Abstract
Low molecular weight heparins (LMWHs), the anticoagulant drug of choice in many indications, had been suggested as novel drug treatment for a range of diseases. Their superior pharmacokinetic properties compared to unfractionated heparin (UFH), motivated scientists to explore new delivery systems for improved therapeutic outcomes. Micro- and nano-carriers, with the versatile nature and characteristics of materials used for their fabrication, are able to surmount the challenges opposed by their native structures. The present review discusses the recent perspectives on the development of micro- and nano-particulate vectors for the delivery of LMWHs through various routes. Special focus on the application of the suggested systems, their characterization and the achieved improved bioavailability will be given throughout the review.
Introduction
Heparin and related compounds that belong to the glycosaminoglycans family are well characterized and have been widely used in the clinic as anticoagulants for more than 50 years (Motlekar & Youan, Citation2006). The structural similarity between heparin and the endogenous macromolecule, heparan sulfate that participates in inflammatory processes was the basis of various literatures suggesting that heparins, including low-molecular-weight heparins (LMWH), possess anti-inflammatory property (Patel et al., Citation2014). Besides, other researches provided an increased understanding of the heparin structure, indicating that specific “tailor-made” sequences based on the heparin template could produce different therapeutic activities. These studies provide the key for novel drug treatments for a range of diseases, including cancer as well as various inflammatory diseases (Lever & Clive, Citation2002). Although commercial, pharmaceutical heparin preparations are strictly obtained from mammalian sources, marine mollusk have recently been identified as a potent alternative source for extraction of several heparin and LMWH-like polymers with high anticoagulant activity (Saravanan & Shanmugam, Citation2010; Periyasamy et al., Citation2013). Heparins [both unfractionated (UFH) and low molecular weight (LMWH) derivatives] remain as the agents of choice for pharmacologic thromboprophylaxis, treatment of thromboembolism during pregnancy, with pediatric patients and in myocardial infarction, cardiovascular surgery, coronary angioplasty and numerous other conditions (Eldor, Citation2002; Pineo et al., Citation2004; Greer, Citation2005; Baldwin et al., Citation2014; Molinari et al., Citation2014).
Drug delivery systems, such as micro/nanoparticulate systems are one of the most challenging in pharmaceutical sciences offering uncountable benefits among them, controlled biodistribution and elimination patterns. Besides, by the new indications of UFH and LMWH, it deemed necessary to convert the outcome of these drugs to achieve improved desirable therapeutic effects. These drug carriers have to navigate through multiple physiological conditions, target a specified tissue, and finally be uptaken intracellularly (Yoo et al., Citation2011).
In comparison with UFH, LMWH has a more homogeneous molecular weight, ranging from 2000 to 8000 Da (average of 4500) with a more predictive anticoagulant response requiring less coagulation level monitoring (Oliveira et al., Citation2011). Heparins’ structure and stability has been the central core of many studies to find suitable drug delivery systems for their administration (Scala-Bertola et al., Citation2009). The drug load and release profiles obtained from studies on poly-lactic-co-glycolic acid polymer (PLGA)-based microspheres and nanoparticles (NPs) were not encouraging (Kreitz et al., Citation1997; Jiao et al., Citation2001; Hoffart et al., Citation2003, Citation2006) motivating other researchers to prepare microspheres with more satisfactory results (Rawat et al., Citation2008).
To the best of our knowledge, no attempt for a comprehensive manuscript on particulate delivery of LMWHs, in specific, through the various routes has been made. Accordingly we aimed, by this review, to be able to focus on recent perspectives on the development of micro- and nano-particulate carriers for the delivery of LMWHs through various routes, to help improving the outcome with future particulate delivery systems.
