUR conjugate micelles. Collectively, these results indicated that F68–CUR conjugate micelles significantly increase water solubility of CUR, which may improve the anti-cancer activity of CUR.
The stability of micelles
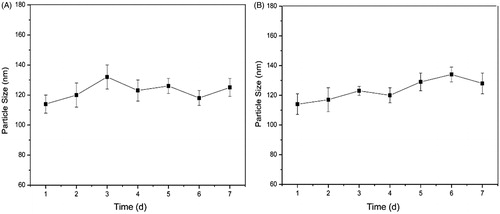
Long-term stability is important for micelles to have consistent pharmacological activities. To evaluate the stability of micelles, the particle size of F68–CUR conjugate micelles was measured during a seven-day storage in PBS at 37 and 4 °C. The particle size ranged from 114 ± 6 to 132 ± 8 nm and from 114 ± 7 to 134 ± 5 nm at 37 and 4 °C, respectively (). The small particle size facilitates micelles to accumulate in tumor tissues but not in normal tissues due to the EPR effect (Fang et al., Citation2011). In addition, CUR was not released from the micelles during storage. Both of these results indicated that F68–CUR conjugate micelles are stable over time without aggregation and dissociation.
In vitro drug release
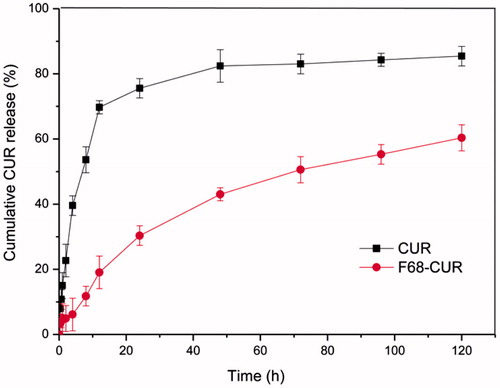
When F68–CUR conjugate micelles enter cancer cells, the conjugates are hydrolyzed to release CUR for anti-cancer therapy. In this study, in vitro drug release studies were conducted on F68–CUR conjugate micelles and CUR to simulate CUR release in cancer cells (). For free CUR, there was a burst drug release in 10 h after dialysis, and the cumulative release of CUR reached 80% of total within 40 h. In contrast, the cumulative release of CUR from F68–CUR conjugate micelles was less than 20% at 12 h and slowly increased to 60% at 120 h. The difference in drug release is due to the formation of micelles that slow down drug diffusion (Muthu et al., Citation2015). This result illustrated that F68–CUR conjugate micelles have sustained and stable release of CUR, which is important to maintain a constant serum concentration of CUR during the treatment period and avoid drug overdose.
Cytotoxicity of F68–CUR conjugate micelles
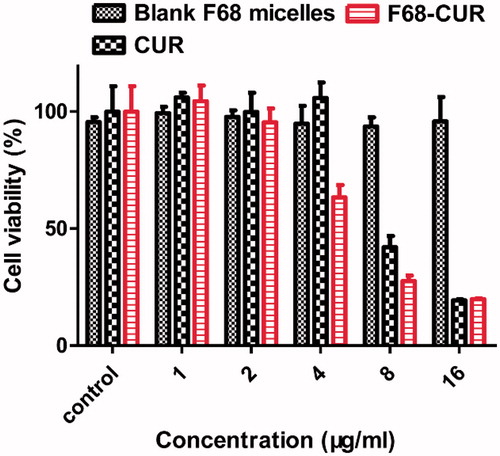
The cytotoxicity of F68–CUR conjugate micelles was evaluated by measuring their half maximal inhibitory concentration (IC50) value in MDA-MB-231 cells. Blank F68 micelles were noncytotoxic as their effects on cell survival rate were similar to those in the control group (). However, CUR and F68–CUR conjugate micelles had strong cytotoxicity against MDA-MB-231 cells (). Cells treated with F68–CUR conjugate micelles at 8 μg/mL had a lower viability rate compared to cells treated with the same concentration of free CUR (). The IC50 value of F68–CUR conjugate micelles was 4.229 μg/mL, which was 1.95-fold lower than that of free CUR (IC50 = 8.27 μg/mL). The greater cytotoxicity of F68–CUR conjugate micelles compared with free CUR may be due to the more stable nanostructure and easier endocytosis of micelles.
