?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
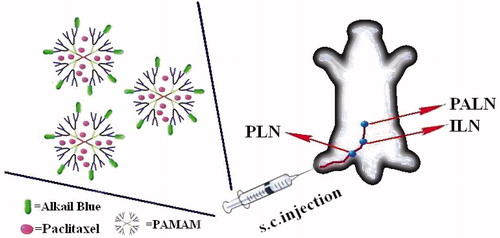
In this study, paclitaxel (PTX)-loaded polyamidoamin-alkali blue (PTX-P-AB) was prepared in order to investigate the intralymphatic targeting ability and anti-cancer effect after subcutaneous (s.c.) administration. The physicochemical properties and in vitro drug release were evaluated. The lymphatic drainage and lymph nodes (LNs) uptake were examined by pharmacokinetics and distribution recovery of PTX in plasma, LNs, injection site (IS) and tissues after s.c. injection in healthy mice and in tumor-bearing mice. The osmotic pressure of PTX-P-AB affecting the lymphatic targeting was studied. The anti-tumor activity of PTX-P-AB was investigated in mice bearing S180 metastatic tumors. Results showed that PTX-P-AB with suitable and stable physicochemical properties could be used for in vivo lymphatic studies, and displayed the more rapid lymphatic absorption, the higher AUC value in LNs, the longer LNs residence time and the higher metastasis-inhibiting rate compared with Taxol®. Enhanced lymphatic drainage from the IS and uptake into lymph by increasing the osmotic pressure of PTX-P-AB indicated that PTX-P-AB possesses the double function of lymphatic tracing and lymphatic targeting, and suggested the potential for the development of lymphatic targeting vectors and the lymphatic tracer for treatment and diagnosis.
Introduction
The lymphatic system is a major conduit for the proliferation and spread of metastatic cancer (Kodama et al., Citation1979; Benedetti-Pancini et al., Citation1996; Dowlatshahi et al., Citation1997). Traditionally, lymph-resident diseases have been treated indirectly, via drug delivery to the systemic circulation, equilibration into the extracellular space and subsequent drainage from the extracellular space into the lymphatic capillaries (Kaminskas & Porter, Citation2011). The importance of tumor lymphatics in disease outcome for cancer patients is underlined by the major prognostic role played by the presence of lymph node (LN) metastases for many different types of tumor (Leong et al., Citation2006). Consequently, more attention paid to lymphatic targeted delivery strategies became the potential urgency. Lymphatic targeted delivery increased drug concentrations directly at the site of pathogenesis, and then retained broader systemic exposure via the eventual drainage of lymph into the vasculature (Nishioka & Yoshino, Citation2001; Xie et al., Citation2009).
The twentieth century has witnessed impressive innovations in polymer synthesis and advances in the design of biodegradable chemistries. The first reports on dendrimers synthesis are attributed to Vogtle group (Buhleier et al., Citation1978) in the late 1970s, followed by the work of Tomalia et al. (Citation1985) in the early 1980s. Dendrimers with a number of synthetic scaffolds have previously been shown to promote passive tumor targeting via the enhanced permeation and retention effect (Tomalia et al., Citation2007; Lei et al., Citation2008; Lim et al., Citation2009). Due to similarities in size and highly branched, monodisperse three dimensional globular structure in nanometer scale, dendrimers have, in several instances, been referred to as “artificial proteins” (Tam & Spetzler, Citation2001; Chen et al., Citation2003). Consequently, dendrimers have the potential to exhibit similar physicochemical properties to proteins but to show improved stability to conditions that would otherwise promote rapid and uncontrolled degradation. Multifunctional dendrimer surfaces are amenable to a wide range of chemical modifications, and the interior is characterized by the availability of a substantial amount of solvent-filled void space that might be suitable for host–guest chemistry. Dendrimers are multivalent owing to the presence of high multiplicities of reactive surface ending groups, making them ideal drug carriers with higher drug payload capacities (Yang & Lopina, Citation2007). Recently, studies have demonstrated the utility of dendrimers as therapeutic and imaging agents and reported good lymphatic uptake of draining lymphatics, making them ideal candidates as lymph targeted delivery systems (Kobayashi et al., Citation2001, Citation2004, Citation2007; Mounzer et al., Citation2007).
A novel polyamidoamin-alkali blue (PAMAM-AB) dendrimer was synthesized by bonding the carboxyl ending groups of PAMAM-G3.5-COOH and amino groups of alkali blue (AB) by a N,N′-dicyclohexylcarbodiimide (DCC) coupling reaction in our previous study (Yang et al., Citationin press). UV–vis, FT-IR, NMR and HPLC characterization were performed to prove the successful synthesis of PAMAM-AB. The calculated AB payload of PAMAM-AB conjugate was seven per dendrimer molecule (27.16% by weight). The dark-blue PAMAM-AB could obviously blue-stain right popliteral lymph nodes (PLNs), iliac lymph nodes (ILNs) and paraaortic lymph nodes (PALNs) within 10 min after subcutaneous (s.c.) administration in mice, indicating the potential application as a lymphatic tracer for sentinel lymph node biopsy, which was introduced for the management of cutaneous melanoma patients without clinical evidence of draining lymph node metastases (Morton et al., Citation1992; Alex et al., Citation1993).
In this study, paclitaxel (PTX) as model drug was loaded in PAMAM-AB, and this study aims at (1) preparing PTX-loaded PAMAM-AB (PTX-P-AB) and studying physicochemical characterizations; (2) establishing mimic s.c. and lymphatic in vitro release system and investigating in vitro release behavior; (3) examining lymphatic drainage and LN uptake; (4) evaluating the influence of the osmotic pressure on lymphatic targeting and (5) studying on lymphatic distribution and anti-cancer effect ().