LMWHs: chemistry and pharmacological action
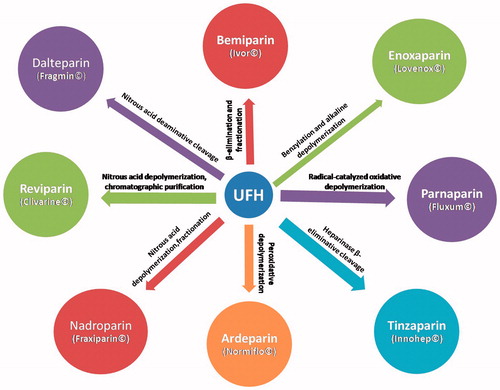
Low-molecular-weight heparins are negatively charged, water soluble, highly sulfated oligosaccharride fragments derived from commercial grade heparin by controlled depolymerization (chemical or enzymatic) (Köse et al., Citation1998). The most commonly used commercial LMWHs and their methods of preparation are shown in (Paliwal et al., Citation2012a). All LMWHs exert their pharmacological action by binding to antithrombin, thereby catalyzing the inactivation of factor Xa (Paliwal et al., Citation2012a). This inhibits thrombin and suppresses the cascade of reactions that lead to blood clotting (Moon et al., Citation2006). However, since they are prepared by different depolymerization methods, the members differ in their pharmacokinetic properties, anticoagulant profile and may not be used interchangeably (Hirsh et al., Citation2001; Bisio et al., Citation2009).
The pharmacokinetic differences between LMWHs and UFH can be simply explained by the increased tendency of the later to bind to non-anticoagulant plasma proteins, endothelial cells and macrophages decreasing the available amount to interact with antithrombin (Lane et al., Citation1989; Young et al., Citation1992; Weitz, Citation1997; Moon et al, Citation2006; Song & Kim, Citation2006). Moreover, binding of UFH to osteoblasts and platelet factor 4 results in increased tendency to induce osteoporosis (Garcia et al., Citation2012) and thrombocytopenia (Grace, Citation2001), respectively. The unpredictable anti-coagulant response to UFH reflects the wide variability in plasma concentrations of heparin binding proteins needing careful laboratory monitoring during UFH treatment (Hirsh et al., Citation1976; Young et al., Citation1992). Finally, the lower bleeding propensity associated with LMWH is attributed to its better antithrombotic (anti-Factor Xa) to anticoagulant (anti-factor IIa) ratio compared to UFH (4:1 to 2:1 for LMWH and 1:1.5 for UFH) (Eldor, Citation2002; Mustafa et al., Citation2004; Moon et al., Citation2006).
To sum up, many of the untoward effects associated with UFH are caused by its binding to secondary locations, an interaction which is not seen with LMWHs and which gives them a better safety profile. summarizes some of the main advantages which led to the increasing use of LMWHs since their introduction into the clinical practice some 25 years ago.
Table 1. Clinical advantages of LMWHs over UFH.
Therefore, LMWHs have received widespread acceptance as the anticoagulant of choice not only for short-term treatment of deep venous thrombosis (DVT) and pulmonary embolism (PE) (Bai & Ahsan, Citation2010) but also for prolonged duration thromboprophylaxis in certain pathologic conditions, such as cancer (Schulman, Citation2003; Kher & Samama, Citation2005; Debourdeau et al., Citation2008; Walter et al., Citation2014). Moreover, LMWHs have been used for maintenance of vessel patency during hemodialysis and artery bypass grafting (Patel et al., Citation2009), prevention of acute bronchoconstrictor responses and airway hyper-responsiveness in asthma (Campo et al., Citation1999; Ahmed et al., Citation2000) and regulation of growth factor activity in various vascular disorders and angiogenesis (Reyes-Ortega et al., Citation2013). Nevertheless, their high anionic charge density, large molecular weight and low stability in acidic stomach pH limit their gastrointestinal absorption, as well as, absorption from other non-invasive routes (Bai et al., Citation2007; Scala-Bertola et al., Citation2009; Fan et al., Citation2014; Hwang & Byun, Citation2014) rendering the parenteral route the most efficient delivery way so far. However, the need for daily subcutaneous injections (SC) limits its outpatient use (Baldwin et al., Citation2014). A summary of the recent perspectives on the delivery of LMWHs using micro- and nano-particulate approaches via the different routes is presented below.