Apoptotic cell analysis
It has been reported that CUR is a promising agent to prevent and treat cancer (Shishodia et al., Citation2007) and it can induce apoptosis in MDA-MB-231 cells (Hong et al., Citation2014). In this study, changes in MMP, cell morphology and apoptotic cell population were measured in MDA-MB-231 cells treated with free CUR or F68–CUR conjugate micelles.
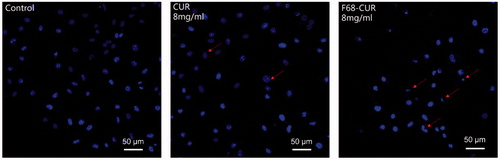
The Hoechst 33342 staining results showed that the morphology of cells treated with CUR or F68–CUR conjugate micelles had a smaller nuclear size and more fragmentary cells compared with control cells (). Notably, cells treated with F68–CUR conjugate micelles had less cell numbers and more fragmentary cells compared to the cells treated with CUR, indicating that F68–CUR conjugate micelles have a higher pro-apoptotic effect than CUR.
Figure 8. Nuclear morphology of MDA-MB-231 cells treated with free CUR or F68–CUR conjugate micelles for 24 h. Untreated cells served as control. Cells were stained with Hoechst 33342 (blue) and imaged with a laser scan confocal microscope. Red arrows indicate apoptotic cells with smaller nuclear size, more fragmentary cells and less cell number.
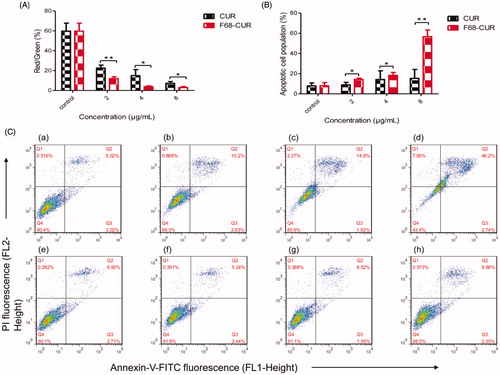
To further study the pro-apoptotic effect of F68–CUR conjugate micelles, MMP was measured in MDA-MB-231 cells. In general, decreased MMP is correlated with cytochrome c release and cell apoptosis (Scarlett et al., Citation2000). We found that the red/green ratio, representing MMP, was decreased in MDA-MB-231 cells treated with CUR or F68–CUR conjugate micelles. At equivalent CUR concentrations, MMP in cells treated with F68–CUR conjugate micelles declined further than that in cells treated with CUR, indicating that F68–CUR conjugate micelles have stronger ability to damage mitochondria and decrease MMP than free CUR, leading to early apoptosis of cells.
In addition, Annexin V/PI staining was used to detect early and late apoptotic cells. Annexin V is a sensitive index for apoptosis and binds to the phosphatidylserine in early apoptotic cell membrane with high affinity. Meanwhile, PI has transmembrane ability and can detect late apoptotic and necrotic cells (Wang et al., Citation2012). In our study, few apoptotic MDA-MB-231 cells were identified in the control group, but both F68–CUR conjugate micelles and CUR induced cell apoptosis in MDA-MB-231 cells in a concentration-dependent manner. Notably, treatment with F68–CUR conjugate micelles at 8 μg/mL markedly increased the population of apoptotic cells to 56.63%, whereas the apoptotic cell percentage was 3.70-fold lower in the 8 μg/mL free CUR group. Therefore, F68–CUR conjugate micelles have a stronger pro-apoptotic effect than free CUR ().
Figure 9. MMP was decreased in MDA-MB-231 cells treated with F68–CUR conjugate micelles or CUR at concentrations ranged from 2 to 8 μg/mL for 24 h (A). Annexin V/PI staining followed by FCM analysis was used to detect early (B) and late (C) apoptosis in MDA-MB-231 cells treated with F68–CUR conjugate micelles or CUR. FCM analysis detected apoptosis in control cells (C (a), (e)) and cells treated with F68–CUR conjugate micelles (C (b), (c), (d)) or free CUR (C (f), (g), (h)) for 24 h at equivalent CUR concentrations of 2, 4, 8 μg/mL.