Materials and methods
Materials
PAMAM-G3.5-COOH with molecular weight 11 520 g/mol (64 carboxyl end groups) was purchased from Weihai CY Dendrimer Technology Co., Ltd. (Weihai, China). AB was supplied by the Shanghai Sanaisi Agent Company (Shanghai, China). N,N′-Dicyclohexylcarbodiimide (DCC), 4-dimethylaminopyridine and dimethyl sulfoxide were purchased from Sinopharm Chemical Reagent Co., Ltd. (Beijing, China). PTX, the purity of which was over 99.5%, was purchased from Shenyang Pharmaceutical University Pharmaceutical Co., Ltd. (Shenyang, China). Taxo1® was supplied by Bristol-Myers Squibb Co., New York, NY. Other reagents of HPLC grade were obtained from Dikma Technologies Inc. (Beijing, China).
Animals
Kunming mice (male, 20 ± 2 g) were purchased from the Experimental Animal Center, Shenyang Pharmaceutical University (Shenyang, China). The mice were housed in a room temperature and humidity, and had access to water and food ad libitum. Animals were kept under fasting overnight prior to the experiment. All animal experiments were carried out according to guidelines “Principles of Laboratory Animal Care” (NIH publication #85-23, revised in 1985) and approved by the Animal Ethics Committee of Shenyang Pharmaceutical University.
Preparation and characterization of PTX-P-AB
PAMAM-AB was synthesized by the acylation method which was mentioned according to the paper reported previously by our group (Yang et al., Citationin press). PTX-P-AB dendrimer was prepared. Briefly, 3 mg PAMAM-AB was dissolved in the distilled water, followed by the addition of 3 mg PTX. The mixed solution was allowed to incubate with constant magnetic stirring for 24 h at room temperature. The mixture was filtered to remove free drugs from the formulations, and the free PTX was redissolved in methanol to be examined the unloaded PTX to determine indirectly the amount of drug bound by the system. The amount of unloaded PTX was determined by HPLC method. HPLC characterization of PTX was carried out with HITACHI L-2000 series (Tokyo, Japan). HPLC instrument equipped with a L-2130 pump, a L-2200 autosampler and a L-2400 UV-detector. Data were collected by a D-2000 Elite HPLC chromatography workstation. PLATISILTM ODS C18 reversed-phase column (5 μm, 250 mm × 4.6 mm, Dikma) was used for the characterization of the conjugate. The separation was carried out with the mobile phase consisting of acetonitrile–water (55:45, v/v) at a flow rate of 1.0 ml/min. Mobile phases were freshly prepared, filtered and degassed prior to the use. The UV absorbance detector was used at 228 nm and the temperature of the column was kept at 25 °C.
The osmotic pressure of PTX-P-AB was measured by a Vapor Pressure Osmometer (Wesor, Inc.). The zeta potential was measured by a Zetasizer Nano ZS90 (Malvern, UK). The encapsulation efficiency (EE) and drug loading (DL) were calculated using the following equations:
where Wadded was the total weight of PTX added; Wunloaded was the weight of the unloaded PTX; WPAMAM-AB was the total weight of PAMAM-AB added. Each measurement was carried out in triplicate.
Stability and leakage test in vitro
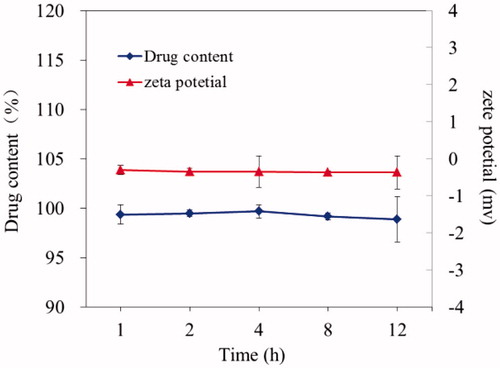
The stability and leakage in vitro of PTX-P-AB were investigated by the drug content and the zeta potential. PTX-P-AB (1 mg/ml) samples were stored at room temperature and 0.1 ml samples of PTX-P-AB were taken at 1, 2, 4, 8, 12 h post. The drug content was measured by the method mentioned in the section “Preparation and characterization of PTX-P-AB”. The zeta potential was measured by laser light scattering using a Zetasizer Nano-ZS90 at 25 °C.
Release studies in vitro
This article designed an in vitro release method in the application of dissolution tester and dialysis method to simulate lymphatic system (Ye et al., Citation2014). The in vitro release behaviors of PTX-P-AB were, respectively, investigated in mimic s.c. and lymphatic system.
Subcutaneous release study
In vitro s.c. release of PTX-P-AB was evaluated using a membrane dialysis method in dissolution tester (ZRS-6G, TDTF, Tianjin, China) (Kumer et al., Citation2007). Briefly, an aliquot (1 ml) of PTX-P-AB (1 mg/ml PTX) was placed in the dialysis bag (molecular weight 7000 cut-off). Then, the dialysis bag was put into 10 ml stoppered glass tubes containing 8 ml of pH 5.0 PBS, pH 5.8 PBS, pH 6.8 PBS and pH 7.4 PBS (with 0.1% Tween-80), respectively. The tubes were fixed with paddles in dissolution cups. Then the tubes horizontally rotated with the paddles at 30 rpm and were incubated at 37 °C. At various pre-determined time intervals, all release medium was withdrawn and the same volume of fresh release medium was added. PTX content was analyzed by HPLC method described previously. Drug release profiles (cumulative release versus time) were plotted. The percentage of cumulative release was calculated by the following equation:
where Q, V0, C and W are the percentage of cumulative release, release medium volume, drug concentration of each interval and the weight of total drug, respectively.
Lymphatic system release study
In vitro lymphatic system release of PTX-P-AB was analyzed by the membrane dialysis method. Briefly, a 1 ml aliquot of PTX-P-AB (1 mg/ml PTX) was loaded in the dialysis bag (molecular weight 7000 cut-off). Then, the dialysis bags were put into dissolution cups with gentle stirring and incubated at 37 °C with 200 ml of pH 5.0 PBS, pH 5.8 PBS, pH 6.8 PBS and pH 7.4 PBS (with 0.1% Tween-80), respectively. In addition, the PTX release from Taxo1® was investigated at pH 7.4 PBS (with 0.1% Tween-80) as the control. At various time points, 5 ml release medium was withdrawn and the same volume of fresh release medium was added. The PTX content was assayed by HPLC method and drug release profiles were plotted. The percentage of cumulative release was calculated by the following equation:
where Q, V0, C, V and W are the percentage of cumulative release, release medium total volume, drug concentration of each interval, release medium volume of each interval and the weight of total drug, respectively.