Micro-delivery platforms for LMWHs
Over the past decades, microencapsulation of LMWHs within different polymeric carriers has drawn considerable attention, for mucosal delivery through different routes. Pulmonary administration of LMWH-loaded microparticles (MPs) containing 60% dipalmitoyl phosphatidylcholine prepared by spray drying or mechanical grinding produced therapeutic levels of the drug (>0.2 IU/ml) and a very rapid onset of action compared to SC route (Qi et al., Citation2004). Differently sized particles with geometric diameters of 20–80 μm indicated that absorption and bioavailability were particle size independent in this range. Moreover, imaging of fluorescently labeled LMWH aerosol particles confirmed that these particles did not reach the deep lung, but in fact deposited in the upper airway and bronchi, from where they were rapidly absorbed into the bloodstream. To improve respirability and enhance residence time in the lungs, Rawat et al., (Citation2008) used PLGA to develop particles with mass densities <0.4 g/cm3 and geometric diameter >5 μm. They prepared the large porous PLGA microspheres of LMWH by a double-emulsion solvent evaporation technique using different additives. Microspheres modified with either stearylamine or polyethyleneamine were able to increase plasma half-life of the drug by up to 5–6-fold compared to SC LMWH following intra-tracheal instillation into rats (Rawat et al., Citation2008). Hence, the authors proposed PLGA-based large porous microspheres as a viable option for the delivery of LMWH via the pulmonary route for a prolonged release effect. Aiming at proving the anti-inflammatory activity of LMWH, Patel et al., (Citation2013) found that drug-loaded large porous PEG-PLGA MPs, administered intra-tracheally, showed promising anti-asthmatic activity when tested in sensitized rat animal model of asthma.
The nasal route was also investigated for the systemic delivery of LMWHs by several groups. Arnold et al. (Citation2002) formulated nasal drops of a LMWH with tetradecylmaltoside (TDM), a non-ionic surfactant and a potent absorption enhancer. Yang et al. (Citation2004) exploited the effect of dimethyl-beta-cyclodextrin in LMWH-cyclodextrin formulations on tight junctions’ permeability. Both groups reported enhanced nasal drug absorption mediated through opening of cell–cell tight junctions. The effect was reversible and diminished with time indicating safety of the formulations.
Several approaches for the preparation of orally-active LMWH-loaded polymeric MPs were attempted. Complex coacervation strategy was employed to overcome the strong negative charge of tinzaparin (Lamprecht et al., Citation2007). In this study, tinzaparin/acacia gum mixture was coacervated with gelatin (A or B) to produce MPs that can protect the drug from gastric juice. The system did not provide sustained release, but rather, either efficient retention of tinzaparin inside the MP matrix at pHs < 4 or fast and complete release within 15 min at higher pHs triggering the dissociation of tinzaparin/acacia gum mixture and gelatin. However, in-vivo results demonstrated a poor oral bioavailability of only 4.2%. This was attributed to the exclusion of paracellular transport and intracellular uptake of the MPs due to their large size (>5μm) and reduced probability of uptake via gut-associated lymphatic tissue as the rabbit model used have only few Peyer’s patches. In another study, colon-targeted enoxaparin-loaded microspheres were prepared using the pH-sensitive polymer Eudragit P4135F (Meissner et al., Citation2007). Here, the anti-inflammatory, rather than the anti-coagulant, effect of the drug was pursued for the treatment of inflammatory bowel disease. High entrapment efficiencies (up to 78.2%) and satisfactory pH-dependent in-vitro release profiles were observed although no proper in-vivo study was performed to confirm the clinical efficacy of this delivery system. Another pH-sensitive Eudragit grade, Eudragit S100, was utilized to prepare ardeparin-loaded microspheres for oral delivery by spray drying technique. Good production yields of powders with particle size in the range of 19–60 μm were reported but again with no referral to any in-vitro release or in-vivo studies (Motlekar & Youan, Citation2008). Furthermore, Viehof & Lamprecht (Citation2013) formulated two LMWHs (enoxaparin and nadroparin) in Eudragit RL and RS coacervates using polyethylene glycol solvent having size around 40 μm and high encapsulation efficiencies (>90%). However, a very poor oral bioavailability not exceeding 6% even after doubling the dose was reported. The only plausible absorption mechanism of LMWH from the coacervates, according to the authors, was a non-specific particle adhesion to the epithelial barrier followed by replacement of the drug on the particle surface by mucin (Viehof & Lamprecht, Citation2013). The free drug then diffused through the mucus layer and was actively either taken up into or transported through the absorption barrier. No effect of the polymers on the tight junction could be detected and no particle translocation across the Peyer’s patches was expected due to the large size of the coacervates, which might explain the low values of bioavailabilities obtained.
