?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Withania somnifera Dunal is an Indian medicinal plant with significant pharmacological properties, such as adaptogenic, anti-inflammatory, anti-oxidant, anti-platelet, anti-hypertensive, hypoglycemic and hypolipidemic effects. Several chemotypes of W. somnifera include NMITLI-101, NMITLI-118 and NMITLI-128. The present work elaborates the optimization and development of a liposomal delivery system for efficient delivery of NMITLI118RT+ [a standardized ethanolic extract of a new chemotype of W. somnifera Dunal (NMITLI-118) roots] against cerebral stroke in rats. Liposomal systems were prepared using thin-film hydration method and characterized on the basis of size, zeta potential, physical stability, FT-IR, DSC-TGA analysis and surface morphological studies by TEM. NMITLI118RT+ and its formulations (NMITLI118RT+LF) were evaluated for biological activity utilizing middle cerebral artery occlusion model in rats. The Z average of the developed liposomal formulation was about 142.6 ± 0.09 nm with a zeta potential of −31.20 ± 1.0 mV. Results of TEM revealed spherical particles in the range of 200 nm. The entrapment efficiency was found to be 94.603 ± 2%. The formulation was found to be physically stable over a 3-week period. Results were suggestive of the fact that both NMITLI118RT+ and its delivery system possess significant neuroprotective activity in cerebral ischemia. The liposomal system largely exhibits better performance over NMITLI118RT+ precisely in the post-treatment group. The present studies could elucidate the successful development of a delivery system for NMITLI118RT+ and demonstrate their beneficial neuro-protective potential in overcoming and reversing the consequences of I/R injury following stroke.
Introduction
Stroke is a leading cause of morbidity and disability across the globe and according to an estimate by 2020; the overall cost on the management of stroke could account to 6.2% of the total burden of illness (Demaerschalk et al., Citation2010). Stroke occurs either when one of the arteries to the brain is blocked or it bursts and the part of the brain is deprived of the blood it needs.
There are mainly two kinds of ischemic stroke: embolic stroke and thrombotic stroke. Another kind of stroke, the hemorrhagic stroke happens when a blood vessel in the brain bursts and spills blood into or around the brain (National Stroke Association, Citation2013). However, ischemic strokes are the most prevalent representing approximately 80% of all strokes, whereas intra-cerebral haemorrhage represents about 10–15% of all strokes.
The pathophysiological changes occurring in response to stoke include free radical production, excitotoxicity, disruption of sodium and calcium influx, enzymatic changes, stimulation of the inflammatory processes, endothelin release, platelets and leukocytes activation, delayed coagulation and endothelial dysfunction. All these together contribute to the brain injury following onset of stroke (Gupta et al., Citation2010). Owing to the gross complexities associated with the pathophysiology of stroke; possibly drugs of different mechanisms of action may be more effective than an approach based on an individual candidate (Lapchak et al., Citation2010).
The only FDA approved drug for stroke treatment is r-tPA but with a narrow therapeutic time window of 3 h, thus failing to demonstrate unequivocal benefit using later time points in stroke patients in recent clinical trials. Hence, there is an urgent need to screen agents that might be beneficial in effective management of stroke and to come up with their efficient delivery systems. Several herbal candidates, such as Withania somnifera, Centella asiatica, Shilajit, Tinospora cordifolia and Convolvulus pluricaulis, have been evaluated as prophylactic treatment in stroke (Chaudhary et al., Citation2003; Gupta et al., Citation2010).
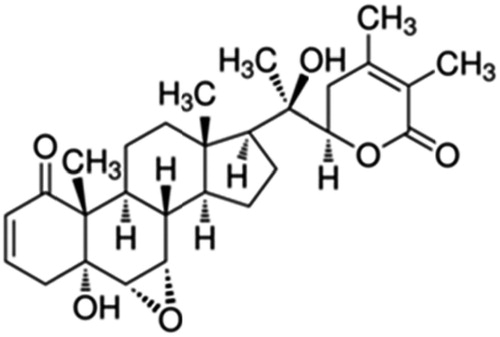
Withania somnifera Dunal (Ashwagandha) is an ancient Indian medicinal plant. Its use has been indicated traditionally in bronchitis, asthma, ulcers, insomnia, etc. It is associated with the presence of unique classes of steroidal lactones called Withanolides (including Withanolide A (
), Withaferin A, Withanolide D, Withanone, etc.) (Dhar et al., Citation2006; Chaurasiya et al., Citation2009; Rajasekar & Elango, Citation2011).NMITLI118RT+ signifies standardized concentrated aqueous ethanolic extract of roots of NMITLI118, a new chemotype of W. somnifera, which is unique in having high root yield, possessing a uniform crop canopy and uniform composition with respect to its secondary metabolites – withanolides. It is phytochemically characterized by particular abundance of two withanolides (i.e. Withanolide A and Withanone) and its delivery systems were evaluated for their therapeutic neuro-protective action in rats (Chaurasiya et al., Citation2009).
To overcome the complexities associated with drug delivery of anti-stroke agents, liposomal systems were thought of as an alternative. Liposomes are spherical particles constructed of polar lipids with one or multiple concentric membranes in which a fraction of the solvent can be encapsulated (Gortzi et al., Citation2007). They can have better pharmacological effect of the neuro-protectant as encapsulation of actives enhances their bioavailability to brain. Cytidine-5 V-diphosphocholine, asialoerythropoietin and PEGylated asialoerythropoietin are some of the therapeutic agents that have recently been developed as liposomal systems in the treatment of stroke (Rao et al., Citation2005; Ramos-Cabrer et al., Citation2011; Ishii et al., Citation2012, Citation2013).