Osmotic pressure study of PTX-P-AB affecting PTX release in vitro
In vitro lymphatic system release of PTX-P-AB (1 mg/ml PTX) with the osmotic pressure of 100, 300, 500 and 700 mmol/kg (sodium chloride as osmolyte) in pH 5.8 PBS was examined to investigate the influence of different osmotic pressure on PTX release.
Tumor implantation
S180 cancer cells were extracted from the abdominal dropsy of mice bearing cancer cells and diluted with saline to achieve a cell concentration of 1 × 107 per ml. A 0.02-ml aliquot of cell suspension was subcutaneously injected into mice under the right hindpaw. In addition, tumors of PLNs were allowed to develop for 7 days to reach about 8 mm3 in volume, and mice weighing 20 ± 2 g were ready for pharmacokinetic and pharmacodynamic studies.
Lymphatic uptake and drainage
Lymphatic uptake and drainage in healthy mice
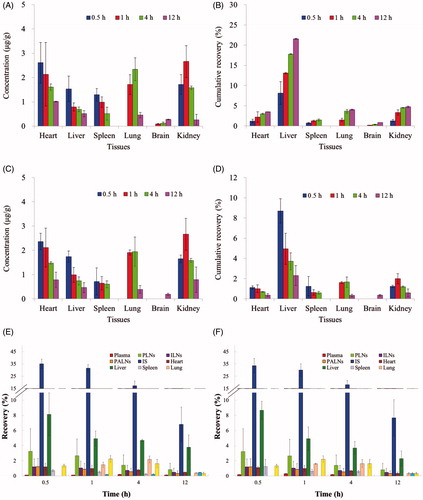
KM mice were used for lymphatic uptake and drainage studies. PTX-P-AB with the osmotic pressure of 300, 500, 700 mmol/kg (OSM-300, OSM-500 and OSM-700) and Taxo1®, were selected for the study and were administered subcutaneously to the footpad of the right hindpaw of the mice at the dose of 1 mg/kg, respectively. Time taken for administration was 30 s. Blood samples were drawn by retro-orbital venous plexus puncture at 5 min, 10 min, 30 min, 1 h, 2 h, 4 h, 8 h and 12 h post s.c. dose. The samples were put into heparinized micro-centrifuge tubes and followed by centrifuging at approximately 14 000 rpm for 10 min, and the serum was collected for analysis. After blood samples were got, the mice were sacrificed. Separately, the right PLN, ILN, PALN and right hindpaw were excised immediately, then washed in normal saline and blotted dry with filter paper and weighed for analyzing the drug concentration. An aliquot (100 μl) of plasma sample, LN and hindpaw homogenates was put into an Eppendorf tube followed by the addition of 200 μl acetonitrile and 10 μl docetaxel solution (59.1 μg/ml) as the internal standard. The mixture was vortexed for 2 min and centrifuged at 14 000 rpm for 10 min. PTX extracted of the supernatants was detected by HPLC method with a little modification as below: LATISILTM ODS C18 reversed-phase column (5 μm, 250 mm × 4.6 mm, Dikma) was used and the separation was carried out with the mobile phase consisting of acetonitrile–water (50:50, v/v) at a flow rate of 1.0 ml/min. The detection wavelength was set at 228 nm and the temperature of the column was kept at 25 °C. Non-compartmental PK parameters were carried out by Phoenix WinNonlin 6.3 (Pharsight Co.). The fraction of the dose that was recovered in the LN, IS, systemic plasma at pre-determined time was calculated by recovering the amount of drug divided by administration dose (% dose) (Ye et al., Citation2014). In the tissue distribution experiments, mice were s.c. administered PTX-P-AB with the osmotic pressure of 300 mmol/kg and were sacrificed after 0.5, 1, 4 and 12 h. The tissues (heart, liver, spleen, lung, kidney and brain) were then removed, weighed and disrupted to homogenates with saline following the same procedure used for plasma samples. Separately, the recovery and the cumulative recovery of tissues were calculated.
Lymphatic uptake and drainage in tumor-bearing mice
KM mice with the expected tumor volume were divided into four groups with five mice in each group. OSM-300, OSM-500 and OSM-700 and Taxo1® were selected for the study and were administered subcutaneously to the footpad of the right hindpaw of the tumor-bearing mice at the dose of 1 mg/kg, respectively. Lymphatic pharmacokinetics and tissue distribution were investigated as the method described above.
Osmolyte study affecting PTX-P-AB in vivo lymphatic targeting
The influence of different osmolytes on the lymphatic targeting was investigated by analyzing LN s uptake. Separately, sodium chloride (NaCl), glucose and mannose were selected as the osmolyte with the osmotic pressure of 300 mmol/kg. The PTX concentrations in right PLN and ILN at 0.5, 1, 4 and 12 h time point were studied by the above described method.
Pharmacodynamic studies of PTX-P-AB
Mice with the expected tumor volume were divided into six groups with 15 mice in each group. The mice of Group I were s.c. injected to the footpad of the right hindpaw with saline as the blank control, and the mice of Group II were injected with Taxo1® diluted with saline to the PTX concentration of 1 mg/ml as the positive control. The mice of Group III, IV and V were administrated with OSM-300, OSM-500 and OSM-700 (1 mg/ml PTX, 1 mg PTX/kg dose) as the experimental groups, respectively. The mice of Group VI were injected with blank PAMAM-AB alone (3 mg PAMAM-AB/kg dose) as the carrier control.