Conclusion
In this study, F68–CUR conjugate micelles were prepared by the esterification and solvent evaporation method. These micelles have an optimal particle size and high DL%, leading to an EPR effect. The in vitro drug release and stability studies showed that the micelles have the excellent sustained release behavior and have great physical stability. The cytotoxicity of F68–CUR conjugate micelles on MDA-MB-231 cells was higher than that of free CUR, and blank F68 micelles have no cytotoxicity. The apoptotic cell analyses demonstrated that F68–CUR conjugate micelles enhance the pro-apoptotic effect of CUR on MDA-MB-231 cells. Hence, F68–CUR conjugate micelles have higher solubility and stability than free CUR and are a promising drug delivery system to improve the clinical application of CUR against cancer.
Declaration of interest
This study was supported by the Macao Science and Technology Development Fund (062/2013/A2) and the Research Fund of the University of Macau (MYRG2014-00033-ICMS-QRCM, MYRG2014-00051-ICMS-QRCM, MRG004/CMW/2014/ICMS), and the National Natural Science Foundation of China (81403120).
References
- Agrawal DK, Mishra PK. (2010). Curcumin and its analogues: potential anticancer agents. Med Res Rev 30:818–60
- Anand P, Kunnumakkara AB, Newman RA, et al. (2007). Bioavailability of curcumin: problems and promises. Mol Pharm 4:807–18
- Anitha A, Deepa N, Chennazhi KP, et al. (2014). Combinatorial anticancer effects of curcumin and 5-fluorouracil loaded thiolated chitosan nanoparticles towards colon cancer treatment. BBA Gen Subjects 1840:2730–43
- Arunraj TR, Rejinold NS, Mangalathillam S, et al. (2014). Synthesis, characterization and biological activities of curcumin nanospheres. J Biomed Nanotechnol 10:238–50
- Batrakova EV, Kabanov AV. (2008). Pluronic block copolymers: evolution of drug delivery concept from inert nanocarriers to biological response modifiers. J Control Release 130:98–106
- Basniwal RK, Buttar HS, Jain VK, Jain N (2011). Curcumin nanoparticles: preparation, characterization, and antimicrobial study. J Agric Food Chem 59:2056–61
- Bildstein L, Dubernet C, Couvreur P. (2011). Prodrug-based intracellular delivery of anticancer agents. Adv Drug Deliv Rev 63:3–23
- Byagari K, Shanavas A, Rengan AK, et al. (2014). Biocompatible amphiphilic pentablock copolymeric nanoparticles for anti-cancer drug delivery. J Biomed Nanotechnol 10:109–19
- Chen X, Zhou ZW, Xue CC, et al. (2007). Role of P-glycoprotein in restricting the brain penetration of tanshinone IIA, a major active constituent from the root of Salvia miltiorrhiza Bunge, across the blood–brain barrier. Xenobiotica 37:635–78
- Dey S, Sreenivasan K. (2014). Conjugation of curcumin onto alginate enhances aqueous solubility and stability of curcumin. Carbohyd Polym 99:499–507
- Du Q, Hu B, An HM, et al. (2013). Synergistic anticancer effects of curcumin and resveratrol in Hepa1–6 hepatocellular carcinoma cells. Oncol Rep 29:1851–8
- Ediriwickrema A, Saltzman WM. (2015). Nanotherapy for cancer: targeting and multifunctionality in the future of cancer therapies. ACS Biomater Sci Eng 1:64–78
- Fang J, Nakamura H, Maeda H. (2011). The EPR effect: unique features of tumor blood vessels for drug delivery, factors involved, and limitations and augmentation of the effect. Adv Drug Deliv Rev 63:136–51