Materials
NMITLI118RT+ and its marker compound Withanolide A (Spectroscopic identification provided as supplementary data, Figures S1--S5) were obtained from CSIR-CIMAP (Central Institute of Medicinal and Aromatic Plants, Lucknow, India). All solvents used in the study were of HPLC grade; supplied by Merck India Ltd., Mumbai, India. Milli-Q pure water was obtained from a Millipore Elix water purification system purchased from Millipore India Pvt. Ltd., New Delhi, India. Other reagents used were of analytical grade. Soy phosphatidylcholine (soy lecithin) and egg phosphatidylcholine (EPC) were supplied by Sigma Aldrich, Seelz, Germany, cholesterol (Himedia Pvt Ltd., Mumbai, India), potassium dihydrogen phosphate (Merck Specialities Pvt Ltd., Mumbai, India), sodium hydroxide (Fisher Scientific India Pvt Ltd., Mumbai, India).
Methods
NMITLI118RT+ (preparation and standardization)
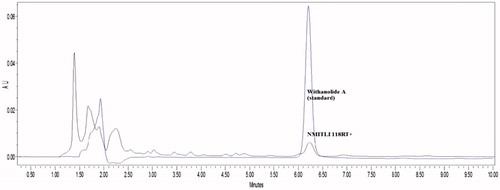
The ethanolic extract was prepared from the roots of a new chemotype of W. somnifera Dunal (NMITLI118) as described by the authors in their Indian patent application (Dwivedi et al., Citation2014). The concentrated extract was lyophilized to dry powder stage, sealed and immediately stored at −80 °C. Aliquots of the lyophilized powder were kept separately and an HPLC fingerprint was established (
). The average yield of extract was 62 g/kg fresh weight of root.The authors have worked upon and previously reported a detailed stability protocol (photostability studies, drug-excipient studies and forced degradation studies) for NMITLI118RT+, its fingerprint pattern, estimation of Withanolide A content also stating the recommended storage conditions of the same (Ahmad et al., Citation2014). To ensure an efficient delivery of NMITLI118RT+ for neuro-protection, the authors adopted the development of a novel drug delivery system (liposomal preparation in the present case), which could minimize the dose requirements providing beneficial effects at a dose lesser than NMITLI118RT+ itself.
Development of liposomal delivery systems of NMITLI118RT+ (NMITLI118RT+LF)
Liposomes were prepared by thin-film hydration method (Park et al., Citation2013). Briefly, the lipid composition in a fixed optimized ratio and the drug were solublized in the selected solvent system. These contents were transferred to a round bottom flask and dried under vacuum (rotary evaporator) to evaporate off the solvent and also to obtain a thin film. The film was allowed to dry for 30 min and later rehydrated with an appropriate aqueous medium (buffer solution) to prevent separation of the phospholipid membrane. It was maintained under hydration for sufficient time and then homogenized with a probe sonicator and filtered with a 0.45 µ syringe filter (Minisart, Bohemia, NY). The liposomal dispersions were stored under refrigeration until further evaluations.
Lipid composition
Initial trials were conducted with mixtures of EPC and cholesterol (C) and later with mixtures of soy phosphatidylcholine (S) and cholesterol (C). Various compositions of (S) and (C) used were 80 + 20, 55.55 + 44.44, 88.88 + 11.11 and 95.25 + 4.76 on % w/w basis.
Choice of solvent system
A suitable solvent system is one which solubiliszs both the drug as well as the lipid mixture. Owing to the characteristic solubility of NMITLI118RT+ in alcohol, a binary system of alcohol with chloroform/dichloromethane was used for film formation.
Preparation and use of phosphate buffer
Potassium dihydrogen phosphate (0.680 g) was dissolved in 500 mL distilled water and then filtered. The pH was adjusted to 7.0 with the use of 0.1 N sodium hydroxide solution.
Hydration
This buffer medium was used to simulate the aqueous environment and for hydrating the film obtained after rotary evaporation. Temperature of buffer and volume of buffer used for hydration were important factors affecting optimization design.
Optimization and development of blank and NMITLI118RT+-loaded liposomal systems (NMITLI118RT+LF)
Several permutations and combinations were attempted by variations in the lipid composition and conditions of rotation (rpm and temperature) to obtain an optimized blank formulation which results in a continuous uniform film with acceptable size and PDI values. Based on the leads from development of blank liposomal preparations, drug-loaded liposomes were formulated (
Table 1. Development of blank and drug-loaded formulations and their particle size measurements.
Evaluation and characterization of NMITLI118RT+LF
Determination of mean particle size and poly dispersity index and zeta potential
The mean particle size and particle size distribution of blank formulations as well as NMITLI118RT+LF were evaluated by a laser diffraction particle size analyzer Zeta-sizer Nano ZS model (Malvern Instruments). The sample was dispersed in TDW and sonicated at 10% amplitude for 30 s. Optical properties of the sample were defined as follows: refractive index 1.47 and absorption 1.00. Each sample was measured in triplicate. The Z average (d. nm) and the poly-dispersity index were determined. Zeta potential was also determined by laser Doppler anemometry using a Malvern Zeta-sizer.