The first administration day was recorded as day 0. The six groups were subjected to the formulations every other day for 14 days. Five mice of each group selected randomly were sacrificed on day 5, 10 and 15. Body weight of mice was measured by an electronic digital scale, and the metastatic tumor in right PLNs was immediately excised for experimental data. Tumor sizes were measured in two perpendicular dimensions using a caliper, and tumor volume (V) was calculated with the formula, V = 0.5 L × W2, where L is the largest superficial diameter and W is the smallest superficial diameter of the tumor. The tumor inhibitory rate based on the following equation:
where Wblank and Wtest are the tumor average weight of the control group and the test groups, respectively. Statistically significant differences in multiple groups were determined using one-way analysis of variance, and a p value of 0.05 was considered to be statistically significant.
Results and discussion
Preparation and characterization of PTX-P-AB
In our previous study, the synthesis of water-soluble PAMAM-AB was briefly described (Yang et al., Citationin press). In this study, PTX was encapsulated in the dendritic interiors of PAMAM-AB to increase the hydrophilicity. The encapsulation efficiency, drug loading, zeta potential and the osmotic pressure are shown in . As shown in , the zeta potential and drug content of PTX-P-AB essentially showed no apparent change after 12 h comparing. This result indicated that the drug in a PAMAM-AB dendrimer delivery system was stable which benefited in vivo evaluation.
Table 1. Physicochemical properties of PTX-P-AB (n = 3).
Release studies in vitro
In vitro release study of PTX-P-AB and Taxol® was evaluated by the dialysis method. Due to the poor solubility of PTX in water (Mitra et al., Citation2001), PBS containing 0.1% Tween 80 (Moghimi & Szebeni, Citation2003) was selected as a release medium to reach the sink condition, which could improve the solubility of PTX and avoid PTX attaching to the dialysis bag. The cumulative percentage release profiles of simulative s.c. condition and simulative lymphatic system condition are presented in . As shown in , PTX completely release from Taxol® within 4 h, and it indicated that PTX release from PTX-P-AB was slower than that from Taxol®. The release profile of PTX was prominently prolonged (p < 0.05) by being loaded in PAMAM-AB dendrimer system. The solubility of PTX was dependent on pH and was higher under acidic conditions than under neutral conditions. Hence, the observed release rate of PTX-P-AB was quite low in pH 7.4 PBS. The release rate was in descending order of pH 5.8 PBS, pH 6.8 PBS, pH 5.0 PBS and pH 7.4 PBS. Because tumors generally have a low pH, the fastest release of PTX-P-AB dendrimers in pH 5.8 PBS is favorable for cancer therapy. The difference between s.c. and lymphatic system was significant. 18.74 ± 3.46%, 30.41 ± 3.36%, 23.29 ± 2.69% and 15.76 ± 2.04% of PTX from PTX-P-AB were, respectively, released in pH 5.0 PBS, pH 5.8 PBS, pH 6.8 PBS and pH 7.4 PBS within 8 h under mimic s.c. condition, whereas the percentage of PTX released from PAMAM-AB dendrimer system in mimic lymphatic condition, respectively, were 49.23 ± 5.62%, 81.47 ± 9.59%, 61.67 ± 3.68% and 46.11 ± 7.53%. The results showed that PTX released from PTX-P-AB dendrimer system in mimic s.c. release condition was significantly slower than in mimic lymphatics (p < 0.05). As shown in , PTX release from PTX-P-AB gradually speeded up with the increase of the osmotic pressure of PTX-P-AB, and the cumulative release rate was 81.47 ± 9.59%, 88.10 ± 2.76%, 88.61 ± 4.15% and 94.10 ± 3.33% with the osmotic pressure of 100, 300, 500 and 700 mmol/kg at the sampling time of 8 h, respectively.
Figure 3. In vitro release profiles of (A) PTX from Taxol® and PTX-P-AB in pH 5.0 PBS, pH 5.8 PBS, pH 6.8 PBS and pH 7.4 PBS under mimic subcutaneous (s.c.) condition and lymphatic system (l.s.) condition; (B) PTX from PTX-P-AB with the osmotic pressure of 100, 300, 500 and 700 mmol/kg (n = 3).
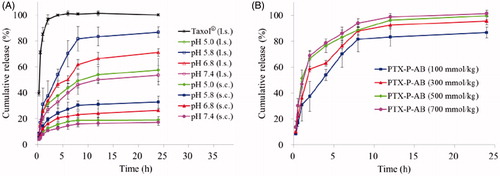
In this study, PTX s.c. and lymphatic mimic release in vitro was investigated. Because of the little s.c. humoral fluid and limited contact areas compared to gastrointestinal and hematological system, dissolution and the absorption rate of the drug administrating s.c. injection were limited by comparison with oral or intravenous administration (McDonald et al., Citation2010; De Campos et al., Citation2013). The lymphatic system was the network of liquid system which was similar to hematological system. But different from the hematological system, lymphatic system contained a large number of lymphocytes and the lymph flow was slow which was one-tenth of the venous flow velocity. Due to the different characteristics of the s.c. and lymphatic system, the mimic release device and conditions of the s.c. and lymphatic system were improved on the base of intravenous administration.