Co-encapsulation of an absorption enhancer (papain) with LMWH into albumin microspheres followed by enteric coating with Eudragit L100-55 and polyethylene glycol 8000 using spray drying was studied for oral delivery of LMWH (Lanke et al., Citation2009). The spray dried microspheres were all around 5 μm in size and possessed high encapsulation efficiencies (66–78%). In-vivo assessment of the formulations revealed the achievement of therapeutic levels of the drug at the jejunum (the target site) with prolongation of half-life six-fold compared to SC route. This was attributed to the effect of papain which caused opening of the tight junctions. However, the maximum relative bioavailability achieved was 21%.
Given the poor clinical efficacy of non-invasive microparticulate delivery systems of LMWH and the therapeutic advantage of parenteral delivery (regarding bioavailability and accurate dosage control), the search for the greater potential of the invasive route never ceased. Firoz et al. (Citation2009) and Oliveira et al. (Citation2011) developed long acting parenteral polymeric microparticulate delivery systems of enoxaparin based on PLGA 85:15 and 50:50 lactide to glycolide ratios, respectively. High encapsulation efficiencies (>50%) were reported in the relatively hydrophobic polymer by optimization of fabrication techniques. The former group conducted an in-vivo study on rabbit model from which they concluded that the formulation was able to prolong the duration of action of the drug. Moreover, in-vitro release study performed by the later group showed a prolonged (but incomplete) release of the drug for 35 days in phosphate buffered saline (pH 7.4).
Liposomes are another class of colloidal carriers that can be produced in the micro- or nano-size range according to constituents used and the preparation procedure. Multilamellar vesicles conjugated to LMWH were prepared by thin film hydration method followed by conjugation via carbodiimide chemistry (Köse et al., Citation1998). The vesicles showed good hemocompatibility and were assumed to have potentially long half-lives in the blood circulation.
Nano-delivery platforms for LMWHs
Nano-delivery systems are the most explored novel drug delivery approaches, because of their ability to improve drug bioavailability, solubility and retention time owing to their small size, surface structure and high surface area (Hallan et al., Citation2014). These colloidal carriers can be fabricated from a wide range of polymers, lipids, surfactants, dendrimers or combinations in a wide range of sizes and can sustain localized therapeutic activity for weeks in addition to their penetration capability through various biological barriers (Nitta & Numata, Citation2013; Reyes-Ortega et al., Citation2013; Hallan et al., Citation2014). Despite the number of challenges posed by the versatile nanotechnology platforms including scaling-up difficulty, low-drug loading capacity, wide size distribution added to the nano-toxicological concerns (Nitta & Numata, Citation2013); nevertheless, the potential of nano-carrier vectors for the delivery of LMWHs via the different routes has been embraced by many researchers and has shown significant progress.
Long lived pegylated and conventional liposomes encapsulating ardeparin were prepared in the nanosize range (104.8 and 113 nm, respectively) by hydration method (Bai et al., Citation2009; Bai & Ahsan, Citation2010). They were successfully tested for their pharmacological efficacy in rodent models of PE and DVT following pulmonary administration. A once-every 48 h inhaled dose showed similar therapeutic efficacy to a once-daily SC dosing regimen without any significant toxicity to lung tissues. Pegylation helped in avoiding the reduced half-life and bioavailability seen after three repeated dosings of the conventional liposomes.