Determination of encapsulation efficiency
Unloaded NMITLI118RT+ was recovered from a 2 mL volume of liposomal solution by passing the liposomal solution through a 0.45-µm filter, and degrading the solution with 10 mL of ethanol. The ethanol was evaporated off in a rotary evaporator and NMITLI118RT+ was then re-dissolved in 2 mL of ethanol. The concentration of Withanolide A (major quantifiable marker in NMITLI118RT+) was determined using HPLC method previously reported on a Waters HPLC (Milford, MA) system used was equipped with a binary gradient pump (model 515, Waters), an auto sampler injector (Model 2707, Waters) and a diode array detector (Model 2998, Waters). Waters HPLC interface and Empower 2 (Miliford, MA) software was utilized for data acquisition (14) and the following equation was used to calculate the encapsulation efficiency of NMITLI118RT+ in liposomes on the basis of Withanolide A content
where Ce is the concentration of encapsulated drug and Ci, is the initial concentration of drug.
Physical stability of NMITLI118RT+LF
Assessment of physical stability is an important parameter as it ensures that the formulation is pharmaceutically acceptable. Physical stability can be assessed by changes in particle size, PDI, size distribution and zeta potential. The final formulation was prepared in three batches and stored at 4 °C and assessed over a 3-week study period to observe any changes in particle size and zeta potential.
Compatibility studies
Infrared spectroscopy identifies various functional groups in unknown substances through the identification of different covalent bonds that are present in the compound and thereby is useful in establishing the identity of compounds and to bring about comparisons between the pure compound and altered spectrum due to modifications in a formulation developed from the pure drug. Spectroscopic studies were carried out by appropriately diluting the sample with dried potassium bromide and acquiring infrared (IR) spectrum in the range of 400–4000 cm−1 with 2 cm−1 resolution. IR spectra of both NMITLI118RT+ and NMITLI118RT+LF (NMITLI118RT+-loaded liposomal formulation) were obtained. DSC-TGA curves of the samples of NMITLI118RT+ and NMITLI118RT+LF were collected with a thermo-analyzer (Pekin Elmer, model-Pyris-7, TGA, Perkin Elmer, Shelton CT) within a temperature range of 50–700 °C with heating and cooling rate of 10 °C/min in a nitrogen gas environment.
TEM studies
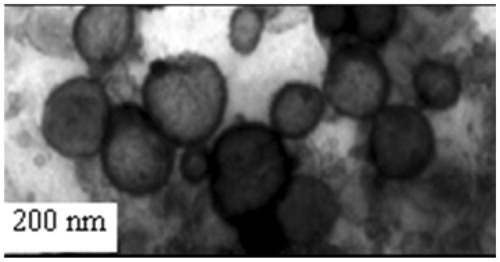
The morphology of the NMITLI118RT+LF was examined by transmission electron microscopy (TEM). TEM (TEM; 400T TEM, Jeol, JEM 1400 Plus, Jeol, Pleasanton, CA) studies were carried out using phosphotungstic acid as a negative stain. TEM was carried out to determine the surface characteristics of the optimized formulations in aqueous medium using a 3 mM forman (0.5% plastic powder in amyl acetate)-coated copper grid (300 mesh) at 60 kV using negative staining by 2% phosphotungstic acid at various magnifications.
Evaluation of neuro-protective potential of NMITLI118RT+ and NMITLI118RT+LF in cerebral stroke by MCAO model in rats
Animals
Male Sprague-Dawley rats (240–260 g) were procured from the National Laboratory Animal Centre of CDRI, Lucknow, India and experiments were performed after necessary approval vide IAEC/2010/71 dated 205-2014 of the Institute Animal Ethical Committee. Rats were allowed free access to food and water.
Animal grouping
Male Sprague-Dawley rats were divided in the following groups (n = 8) for study:
Experimental protocol
The middle cerebral artery occlusion (MCAO) model is the most preferred model to study cerebral stroke, because majority of human ischemic strokes results from occlusion of the middle cerebral artery, and thus MCAO model simulates the clinical situation.
Surgical procedure for induction of cerebral ischemia by MCAO
Chloral hydrate (300 mg/kg i.p.) was used to anaesthetize Sprague-Dawley rats (260 ± 20 g) and then rats were placed in a supine position over a pre-heated operation table to maintain the body temperature at 37 ± 0.5 °C. Through a midline incision in the neck region the left common carotid artery was exposed. The neck muscles were separated further to expose external carotid artery (ECA) and internal carotid artery (ICA). A silk suture knot was tied on the ICA loosely close to the bifurcation region. A (3-0) nylon monofilament suture (Ethicon, Johnsons & Johnsons Ltd., Mumbai, India) was introduced into the ECA lumen through a nick given in the ECA. The intraluminal suture blocked the origin of the MCA, occluding all sources of the blood flow from the ICA, anterior cerebral artery and posterior cerebral artery. The suture was pulled back after 2 h of ischemia to re-establish the cerebral blood flow.
After the designated time points of ischemia/reperfusion rats were examined for neurologic deficit, re-anaesthetized with ether and about 3.5 mL of blood was taken out retro-orbitally to determine the MDA and GSH levels in the blood followed by TTC staining to assess the brain damage and infarction volume.
Assessment of I/R injury
Neurobehavioral assessment. The neurological deficit was scored on a 10-point scale following the methods of Longa and Bederson with modifications (Bederson et al., Citation1986; Longa et al., Citation1989).