Lymphatic uptake and drainage in vivo
Plasma–PTX concentration and pharmacokinetics
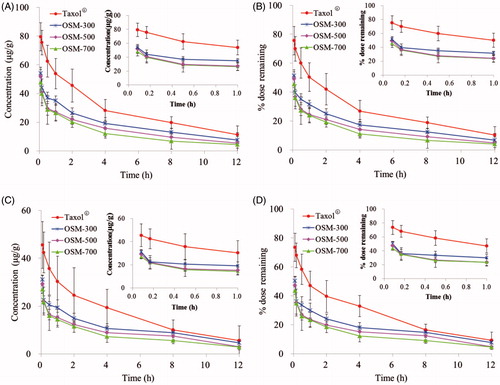
The concentration versus time curves generated for PTX in healthy mice and tumor-bearing mice after Taxol®, OSM-300, OSM-500 and OSM-700 administration are shown in and the pharmacokinetic parameters are summarized in . According to the data got from HPLC analysis shown in , after a s.c. injection of Taxol®, peak plasma levels for PTX of 203.450 and 208.150 µg/ml were reached immediately and decreased to 29.603 and 26.711 µg/ml within 4 h in healthy mice and tumor-bearing mice, respectively. Compared to Taxol®, following OSM-300, OSM-500 and OSM-700 s.c. administration, peak plasma levels of 31.222, 37.076, 40.076 µg/ml in healthy mice and 51.889, 54.410, 61.576 µg/ml in tumor-bearing mice for PTX were reached at 1 h and decreased gradually to 8 h, which were significantly lower than that in Taxol® group. After Taxol® administration, no drugs were detected in plasma after 4 h. However, for OSM-300, OSM-500 and OSM-700 groups, PTX could be examined for 8 h. The rapid decay of PTX from Taxol® led to a much shorter half-life of 1.381 and 1.270 h in healthy mice and tumor-bearing mice, respectively, when compared with that of 6.386 h (OSM-300), 6.045 h (OSM-500), 6.289 h (OSM-700) in healthy mice and 6.034 h (OSM-300), 6.051 h (OSM-500), 6.067 h (OSM-700) in tumor-bearing mice. Results indicated that PTX-P-AB prolonged PTX residence time in plasma circulation. But AUC(0–12 h) of Taxol® group was significantly higher than other groups, which demonstrated that PTX-P-AB decreased PTX plasma exposure after s.c. administration. The cumulative recovery in is also shown that PTX-P-AB after s.c. administration less entered the blood circulation than Taxol®. In addition, it can be concluded that PTX plasma exposure was prolonged with the increase of the osmotic pressure, but no significant differences were found among the three groups. Besides, plasma concentrations of PTX in tumor-bearing mice were slightly higher than that in healthy mice, resulting in larger PTX exposure to a small extent.
Figure 4. Plasma concentrations in (A) healthy mice and (C) tumor-bearing mice; plasma cumulative recovery % of total dosed PTX-P-AB recovered in (B) healthy mice and (D) tumor-bearing mice following s.c. dosing of 1 mg/kg (n = 5).
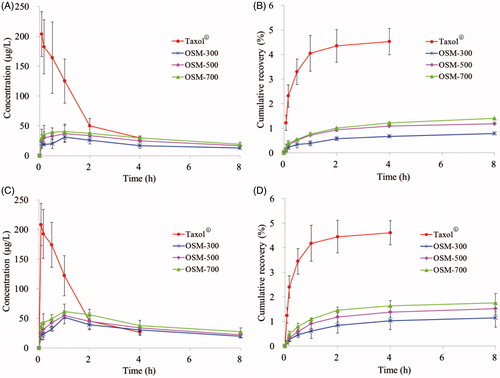
Table 2. Non-compartmental pharmacokinetic parameters for plasma, IS, PLNs, ILNs and PALNs after PTX-P-AB s.c. administration at a dose of 1 mg/kg (n = 5).
Lymphatic drainage from the IS
The injecting site clearance profiles were compared over a 12-h time course following s.c. administration of PTX-P-AB and Taxol® in healthy mice and tumor-bearing mice. As shown in and , AUC(0–12 h) of PTX-P-AB in OSM-300 (220.989 h µg/g), OSM-500 (175.943 h µg/g), OSM-700 (148.493 h µg/g) in the IS was lower than Taxol® (342.971 h µg/g) in healthy mice and in OSM-300 (130.773 h µg/g), OSM-500 (106.987 h µg/g), OSM-700 (92.377 h µg/g) in the IS was lower than Taxol® (195.985 h µg/g) in tumor-bearing mice. Injection site remaining (% dose) is shown in . Majorities of formulations were removed within 8 h, and at 12 h after administration, nevertheless, about 6.81% of OSM-300, 4.84% of OSM-500, 3.93% of OSM-700, 10.20% of Taxol® remained at IS in healthy mice, and about 7.68% of OSM-300, 4.86% of OSM-500, 4.73% of OSM-700, 9.24% of Taxol® remained at IS in tumor-bearing mice, respectively. The drainage from IS was in descending order of OSM-700, OSM-500, OSM-300 and Taxol®. OSM-700 group had the least retention at IS at 12 h post-dosing, possibly as a result of electrostatic interactions with the surrounding tissues. The interstitial space consisted of mucopolysaccharides with low isoelectric points resulting in a negative charge at physiological pH, and PTX-P-AB also negatively charged owing to the large number of −COOH groups on the surface. Consequently, electrostatic interactions between the interstitial space and PTX-P-AB made PTX-P-AB more drainage from the IS than Taxol®. In addition, the increase in the osmotic pressure made PTX more drainage from the IS. Comparison of data in healthy mice and in tumor-bearing mice indicated that PTX-P-AB draining from the IS in tumor-bearing mice faster than that in healthy mice, but no significant differences were noticed.