- Guo QF, Li SL, Yang Y, et al. (2014). Enhanced 4T1 breast carcinoma anticancer activity by co-delivery of doxorubicin and curcumin with core-shell drug-carrier based on heparin modified poly(l-lactide) grafted polyethylenimine cationic nanoparticles. J Biomed Nanotechnol 10:227–37
- Gururaj AE, Belakavadi M, Venkatesh DA, et al. (2002). Molecular mechanisms of anti-angiogenic effect of curcumin. Biochem Biophys Res Commun 297:934–42
- He YW, Wang HS, Zeng J, et al. (2013). Sodium butyrate inhibits interferon-gamma induced indoleamine 2,3-dioxygenase expression via STAT1 in nasopharyngeal carcinoma cells. Life Sci 93:509–15
- Hong R, Wu YQ, Wu Y. (2014). Effect of curcumin in inducing apoptosis of MDA-MB-213 cells by activating endoplasmic reticulum stress. Zhongguo Zhong Yao Za Zhi 39:1495–8
- Hu XL, Jing XB. (2009). Biodegradable amphiphilic polymer–drug conjugate micelles. Expert Opin Drug Deliv 6:1079–90
- Huang YQ, Luo ZY. (2014). Preparation and characterizations of a novel copolymer based on an emulsion solvent evaporation method. Mater Technol 48:355–9
- Ide H. (2012). Prostate cancer and curcumin: current application and future prospect. Int J Urol 19:SI59
- Kabanov AV, Batrakova EV, Alakhov VY. (2002). Pluronic (R) block copolymers as novel polymer therapeutics for drug and gene delivery. J Control Release 82:189–212
- Ke XY, Ng VWL, Gao SJ, et al. (2014). Co-delivery of thioridazine and doxorubicin using polymeric micelles for targeting both cancer cells and cancer stem cells. Biomaterials 35:1096–108
- Liu JB, Zeng FQ, Allen C. (2005). Influence of serum protein on polycarbonate-based copolymer micelles as a delivery system for a hydrophobic anti-cancer agent. J Control Release 103:481–97
- Ma WC, Zhang Q, Li H, et al. (2013). Development of intravenous lipid emulsion of alpha-asarone with significantly improved safety and enhanced efficacy. Int J Pharm 450:21–30
- Mei L, Sun HF, Song CX. (2009). Local delivery of modified paclitaxel-loaded poly(epsilon-caprolactone)/Pluronic F68 nanoparticles for long-term inhibition of hyperplasia. J Pharm Sci 98:2040–50
- Muthu MS, Kutty RV, Luo Z, et al. (2015). Theranostic vitamin E TPGS micelles of transferrin conjugation for targeted co-delivery of docetaxel and ultra bright gold nanoclusters. Biomaterials 39:234–48
- Pan MH, Huang TM, Lin JK. (1999). Biotransformation of curcumin through reduction and glucuronidation in mice. Drug Metab Dispos 27:486–94
- Rejinold NS, Muthunarayanan M, Divyarani VV, et al. (2011). Curcumin-loaded biocompatible thermoresponsive polymeric nanoparticles for cancer drug delivery. J Colloid Interface Sci 360:39–51
- Safavy A, Raisch KP, Mantena S, et al. (2007). Design and development of water-soluble curcumin conjugates as potential anticancer agents. J Med Chem 50:6284–8
- Saha SC, Patel D, Rahman S, et al. (2013). Physicochemical characterization, solubilization, and stabilization of 9-nitrocamptothecin using pluronic block copolymers. J Pharm Sci 102:3653–65
- Salem M, Rohani S, Gillies ER. (2014). Curcumin, a promising anti-cancer therapeutic: a review of its chemical properties, bioactivity and approaches to cancer cell delivery. RSC Adv 4:10815–29
- Sandur SK, Ichikawa H, Pandey MK, et al. (2007). Role of pro-oxidants and antioxidants in the anti-inflammatory and apoptotic effects of curcumin (diferuloylmethane). Free Rad Biol Med 43:568–80
- Scarlett JL, Sheard PW, Hughes G, et al. (2000). Changes in mitochondrial membrane potential during staurosporine-induced apoptosis in Jurkat cells. FEBS Lett 475:267–72