Malondialdehyde estimation in blood. The amount of MDA formed as a by-product of lipid peroxidation was quantitated by reaction with thiobarbituric acid (TBA) in blood serum and has been used as an index of lipid peroxidation (Colado et al., Citation1997). The pink colored MDA–TBA complex formed at low pH and high temperature was measured at 532 nm by spectrophotometer.
GSH estimation in blood. Glutathione was estimated in blood by the 5,5″-dithiobis-(2-nitrobenzoic acid) (DTNB)–glutathione reductase coupled assay essentially as described earlier (Anderson, Citation1985). The yellow color edchromogen formed by reaction of GSH with DTNB was read at 412 nm with spectrophotometer.
Histological study by TTC staining. Brain infarction following cerebral ischemia/reperfusion injury was visualized with 0.5% 2,3,5-triphenyltetrazoliumchloride (TTC) staining (Longa et al., Citation1989). Viable tissue stains deep red, while the infarct remains unstained. Hence, TTC staining is a suitable procedure for the detection of infarcts in brain slices. Rat brain was perfused transcardially with normal saline and carefully isolated in chilled condition immediately. Cerebellum was removed and the brain was kept in refrigerator at −20 °C. Frozen brain was sliced anterio-posteriorly into uniform sections of 2 mm thickness.
Results
Preparation of liposomal systems: thin-film hydration method
Rotary evaporation technique yielded perfect films which were hydrated and maintained in aqueous conditions. Conditions of the rotary evaporator were altered and this formed an important feature of the optimization design. NMITLI118RT+ loading at about 11.9% of the total lipid mass yielded the best result (NMITLI118RT+LF) which was chosen for further evaluation.
Effect of lipid composition
Phosphatidylcholine is the ideal primary ingredient required to make a basic liposomal construct. The unsaturated phosphatidylcholine forms the “container” or vesicle which entraps the solute or drug and architectures the liposome. It happens to be the phospholipid most abundantly found in the cell walls and it is required in sufficient amounts for the proper functioning of our body cells especially in the skin, liver and brain. The cell membrane is semi-permeable and controls the movement of substances in and out of the cell. Sufficient phosphatidylcholine is critical to maintain balanced permeability of the cell membrane. Thus, it provides varied health benefits and is the core material which constructs the phospholipid bilayer in a liposome. Initial trials conducted with EPC resulted in clumping and ridging and the film failed to form even after optimization of EPC amounts. Consequently, soy lecithin (S) was used and various compositions of (S) were attempted with cholesterol (C). Addition of cholesterol provides a certain degree of rigidity to the film. The intravesicle interactions or the fluidity of the liposomes are also altered by incorporating cholesterol in the liposomal system. The cholesterol molecule tends to fill in the free spaces formed due to the kink in the unsaturated lipid chain and thus give rigidity. Liposomes formed with cholesterol, therefore, have the capability to sustain shear stress to a greater extent. Cholesterol offers an added advantage; as it prevents the leakage of the encapsulated drug and retains the entrapped solutes thereby reducing the serum-induced instability caused by binding of serum proteins to liposomal membrane. However, an increase in the cholesterol content results in decreased membrane permeability of the electrolyte as well as non-electrolyte solutes which is a disadvantage in drug targeting. For optimal drug targeting via liposomes, it is essential that the liposome carrier eventually becomes permeable and releases the drug at the desired site; but at the same time it requires high stability in the blood stream. Hence use of cholesterol at an optimal level should be achieved which provides the desired rigidity but does not decrease the permeability to a level where drug targeting is adversely affected. Various compositions of (S) and (C) used were 80 + 20, 55.55 + 44.44, 88.88 + 11.11 and 95.25 + 4.76 on % w/w basis. Addition of cholesterol (C) at 20% resulted in clumping and ridging and the desired film failed to form. 44.44% (C) was also not successful in forming the film, while 11.11% (C) resulted in a film, however, breakage was observed at several places and the film was not continuous. Addition of 4.76% (C) gave the best film which was continuous and even and dispersed with ease in the buffer medium. The ideal film obtained with 4.76% (C) had sufficient strength but also had the desired fluidity required to get dispersed on hydration with the aqueous medium.
Choice of solvent system
A binary system of alcohol with one more organic solvent at an optimized ratio was used for solublizing the lipids and NMITLI118RT+. Ethanol was chosen over methanol due to the relative toxicity associated with methanol. Chloroform and dichloromethane both afforded equally good films. However, a comparison between the two brings out the various advantages of dichloromethane over chloroform. Chloroform is a non-polar solvent with a boiling point of 61 °C, density 1.498 g/mL and dipole 1.04 D, while dichloromethane is a polar aprotic solvent with a boiling point of 40 °C, density 1.326 g/mL and dipole 1.60 D. Thus, dichloromethane requires lower temperatures on rotary evaporation for film formation, and its larger dipole enables salvation of positively charged species. Hence, the final optimized system used was ethanol–dichloromethane mixture (1:4).
Hydration
Phosphate buffer pH 7.0 was used to hydrate the film. Hydration prevents the separation of the phospholipid membrane by hydrolysis. The temperature of the buffer used for hydration should ideally be above the lipid transition temperature and the product should be maintained as a liposome solution for sufficient time. The film was allowed to dry for 30 min before hydration and the temperature of the buffer for hydration was 50 °C. It was maintained under hydration for about 1 h and then homogenized by a probe sonicator. The volume of medium used for hydration accounted for the final concentration of the resulting liposomal formulation. Ideally 5 mL buffer was sufficient to disperse the contents of the flask with ease and amounted to a final concentration of 1 mg/mL of NMITLI118RT+ in the prepared liposomal formulation.