Lymphatic uptake and retention
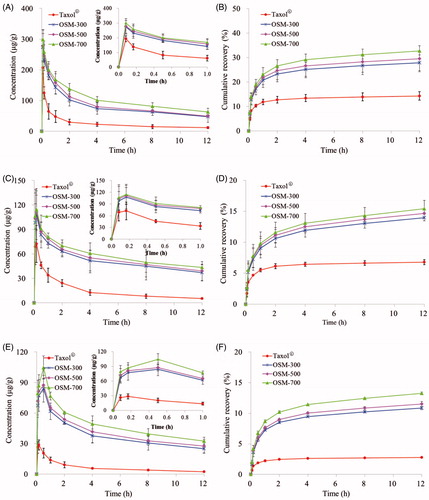
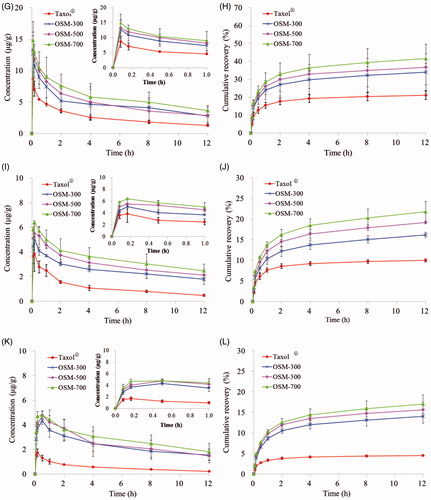
LNs distributions of PTX in OSM-300, OSM-500, OSM-700 and Taxol® were measured in PLNs, ILNs and PALNs. And the non-compartmental pharmacokinetic parameters are shown in . PTX concentrations of PTX-P-AB groups at any time point were obviously higher than Taxol® group. As shown in , the four formulations were quickly absorbed into draining lymphatics, and peak concentrations were both reached immediately at 5 min, with Cmax of 273.950, 277.450, 299.210, 192.670 µg/g in healthy mice and 12.665, 13.227, 14.845, 8.654 µg/g in tumor-bearing mice, respectively. After 5 min post-dosing, the concentration of PTX gradually decreased with time. Twelve hours after administration, the PTX concentration of OSM-700 was higher than OSM-300, OSM-500 and Taxol® group in healthy mice (1.345-, 1.286-, 4.464-fold increase) and in tumor-bearing mice (1.313-, 1.271-, 2.900-fold increase). Separately, AUC(0–12 h) of OSM-700 group was higher than OSM-300, OSM-500 and Taxol® group in healthy mice (1.287-, 1.202-, 3.428-fold increase) and in tumor-bearing mice (1.264-, 1.219-, 2.312-fold increase). The cumulative recovery in PLNs following s.c. administration is shown in , presumably reflecting LNs uptake rate. The cumulative recovery at 12 h time point was in descending order of OSM-700, OSM-500, OSM-300 and Taxol®. OSM-700 increased (1.173-, 1.109- and 2.293-fold) PTX recovery (to 32.724%) when compared to OSM-300, OSM-500 and Taxol® group (to 27.888%, 29.509% and 14.273%) in healthy mice, and similar in tumor-bearing mice. Recovery of OSM-700 was much higher than that of other formulations, indicating more prominent LN retention.
Figure 6. Concentrations of PTX in (A) PLNs, (C) ILNs, (E) PALNs of healthy mice and in (G) PLNs, (I) ILNs, (K) PALNs of tumor-bearing mice; cumulative recovery % of total dosed PTX-P-AB in (B) PLNs, (D) ILNs, (F) PALNs of healthy mice and in (H) PLNs, (J) ILNs, (L) PALNs of tumor-bearing mice following s.c. dosing of 1 mg/kg (n = 5).
Similar to profiles in , curves of concentration versus time in ILNs are shown in . After a s.c. injection of the four formulations, peak concentrations all reached at 10 min and then decreased. PTX levels in ILNs were detected and much lower than that in PLNs at any time point. AUC(0–12 h) of four formulations was in the order of: OSM-700 > OSM-500 > OSM-300 > Taxol®. The cumulative recovery in ILNs for 12 h was in the order of OSM-700, OSM-500, OSM-300 and Taxol® (). OSM-700 increased (1.103-, 1.051- and 2.274-fold) PTX recovery (to 15.410%) when compared to OSM-500 group, OSM-300 group and Taxol® group (to 13.971%, 14.666% and 6.778%) in healthy mice, and similar in tumor-bearing mice.
Profiles in PALNs () differed significantly from that in PLNs and ILNs. Concentrations in PALNs were much lower than that in PLNs and ILNs. With Taxol® administration, the PTX concentration reached the maximum at 10 min both in healthy mice and in tumor-bearing mice. However, PTX encapsulated in PAMAM-AB dendrimer system led tmax of PTX in PTX-P-AB prolonged to 30 min. In PALNs, PTX-P-AB still maintained higher PTX levels after administration (p < 0.05) than Taxol®. PTX-P-AB increased AUC(0–12 h) (over 10-fold) when compared to Taxol® in PALNs. The cumulative recovery in PALNs was in descending order of OSM-700, OSM-500, OSM-300 and Taxol® (). The cumulative recovery of PTX-P-AB was about twofold higher than that of Taxol® (p < 0.05) both in healthy mice and in tumor-bearing mice.
Due to the larger weight of LNs in tumor-bearing mice than that in healthy mice, PTX concentrations in LNs were significantly decreased in tumor-bearing mice. But the cumulative recovery in tumor-bearing mice was slightly higher than that in healthy mice, indicating enhanced lymphatic uptake in tumor-bearing mice because the tumor-induced lymphangiogenesis (Shimoda et al., Citation2003) promotes the lymphatic uptake (Sleeman et al., Citation2009).
The substances injected interstitially must traverse the interstitium, which was the first obstacle of the intralymphatic drug delivery system administered interstitially. The lymphatic targeting drug carrier should have good lymphatic drainage that could traverse from the interstitial space to lymphatic. In the in vivo lymphatic uptake study, PTX-P-AB was found to be superior to Taxol®. Taxol® was rapidly absorbed into lymphatics post-dosing, which eliminate from LNs more rapidly due to the solution state. However, PAMAM-AB is a macromolecular solution, with many hydrophilic groups on the surface and good fluidity, leading to faster lymphatic uptake speed, larger uptake amount and longer LNs residence time. Compared the data got from HPLC analysis, it could be concluded that LN uptake enhanced with the increase of the osmotic pressure of PTX-P-AB. This result could be explained as follows. With the osmotic pressure of PTX-P-AB increasing, the extracellular fluid increased and the transmembrane gradient formed, resulting in enhanced uptake into lymphatics.