- Shah M, Agrawal Y. (2012). Ciprofloxacin hydrochloride-loaded glyceryl monostearate nanoparticle: factorial design of Lutrol F68 and Phospholipon 90G. J Microencapsul 29:331–43
- Shishodia S, Chaturvedi MM, Aggarwal BB. (2007). Role of curcumin in cancer therapy. Curr Prob Cancer 31:243–305
- Song YZ, Tian QJ, Huang ZJ, et al. (2014). Self-assembled micelles of novel amphiphilic copolymer cholesterol-coupled F68 containing cabazitaxel as a drug delivery system. Int J Nanomed 9:2307–17
- Tang HD, Murphy CJ, Zhang B, et al. (2010). Amphiphilic curcumin conjugate-forming nanoparticles as anticancer prodrug and drug carriers: in vitro and in vivo effects. Nanomedicine (Lond) 5:855–65
- Tian Y, Mao SR. (2012). Amphiphilic polymeric micelles as the nanocarrier for peroral delivery of poorly soluble anticancer drugs. Expert Opin Drug Deliv 9:687–700
- Tonnesen HH, Masson M, Loftsson T. (2002). Studies of curcumin and curcuminoids. XXVII. Cyclodextrin complexation: solubility, chemical and photochemical stability. Int J Pharm 244:127–35
- Wang SP, Chen TK, Chen RE, et al. (2012). Emodin loaded solid lipid nanoparticles: preparation, characterization and antitumor activity studies. Int J Pharm 430:238–46
- Wang L, Shen Y, Song R, et al. (2009). An anticancer effect of curcumin mediated by down-regulating phosphatase of regenerating liver-3 expression on highly metastatic melanoma cells. Mol Pharmacol 76:1238–45
- Wang SP, Zhong ZF, Wan JB, et al. (2013). Oridonin induces apoptosis, inhibits migration and invasion on highly-metastatic human breast cancer cells. Am J Chin Med 41:177–96
- Wei XL, Han YR, Quan LH, et al. (2013). Oily nanosuspension for long-acting intramuscular delivery of curcumin didecanoate prodrug: preparation, characterization and in vivo evaluation. Eur J Pharm Sci 49:286–93
- Yang KY, Lin LC, Tseng TY, et al. (2007). Oral bioavailability of curcumin in rat and the herbal analysis from Curcuma longa by LC–MS/MS. J Chromatogr B 853:183–9
- Yang RL, Zhang SA, Kong DL, et al. (2012). Biodegradable polymer–curcumin conjugate micelles enhance the loading and delivery of low-potency curcumin. Pharm Res 29:3512–25
- Yokoyama M, Kwon GS, Okano T, et al. (1992). Preparation of micelle-forming polymer drug conjugates. Bioconjugate Chem 3:295–301
- Zhang J, Li Y, Fang X, et al. (2014a). TPGS-g-PLGA/Pluronic F68 mixed micelles for tanshinone IIA delivery in cancer therapy. Int J Pharm 476:185–98
- Zhang JM, Li YB, Gao W, et al. (2014b). Andrographolide-loaded PLGA–PEG–PLGA micelles to improve its bioavailability and anticancer efficacy. Expert Opin Drug Deliv 11:1367–80
- Zhang W, Bai W, Zhang W. (2014c). MiR-21 suppresses the anticancer activities of curcumin by targeting PTEN gene in human non-small cell lung cancer A549 cells. Clin Transl Oncol 16:708–13
- Zhao X, Liu P. (2014). Reduction-responsive core-shell-corona micelles based on triblock copolymer: novel synthetic strategy, characterization, and application as tumor microenvironment-responsive drug delivery system. ACS Appl Mater Interfaces 7:166–174
- Zheng DH, Li XL, Xu HE, et al. (2009). Study on docetaxel-loaded nanoparticles with high antitumor efficacy against malignant melanoma. Acta Biochem Biophys Sin 41:578–87
- Zhu W., Z. Song, P. Wei, et al. (2015). Y-shaped biotin-conjugated poly (ethylene glycol)-poly (epsilon-caprolactone) copolymer for the targeted delivery of curcumin. J Colloid Interface Sci 443:1–7