Dendrimeric micelles present a promising class of nanocarriers that have also been tested for pulmonary delivery of LMWHs. Unlike negatively-charged dendrimers which had no positive effect on enhancement of enoxaparin bioavailability, pegylated and non-pegylated positively-charged poly(amidoamine) (PAMAM) dendrimers enhanced the relative bioavailability of the drug by 60.6 and 41.3%, respectively (Bai et al., Citation2007; Bai & Ahsan, Citation2009). Moreover, pegylated formulations at a dose of 100 U/kg administered at 48-h intervals, showed very close efficacy to 24-h SC administered drug at a dose of 50 U/kg in rats.
Chitosan-hyaluronic acid NPs loaded with LMWH for pulmonary administration were developed and evaluated for prevention of rat-mast cell degranulation (Oyarzun-Ampuero et al., Citation2009). Although, the nanosystems showed high encapsulation efficiency (up to 70%) and good stability in phosphate-buffered saline for 24 h, they did not show any added advantage over the free drug in prevention of histamine release.
In oral delivery, the uptake of NPs via Peyer’s patches in the gut-associated lymphoid tissue has triggered their use as a potentially useful oral delivery system (Hallan et al., Citation2014). Strategies for oral delivery of LMWHs through the use of nanoparticulate carriers are also based on enhancement of bioadhesion and adsorption to gastro-intestinal tissues, modification of tight junctions and protection against acidic pH of the stomach.
Paliwal et al. (Citation2011) synthesized LMWH-lipid conjugates, which were then encapsulated in phosphatidylcholine-stabilized solid lipid nanoparticles (SLNs) for enhancement of LMWH's oral bioavailability. The chylomicron mimicking biocompatible lipid-based carrier systems improved oral BAV of the therapeutic molecule by following a transcellular mechanism of lipid transport through intestinal lymphatics. Prepared SLNs were found to be free of any gastro-intestinal toxicity and a maximum overall BAV (relative to IV LMWH) of 57% (Paliwal et al., Citation2011).
The most common bioadhesive polymer, which had been used in the fabrication of LMWH-loaded NPs is chitosan and its derivatives (Sun et al., Citation2008, Citation2010; Paliwal et al., Citation2012b). Among these studies, the maximum oral bioavailability, did not exceed 9%, was achieved with Sun et al. (Citation2010). Bagre et al., (Citation2013) compared the oral BAV of chitosan NPs before and after coating with alginate used to overcome solubility problems of chitosan as well as improving oral absorption efficacy at pH values greater than 6.5 found in major parts of the gastro-intestinal tract. A 1.6-fold higher oral bioavailability was attained by the coated NPs compared to uncoated ones although no prolongation in the drug’s half-life was observed.
In another attempt, enoxaparin-loaded NPs possessing both pH-responsive and mucoadhesive properties were formulated using hydroxy propyl methyl cellulose phthalate (HPMCP) as an enteric coating material and thiolated chitosan as mucoadhesive polymer (Fan et al., Citation2014). A pronounced improvement of oral BAV, which reached 21% was reported holding promise for these systems as potential carriers for oral delivery of LMWHs.
Furthermore, poly-electrolyte nanocomplexes of enoxaparin and synthetic chitosan derivatives were thoroughly investigated for oral delivery. Mahjub et al., (Citation2014) synthesized N-trimethyl-O-carboxymethyl chitosan carrying a permanent positive charge on the quaternized amino group and a negative charge on the carboxymethyl group for improved interaction with the LMWH. The nanocomplexes were prepared by self-assembly based on the electrostatic interaction between the positively charged copolymer and negatively charged enoxaparin. High encapsulation efficiency (76.4 ± 5.43%) and prolonged in-vitro release in simulated intestinal fluid for 10 h were observed. MTT cell cytotoxicity studies on Caco-2 cells, however, showed concentration-dependent cytotoxicity after 24 h exposure to NPs.