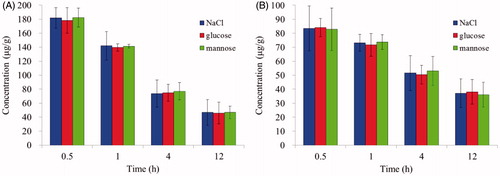
The LN uptake with different osmolytes (NaCl, glucose and mannose) in PLNs and ILNs were studied as shown in . Results indicated that the PTX concentration in PLNs and ILNs did not significantly change (p > 0.05) with different osmolytes. Thus, NaCl was selected to modulate the osmotic pressure for further study.
Tissue distribution
After a s.c. administration of PTX-P-AB, PTX-P-AB was absorbed by blood vessels and lymphatics, then distributed rapidly and peak PTX levels were reached within 0.5 h for the heart, the liver and the spleen investigated (). Tmax of 4 and 1 h was got in lung and kidney, respectively. The highest PTX concentration was found in the kidney, followed by the heart and the lung. The biodistribution of PTX in tumor-bearing mice was similar to that in healthy mice except for the spleen. The amounts of PTX in the spleen of tumor-bearing mice were less than that in healthy mice, whereas the amounts in the kidney and heart were similar. The cumulative recovery in tissues for 12 h was in the order of liver > kidney > lung > heart > spleen > brain (). As shown in , most of PTX-P-AB resided at IS after a s.c. injection, and the rest of drug disposed in plasma, PLNs, ILNs, PALNs and other tissues. Concentration levels were much higher in IS than that in other tissues at any time point.
Figure 8. Concentrations of PTX in tissues in (A) healthy mice and (C) tumor-bearing mice; cumulative recovery % of total dosed PTX-P-AB in (B) healthy mice and (D) tumor-bearing mice; recovery of PTX-P-AB in plasma, PLNs, ILNs, PALNs, IS, heart, liver, spleen, lung, brain and kidney in (E) healthy mice and (F) tumor-bearing mice following s.c. dosing of 1 mg/kg (n = 5).
Tumor implantation and enhanced anti-cancer effect of PTX-P-AB
As is known that tumor-associated lymphatic vessels could drain interstitial fluid containing tumor cells and tumor-derived proteins and other molecules away due to the high internal pressure within primary tumors (Jain, Citation1989). The flow of interstitial fluid drains away from the tumor into the lymphatic vasculature, which may then go on to form secondary tumors within the LN. In our study, the tumor was implanted through the s.c. injection in the hindlimb where the primary tumor was formed. Because of the difficulty dissecting the primary tumor in the hindpaw, the metastatic tumor in PLNs was selected to investigate the anti-cancer effect of PTX-P-AB. The presence or absence of LN metastasis was histopathologically investigated using HE-stained sections. Results showed that 7 days after S180 cancer cell transplant, metastatic cancer cells could be seen in PLNs () and the volume of PLN was about 8 mm3.
Figure 9. (A) HE-stained section of metastasis in PLNs for 7 days after S180 cancer cell transplant; (B) the growth curve of metastasis in PLNs; (C) photographs of metastasis in PLNs on day 15 (n = 5).
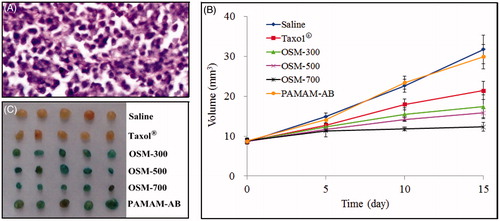
After 15 days administration, the volume of PLNs was in descending order of saline, PAMAM-AB, Taxol®, OSM-300, OSM-500 and OSM-700. Finally, the tumor volumes () of saline, Taxol®, OSM-300, OSM-500, OSM-700 and PAMAM-AB group were 31.74 ± 3.57, 21.42 ± 2.31, 17.36 ± 2.99, 15.84 ± 1.20, 12.30 ± 1.12 and 29.92 ± 3.04 mm3, respectively, and differences are clearly shown in the tumor graphs (). Significant differences of tumor weight on day 15 were seen between any two groups (p < 0.05) except saline versus PAMAM-AB, and OSM-300 versus OSM-500. Interestingly, the body weight shown in of all experimental mice increased by 38.4%, 16.4%, 15.9%, 15.0%, 16.5% and 33.2% for saline, Taxol®, OSM-300, OSM-500, OSM-700 and PAMAM-AB group, respectively. In addition, the saline control group and the PAMAM-AB carrier group had similar tumor volumes during the entire experiment, and no significant difference was discovered, indicating that the PAMAM-AB dendrimer alone had no toxicity. The better anti-cancer activity of OSM-700 (p < 0.01) than OSM-500 and OSM-300 demonstrated the enhanced metastasis-inhibiting effect using PAMAM-AB dendrimer system by increasing the osmotic pressure of PTX-P-AB.
Table 3. The anti-tumor activity of PTX-P-AB on mice bearing S180 tumors (n = 5).
Conclusions
In this current study, PTX-P-AB was prepared to investigate the intralymphatic targeting ability and anti-cancer effect. PTX-P-AB showed better lymphatic targeting ability and higher metastasis-inhibiting effect at the dose of 1 mg/kg compared with Taxol®. Less retention at the IS, higher PTX concentration in LNs showed that PTX-P-AB promote both drainage from the IS and uptake into the lymph by increasing the osmotic pressure of PTX-P-AB in healthy mice and in tumor-bearing mice, and indicated that PTX-P-AB possesses double function of lymphatic tracing and lymphatic targeting, therefore, provide an attractive platform for the development of lymphatic targeting carrier and lymphatic tracer for treatment and diagnosis.
Declaration of interest
We wish to acknowledge the support of Pharmacy Laboratory Centre and Animal Centre of Shenyang Pharmaceutical University. And this work was supported by the Liaoning Province Natural Science Fund Project (No. 2013020187). The authors report no declarations of interest.