Optimization and development of blank systems and NMITLI118RT+LF
Blank liposomal systems were developed and the practical inferences on film formation in each blank formulation could be deduced here: L1: Ridging and clumping of material, absence of film formation and size and PDI values were not within limits. L2: Ridging and clumping of material, absence of film formation and PDI values were not within limits. L3: Film failed to form and size and PDI values were higher than required. L4: Film formed with partial ridging and size and PDI values were not within limits. L5: Film formed with partial ridging and size and PDI values were not within limits. L6: Best observed film with size and PDI values well within range.
Leads from the optimization and development of blank liposomal preparations were suggestive of the fact that the best results were obtained with L6. Thus, NMITLI118RT+-loaded liposomal formulations were attempted on the basis of L6 and practical inferences on film formation suggest that the optimized NMITLI118RT+-loaded liposomal formulation NMITLI118RT+LF afforded the best observed film with size and PDI in good agreement () and it was the final optimized drug-loaded liposomal formulation used for further evaluations.
Evaluation and characterization of NMITLI118RT+
Determination of mean particle size and poly dispersity index and zeta potential
Prepared liposomal formulations were evaluated for size and PDI using a Malvern zeta-sizer and the results are presented in . The optimized liposomal preparation NMITLI118RT+LF exhibited a monodisperse population (later confirmed by TEM) and the size and PDI were within acceptable limits. The zeta potential reflected formation of anionic liposomes with average zeta values approximating −30 mV.
Determination of encapsulation efficiency
Encapsulation efficiency was determined as described under methods. The encapsulation efficiency was found to be 94.603 ± 2%.
Physical stability of NMITLI118RT+LF
Three batches of NMITLI118RT+LF were prepared and evaluated for size, PDI and zeta potential over a period of 3 weeks at 4 °C. Results exhibited that the prepared formulation was stable over the study period, as there were negligible changes in the particle size and zeta potential measurements (
Table 2. Physical stability of developed system as evident from particle size measurement.
Compatibility studies
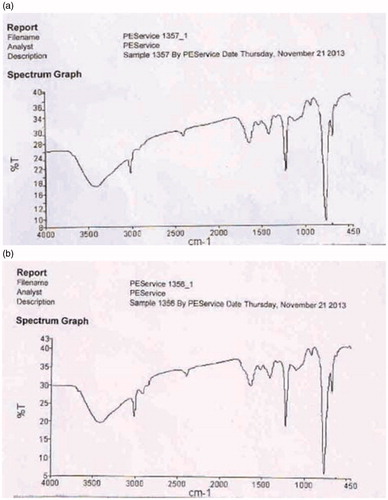
IR spectra of NMITLI118RT+ and NMITLI118RT+LF were obtained. Both were largely similar; however, an additional peak (Peak 3; % T: 27.83; 2928.49 cm−1) corresponding to the altered composition due to lipid entrapment was observed. The peak at 1107.85 is also shifted to 1100.65. This demonstrated that NMITLI118RT+ was well entrapped in the lipid bilayer construct of the liposome so formed (
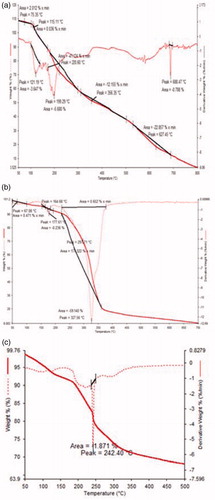
). DSC thermograms were obtained for NMITLI118RT+ and NMITLI118RT+LF. They provided insights into the melting behavior of the extract and the liposomal formulation derived from the extract. The DTA curve for NMITLI118RT+ shows the peculiar melting pattern of the extract. Due to the presence of several components in the extract several peaks is evident, however, three major melting regions could be identified by characteristic peaks at about 121.19, 199.29 and 688.47 °C. The DTA curve for the lipid mixture exhibits melting at about 177.61 and 327.56 °C. The DTA curve for NMITLI118RT+LF shows a single characteristic melting peak at 242.4 °C indicating that physical interaction of ingredients leading to vesicular entrapment of NMITLI118RT+ in the phospholipid bilayer construct of the liposomes. The TG curve for NMITLI118RT+ shows multi-step weight loss where rapid weight loss was observed after 100 °C, a single step weight loss pattern was evident in the TG curve for the lipid mixture and the weight loss pattern for NMITLI118RT+LF exhibited characteristic features of multi-step weight loss, however, it was more defined than NMITLI118RT+ ( ).Evaluation of neuro-protective potential of NMITLI118RT+ and NMITLI118RT+LF and in cerebral stroke by MCAO model in rats
Responses were evaluated on the basis of elevation or depression in the levels of MDA, GSH, evaluation of neurological deficit, reduction in infarct size and a comparative response chart has been shown in
Table 3. Evaluation of protection provided with NMITLI118RT+ and NMITLI118RT+LF in MCAO model.
Assessment of anti-stroke activity
Effect of treatments on MDA level
MDA is a by-product of lipid peroxidation. The MDA levels were measured post-24 h of ischemic/reperfusion injury from blood serum obtained from rat orbital plexus. A significant increase in blood MDA levels (62.83%) was observed in vehicle-treated ischemic rats (MCAO) as compared to control (sham) rats.