Hydrophobic grafting of chitosan with glyceryl monostearate (GM) produced copolymers that exhibited improved mucoadhesion properties up to 18.6% GM substitution degree (Wang et al., Citation2013). Enoxaparin release in simulated gastric fluid (SGF) was very limited with less than 15% released after 6 h, probably due to the strong electrostatic interaction between the positively charged copolymers and negatively charged enoxaparin. In vivo absorption in rat model, after a single peroral administration of different polymer-based enoxaparin nanocomplexes, revealed an enhanced BAV of 12.96% with improved pharmacokinetics compared to oral enoxaparin saline solution. This was attributed to a combination of the superior property of chitosan, the lipophilicity of GM and the distinctive advantages of NP delivery system to enhance the transport of enoxaparin across the intestinal epithelium.
As a different approach, Hoffart et al. (Citation2006) managed to formulate tinzaparin-loaded NPs using a blend of Eudragit RS polyester and a polycationic polymethacrylate polymer. The prepared NPs exhibited a prolonged anti-coagulant effect of up to 8 h with an oral bioavailability of 59%. The success of this nanocarrier system was attributed to the strong and intimate contact of the positively-charged polymeric particles and the negatively-charged gastro-intestinal mucosa. Similarly, novel polymeric NPs encapsulating bemiparin, were fabricated from Eudragit RSPO, PLGA and a new synthetic block copolymer from the polymethylmethacrylate family. The systems showed high encapsulation efficiencies (89–98%) and an enhanced dose-dependent proliferation in cell proliferation assay indicating the applicability of these systems as activating agents of growth factors function (Reyes-Ortega et al., Citation2013).
Interestingly, the topical route had also been considered for the delivery of LMWHs to treat superficial thrombosis and enhance resorption of hematomas. Song & Kim (Citation2006) investigated the use of flexible liposomes (Flexosomes) carrying different surface charges for the topical delivery of LMWH. Flexosomes ranged in size between 81.4 and 167.1 nm depending on surface charge carried. Cationic flexosomes displayed superior properties regarding entrapment efficiency, physico-chemical stability, in-vitro skin penetration and in-vivo localization into deeper skin layers. Nano-sized liposomes were also successfully tested for subconjunctival delivery of LMWH for enhancement of absorption of local hemorrhages in rabbits (Moon et al., Citation2006). A commercial NP suspension composed of Eudragit RS was mixed with different LMWHs, namely enoxaparin, bemiparin, tinzaparin and nadroparin, to produce a gel for topical application (Loira-Pastoriza et al., Citation2012). Quantification of the LMWHs released from gels into skin and plasma samples proved the applicability of the use of gels based on NPs for topical delivery of LMWHs with minimal systemic side effects. In their search for optimum formulations, beside the routine characterization of the developed delivery platforms, various techniques had been used to understand cellular uptake, fate of nanomaterials and mechanisms of enhancement of bioavailability achieved (Bagre et al., Citation2013; Fan et al., Citation2014).
As previously said, the highly anionic nature of LMWH due to the presence of carboxylic acid and sulfate groups in its glycosaminoglycan units made it possible to prepare polyelectrolyte complexes for improvement of its mucosal absorption (Yang et al., Citation2012). A nature which made also the techniques available for the analysis of LMWHs limited and a sort of trouble-some. Common techniques, such as HPLC, ion pair, ion exchange, exclusion chromatography (using ultraviolet (UV)-visible and refractive index detectors) and capillary electrophoresis have all been reported to be unsuccessful in quantification of LMWHs (Oliveira et al., Citation2011). The most commonly employed methods are the nephelometric method (Meissner et al., Citation2007; Oliveira et al., Citation2011), Azure colorimetric method (Sun et al., Citation2008; Bai & Ahsan, Citation2009) and biological quantification of antifactor Xa activity using chromogenic assay kit (Lanke et al., Citation2009). Nephelometric method is based on the detection of turbidity caused by water-insoluble complex between the quaternary ammonium groups of cetylpyridinium chloride and the negatively charged sulfated groups of LMWH. Azure A is a positively charged phenothiazine dye which binds with the negatively charged sulfate groups of the LMWH molecule. This results in a concentration-dependent decrease in the absorbance of the dye at 595 nm due to a negative shift in its λmax (Bai et al., Citation2007). Antifactor Xa assay analyzes LMWH as a complex with antithrombin (AT) present in the blood samples. FXa (in excess) is neutralized in proportion to the amount of LMWH, which determines the amount of [LMWH AT] complex. The remaining amount of FXa hydrolyzes a chromogenic substrate thus liberating the chromophoric group, which is assayed photometrically.