References
- Alex JC, Weaver DL, Fairbank JT, et al. (1993). Gamma-probe-guided lymph node localization in malignant melanoma. Surg Oncol 2:303–8
- Benedetti-Pancini P, Maneschi F, Scambia G, et al. (1996). Lymphatic spread of cervical cancer: an anatomical and pathological study based on 225 radical hysterectomies with systemic pelvic and aortic lymphadenectomy. Gynecol Oncol 62:19–24
- Buhleier E, Wehner W, Vogtle F. (1978). Cascade- and nonskid-chain-like syntheses of molecular cavity topologies. Synthesis 2:155–8
- Chen H, Banaszak Holl M, Orr BG, et al. (2003). Interaction of dendrimers (artificial proteins) with biological hydroxyapatite crystals. J Dent Res 82:443–8
- De Campos JDF, Da Silva JB, Beck ARM, et al. (2013). Subcutaneous administration technique of low-molecular-weight heparins: an integrative review. Clin Nurs Stud 1:36--40
- Dowlatshahi K, Fan M, Snider HC, Habib FA. (1997). Lymph node micro metastases from breast carcinoma: reviewing the dilemma. Cancer 80:1188–97
- Jain RK. (1989). Delivery of novel therapeutic agents in tumors: physiological barriers and strategies. J Natl Cancer Inst 81:570–6
- Kaminskas LM, Porter CJH. (2011). Targeting the lymphatics using dendritic polymers (dendrimers). Adv Drug Deliv Rev 63:890–900
- Kobayashi H, Kawamoto S, Sakai Y, et al. (2004). Lymphatic drainage imaging of breast cancer in mice by micro-magnetic resonance lymphangiography using a nanosize paramagnetic contrast agent. J Natl Cancer Inst 96:703–8
- Kobayashi H, Koyama Y, Barrett T, et al. (2007). Multimodal nanoprobes for radionuclide and fivecolor near-infrared optical lymphatic imaging. ACS Nano 1:258–64
- Kobayashi H, Sato N, Hiraga A, et al. (2001). 3D-micro-MR angiography of mice using macromolecular MR contrast agents with polyamidoamine dendrimer core with reference to their pharmacokinetic properties. Magn Reson Med 45:454–60
- Kodama Y, Inokuchi K, Okamura T. (1979). Tumor cell aggregation and mode of cancer spread in linitis plastics type of gastric carcinoma. Gann 70:721–9
- Kumer D, Tomar RS, Deolia SK, et al. (2007). Isolation and characterization of degradation impurities in docetaxel drug substance and its formulation. J Pharm Biomed 43:1228–35
- Lei XG, Jockusch S, Turro NJ, et al. (2008). EPR characterization of gadolinium (III)-containing-PAMAM-dendrimers in the absence and in the presence of paramagnetic probes. J Colloid Interface Sci 322:457–64
- Leong SP, Cady B, Jablons DM, et al. (2006). Clinical patterns of metastasis. Cancer Metast Rev 25:221–32
- Lim J, Chouai A, Lo ST, et al. (2009). Design, synthesis, characterization, and biological evaluation of triazine dendrimers bearing paclitaxel using ester and ester/disulfide linkages. Bioconjug Chem 20:2154–61
- McDonald TA, Zepeda ML, Tomlinson MJ, et al. (2010). Subcutaneous administration of biotherapeutics: current experience in animal models. Curr Opin Mol Ther 12:461–70
- Mitra S, Gaur U, Ghosh PC, Maitra AN. (2001). Tumor targeted delivery of encapsulated dextran-doxorubicin conjugate using chitosan nanoparticles as carrier. J Control Release 74:317–23
- Moghimi SM, Szebeni J. (2003). Stealth liposomes and long circulating nanoparticles: critical issues in pharmacokinetics, opsonization and protein-binding properties. Prog Lipid Res 42:463–78
- Morton DL, Wen DR, Wong JH, et al. (1992). Technical details of intraoperative lymphatic mapping for early stage melanoma. Arch Surg 127:392–9
- Mounzer R, Shakarin P, Papademetris X, et al. (2007). Dynamic imaging of lymphatic vessels and lymph nodes using a bimodal nanoparticulate contrast agent. Lymphat Res Biol 5:151–8
- Nishioka Y, Yoshino H. (2001). Lymphatic targeting with nanoparticulate system. Adv Drug Deliv Rev 47:55–64
- Shimoda H, Takahashi Y, Kajiwara T, Kato S. (2003). Demonstration of the rat lymphatic vessels using immunohistochemistry and in situ hybridization for 5′-nucleotidase. Biomed Res 24:51–7
- Sleeman J, Schmid A, Thiele W. (2009). Tumor lymphatics. Semin Cancer Biol 19:285–97
- Tam JP, Spetzler JC. (2001). Synthesis and application of peptide dendrimers as protein mimetics. Curr Protoc Protein Sci Chapter 18:Unit18.5. DOI: 10.1002/0471140864.ps1805s17
- Tomalia DA, Baker H, Dewald JR, et al. (1985). A new class of polymers: starburst-dendritic macromolecules. Polym J 17:117–32
- Tomalia DA, Reyna LA, Svenson S. (2007). Dendrimers as multi-purpose nanodevices for oncology drug delivery and diagnostic imaging. Biochem Soc Trans 3:61–7
- Xie Y, Bagby TR, Cohen MS, Forrest ML. (2009). Drug delivery to the lymphatic system: importance in future cancer diagnosis and therapies. Expert Opin Drug Deliv 6:785–92
- Yang H, Lopina ST. (2007). Stealth dendrimers for antiarrhythmic quinidine delivery. J Mater Sci Mater M. 18:2061–5
- Yang R, Xia SX, Ye TT, et al. (2014). Synthesis of a novel polyamidoamine dendrimer conjugating with alkali blue as a lymphatic tracer and study on the lymphatic targeting in vivo. Drug Deliv Early Online: 1--11. DOI: http://dx.doi.org/10.3109/10717544.2014.979515
- Ye TT, Zhang WJ, Sun MS, et al. (2014). Study on intralymphatic-targeted hyaluronic acid-modified nanoliposome: influence of formulation factors on the lymphatic targeting. Int J Pharm 471:245–57