Pre-treatment: A decrease of about 50.66% in the MDA level was observed in rats treated with NMITLI118RT+, whereas NMITLI118RT+LF at a dose of 5 mg/kg p.o. offered a decrease in the MDA level by 42.50% (
Figure 6. (a) Effect of pre-treatment (1 h) and (b) post-treatment (6 h) of NMITLI118RT+ and NMITLI118RT+LF on MDA level following I/R injury.
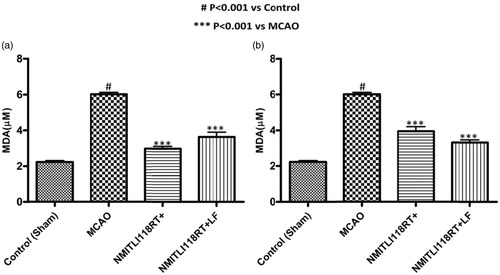
Post-treatment: On the other side in a different set of experiment, when the rats were post-treated with NMITLI118RT+LF at a dose of 5 mg/kg p.o. attenuated the MDA level by 44.83% and, a reduction of 24.83% was observed in rats treated with NMITLI118RT+ at a dose of 50 mg/kg p.o. ()
These studies indicated that the NMITLI118RT+ and its formulation have reduced ischemic damage leading to reduction in MDA content in treated rats.
Effect of treatments on GSH level
GSH levels are markers of oxidative stress. The GSH levels were measured post-24 h of I/R injury in the plasma of ischemic animals. A significant decline in GSH levels generally means an increase in oxidative stress, and was measured post-2/24 h of ischemic injury. A significant decline in blood GSH levels (59.67%) was observed in vehicle-treated ischemic rats (MCAO) as compared to (control) sham rats.
Pre-treatment: Rats treated with NMITLI118RT+ showed an increase in depleted GSH level by about 63.29% at 50 mg/kg p.o. A significant increase of 49.38% was observed in the rats treated with NMITLI118RT+LF at a dose of 5 mg/kg p.o. (
Figure 7. (a) Effect of pre-treatment (1 h) and (b) post-treatment (6 h) of NMITLI118RT+ and NMITLI118RT+LF on GSH level following I/R injury.
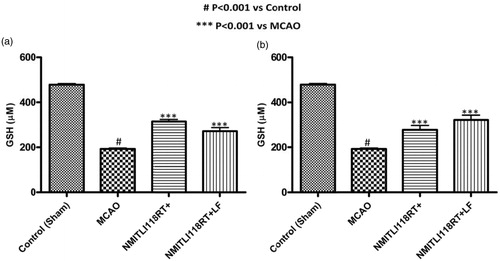
Post-treatment: An overwhelming response was noticed in the rats treated with NMITLI118RT+ and its formulations. An increase in GSH levels of 66.89% and 64.55% were offered by NMITLI118RT+LF and NMITLI118RT+, respectively ().
Thus, the extract and its formulation seem to be a potent antioxidant and counteract oxidative stress due to ischemic insult.
Effect of treatments on neurological deficit score
Neurological deficit was analyzed on the basis of neurological scores obtained after 24 h of reperfusion in all experimental groups. The neurological deficit score in treated group was significantly reduced when compared with the control group (sham group) at 2/24 h of I/R injury.
Pre-treatment: It was found that there was a significant reduction in the neurological score by 69.98% in rats pre-treated with NMITLI118RT+LF, and 64.93% by NMITLI 118RT+ (
Figure 8. (a) Effect of pre-treatment (1 h) and (b) post-treatment (6 h) of NMITLI118RT+ and NMITLI118RT+LF on neurological deficit following I/R injury.
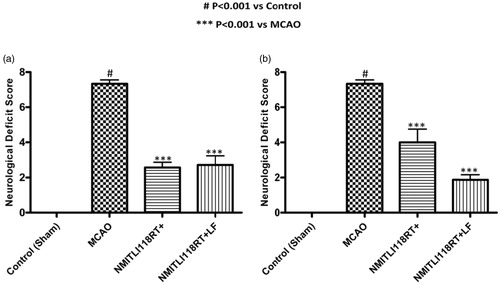
Post-treatment: During this study, it was noticed that NMITLI118RT+LF had significant protective effect in reducing the neurological impairment by 75.44%. But only a decrease by 37.65% was shown by the rats treated with NMITLI118RT+ ().
These studies indicate that treatment with the extract and its formulation was significantly effective in preventing brain damage.
Effect of treatments on cerebral infarct
TTC staining is widely used to assess the cerebral damage resulting from ischemic insult. The rats used for the assessment of ND were sacrificed and thick brain sections (∼2 mm) were made and stained with TTC to assess the infarct area. The TTC unstained area representing infarct was measured and presented as percentage reduction in infract area. Control group (sham group) produced an infarct size of almost 70.74% in ipsilateral side of control rat brain, mostly comprising of striatal and some of the cortical area as well.
Pre-treatment: The pre-treatment of rats with NMITLI118RT+ showed a good reduction in the cerebral infarction by 81% and + and NMITLI118RT+LF closely approximated with a reduction in infarction of about 85% (
Figure 9. (a) Effect of pre-treatment (1 h) and (b) post-treatment (6 h) of NMITLI118RT+ and NMITLI118RT+LF on cerebral infarct following I/R injury.
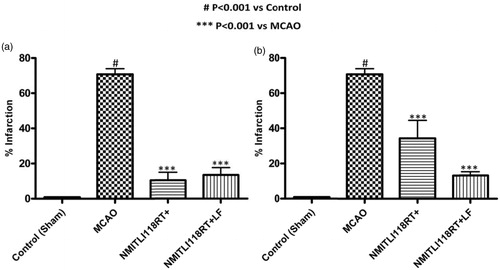
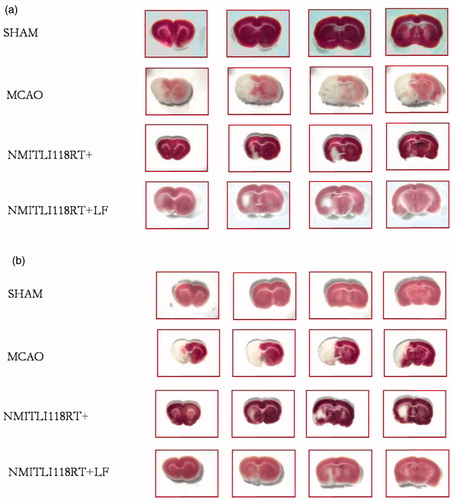
Figure 10. (a) Brain slices stained with TTC showing the effect of pre-treatment (1 h) and (b) post-treatment (6 h) of NMITLI118RT+ and NMITLI118RT+LF following I/R injury.
Post-treatment: The effect of post-treatment with NMITLI118RT+LF was similar to that of the effect shown by pre-treatment on cerebral infarct. It was able to reduce the cerebral damage by 81.41%, whereas a reduction of 44.52% was offered by the NMITLI118R T+ ( and ).
Therefore, these results clearly demonstrated that NMITLI118RT+ and its formulation have a potent neuroprotective activity in cerebral ischemia. NMITLI118RT+LF afforded better activity precisely in the post-treatment and at a reduced dose.
Discussion
Lipid-based drug delivery systems offer the manifold advantages in the delivery of active principles, the most significant being modification or improvement of the drug’s aqueous solubility thereby enhancing its availability at the required site. The greater the available fraction, the better is the intended pharmacological effect of the active principle. With this aim in mind liposomal systems was conceptualized for efficient delivery of NMITLI118RT+ in the event of stroke. Thin-film hydration method was utilized to prepare the intended liposomal formulations and effect of lipid composition, choice of solvent used and medium and conditions of hydration constituted important parameters of the optimization design. A vesicular system loaded with 11.9% NMITLI118RT+ of the total lipid mass and comprising 95.25% (S) and 4.76% (C) was successfully formulated utilizing ethanol–dichloromethane mixture (1:4) by thin-film hydration method and characterized on the basis of size, PDI () zeta potential, encapsulation efficiency, physical stability () IR () DSC () and TEM () studies. The final product afforded a monodisperse population with average particle size around 142.6 ± 0.09 nm, surface morphology studies revealed spherical particles. NMITL1118RT+ exhibited formation of anionic liposomes with a zeta potential of −31.20 ± 1.0 mV; an indicator of stability. Negligible changes in size and PDI values at 4 °C over a 3-week period demonstrated formation of pharmaceutically stable formulation. DSC–TGA curves and FT-IR spectra provided useful information on compatibility and interaction of ingredients. An additional peak (Peak 3; % T: 27.83; 2928.49 cm−1) corresponding to the altered composition due to lipid entrapment was observed and the peak at 1107.85 is also shifted to 1100.65 demonstrating incorporation of NMITLI118RT+ in the lipid bilayer construct of the liposome. The DTA curve for NMITLI118RT+LF showed a single characteristic melting peak at 242.4 °C indicating that physical interaction of ingredients has lead to vesicular entrapment of NMITLI118RT+ in the phospholipid bilayer construct of the liposomes. NMITLI118RT+ and NMITLI118RT+LF were evaluated for their neuro-protective effects on MCAO model in rats. Results demonstrated that both NMITLI118RT+ and NMITLI118RT+LF provided protection against ischemia/reperfusion brain injury as evident from recovery in neurological deficit scores and infract sizes along with MDA and GSH levels (). MDA is a by-product of lipid peroxidation and the significant % decrease in MDA afforded by NMITLI118RT+ and NMITLI118RT+LF deduced their protective effects. GSH levels are markers of oxidative stress; greater the decrement in GSH levels higher is the oxidative stress, thus an increase in their depleted levels exhibited by NMITLI118RT+ and NMITLI118RT+LF provide a link for their neuro-protective potential in reversing the consequences of ischemic brain injury. These were also effective in reducing the neurological deficit as evident by neurological deficit scores. The TTC stained pictures of brain sections demonstrated the extent of infarction and the measurement of percent reduction in the area of cerebral infarcts. Abundance of deep red color demonstrating viable tissue in both the pre- and post-treatment groups showed significant protection provided by NMITLI118RT+ and NMITLI118RT+LF. A comparison drawn between the performances of the two clearly marks that NMITLI118RT+LF exhibited better protective effects as evaluated by all the four parameters in the post-treatment group. Amongst the pre-treatment groups NMITLI118RT+LF exhibited greater reduction of deficit scores, an equivalent response with NMITLI118RT+ in reduction of cerebral infarcts, a 42.50% decrease in MDA levels and a 49.38% increase in GSH levels. However, it can be clearly inferred that NMITLI118RT+LF exhibited significant neuro-protective effect at a dose one-tenth (5 mg/kg p.o) of NMITLI118RT+ (50 mg/kg p.o), also implying that the vesicular entrapment could successfully deliver NMITLI118RT+ and enhance its potential as a neuro-protectant. Both NMITLI118RT+ and NMITLI118RT+LF provide beneficial effects in reversal of I/R injury and in overcoming the pathophysiological damage in the advent of stroke; however, NMITLI118RT+LF demonstrates neuro-protective potential at a reduced one-tenth dose.
Conclusion
NMITLI118RT+ exhibited sufficient scientific promise to provide protection and to reverse the damage due to ischeamic injury and could serve as a neuroprotective agent with beneficial effects in the event of stroke and could be promoted for its use in the therapy following stroke in the paucity of potential anti-stroke agents. Vesicular systems containing NMITLI118RT+ were succefully prepared and their evaluations suggest that the prepared formulation was pharmaceutically acceptable. In the current scenario pertaining to management of stroke rightful delivery of anti-stroke agents assumes greater significance and hence a suitable delivery system such as NMITLI118RT+LF; a vesicular system here that can contain NMITLI118RT+ and provide its therepeutic effects; exhibiting significant improvement against cerebral ischemic injury at a dose that is one-tenth of NMITLI118RT+ seeks relevance to the field.
Supplementary material available online
Supplementary Figures S1-S5
Supplementary Figures S1-S5
Download PDF (658.3 KB)Declaration of interest
The authors are thankful to CSIR-NMITLI, New Delhi, India for financial support and CSIR-CDRI for providing necessary facilities.
Notes
* CDRI Communication No 8970.
References
- Ahmad H, Khandelwal K, Pachauri SD, et al. (2014). Stability indicating studies on NMITLI118RT+ (standardized extract of Withania somnifera Dunal.). Pharmacogn Mag 10:227–33
- Anderson M. (1985). Determination of glutathione and glutathione disulfide in biological samples. Methods Enzymol 113:548–55
- Bederson JB, Pitts LH, Tsuji M, et al. (1986). Rat middle cerebral artery occlusion: evaluation of the model and development of a neurologic examination. Stroke 17:472–6
- Chaudhary G, Sharma U, Jagannathan NR, Gupta YK. (2003). Evaluation of Withania somnifera in a middle cerebral artery occlusion model of stroke in rats. Clin Exp Pharmacol Physiol 30:399–404
- Chaurasiya ND, Sangwan RS, Misra LN, et al. (2009). Metabolic clustering of a core collection of Indian ginseng Withania somnifera Dunal through DNA, isoenzyme, polypeptide and withanolide profile diversity. Fitoterapia 80:496–505
- Colado MI, O’Shea E, Granados R, et al. (1997). Study of the neurotoxic effect of MDMA (“ectasy”) on 5-HT neurons in the brains of mothers and neonates following administration of the drug during pregnancy. Br J Pharmacol 121:827–33
- Demaerschalk BM, Hwang HM, Leung G. (2010). US cost burden of ischemic stroke: a systematic, literature review. Am J Manage Care 16:525–33
- Dhar RS, Verma V, Suri KA, et al. (2006). Phytochemical and genetic analysis in selected chemotypes of Withania somnifera. Phytochemistry 67:2269–76
- Dwivedi AK, Ahmad H, Khandelwal K, et al. (2014). A formulation useful for delivery of neuro protecting agent. Indian Patent Application Number 2773DEL2014, filed on 29.09.2014
- Gortzi O, Lalas S, Chinou I, Tsakins J. (2007). Reevaluation of bioactivity and antioxidant activity of Myrtus communis extract before and after encapsulation in liposomes. Eur Food Res Technol 226:583–90
- Gupta YK, Briyal S, Gulati A. (2010). Therapeutic potential of herbal drugs in cerebral ischemia. Indian J Physiol Pharmacol 54:99–122
- Ishii T, Asai T, Oyama D, et al. (2012). Amelioration of cerebral ischemia-reperfusion injury based on liposomal drug delivery system with asialo-erythropoietin. J control Release 160:81–7
- Ishii T, Asai T, Oyama D, et al. (2013). Treatment of cerebral ischemia-reperfusion injury with PEGylated liposomes encapsulating FK506. FASEB J 27:1362–70
- Lapchak PA, Schubert DR, Maher PA. (2010). Delayed treatment with a novel neurotrophic compound reduces behavioral deficits in rabbit ischemic stroke. J Neurochem 116:122–31
- Longa EZ, Weinstein PR, Cummins R. (1989). Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke 20:84–91
- National Stroke Association. (2013) Explaining stroke. p. 1–18, http://www.stroke.org/site/DocServer/ExplainingStroke_web.pdf?docID=3321 [last accessed 14 Aug 2014]
- Park SN, Lee MH, Kim SJ, Yu ER. (2013). Preparation of quercetin and rutin-loaded ceramide liposomes and drug-releasing effect in liposome-in-hydrogel complex system. Biochem Biophys Res Commun 435:361–6
- Rajasekar S, Elango R. (2011). Estimation of alkaloid content of Ashwagandha (Withania somnifera) with HPLC methods. J Exp Sci 2:39–41
- Ramos-Cabrer P, Agulla J, Argibay B, et al. (2011). Serial MRI study of the enhanced therapeutic effects of liposome-encapsulated citicoline in cerebral ischemia. Int J Pharm 405:228–33
- Rao MA, Hatcher JF, Tureyen K. (2005). CDP-choline liposomes provide significant reduction in infarction over free CDP-choline in stroke. Brain Res 1058:193–7