To sum up, motivated by the versatile applications and advantages of LMWHs, the search for delivery systems with tailored release and target profiles will continue to grow to overcome the challenges opposed by its nature and surmount the biological barriers.
Conclusion
The use of LMWHs had not been limited to their anticoagulant effect, but extended to encompass new indications including anti-inflammatory and anti-cancer properties. LMWHs provide more predictive and stable anti-coagulant therapy in many serious indications compared to UFH. The major drawback of the current therapy with LMWHs is, not only the necessity of administration through the patient-inconvenient parenteral route, but also their relatively short duration of action, anionic character and high molecular weights. Micro- and nano-delivery systems offer potential pathways for a more efficient LMWH delivery, especially with the evolution of new polymers and techniques of nanotechnology and its adaptation in the biomedical field. Successful engineering of carriers for LMWH delivery, able to surmount the biological barriers, requires a good understanding of the physico-chemical properties of each member of the LMWH family as well as better exploitation of the various targets and routes of administration.
Declaration of interest
The authors report no conflicts of interest.
References
- Ahmed T, Ungo J, Zhou M, Campo C. (2000). Inhibition of allergic late airway responses by inhaled heparin-derived oligosaccharides. J Appl Physiol 88:1721–9
- Arnold J, Ahsan F, Meezan E, Pillion DJ. (2002). Nasal administration of low molecular weight heparin. J Pharm Sci 91:1707–14
- Bagre AP, Jain K, Jain NK. (2013). Alginate coated chitosan core shell NPs for oral delivery of enoxaparin: in vitro and in vivo assessment. Int J Pharm 456:31–40
- Bai S, Ahsan F. (2009). Synthesis and evaluation of pegylated dendrimeric nanocarrier for pulmonary delivery of low molecular weight heparin. Pharm Res 26:539–48
- Bai S, Ahsan F. (2010). Inhalable liposomes of low molecular weight heparin for the treatment of venous thromboembolism. J Pharm Sci 99:4554–64
- Bai S, Gupta V, Ahsan F. (2009). Cationic liposomes as carriers for aerosolized formulations of an anionic drug: safety and efficacy study. Eur J Pharm Sci 38:165–71
- Bai S, Thomas C, Ahsan F. (2007). Dendrimers as a carrier for pulmonary delivery of enoxaparin, a low-molecular weight heparin. J Pharm Sci 96:2090–106
- Baldwin AD, Robinson KG, Militar JL, et al. (2014). In situ crosslinkable heparin-containing poly(ethylene glycol) hydrogels for sustained anticoagulant release. J Biomed Mater Res A 100:2106–18
- Bisio A, Vecchietti D, Citterio L, et al. (2009). Structural features of low-molecular-weight heparins affecting their affinity to antithrombin. Thromb Haemost 102:865–73
- Campo C, Molinari JF, Ungo J, Ahmed T. (1999). Molecular-weight-dependent effects of nonanticoagulant heparins on allergic airway responses. J Appl Physiol 86:549–57
- Debourdeau P, Elalamy I, de Raignac A, et al. (2008). Long-term use of daily subcutaneous low molecular weight heparin in cancer patients with venous thromboembolism: why hesitate any longer? Support Care Cancer 16:1333–41
- Eldor A. (2002). The use of low-molecular-weight heparin for the management of venous thromboembolism in pregnancy. Eur J ObstetGynecol Reprod Biol 104:3–13
- Fan B, Xing Y, Zheng Y, et al. (2014). pH-responsive thiolated chitosan NPs for oral low-molecular weight heparin delivery: in vitro and in vivo evaluation. Drug Deliv 28:1–10
- Firoz S, Sarasija S, Yajaman S. (2009). Long acting parenteral formulation of heparin. J Pharm Res 2:1547–49
- Garcia DA, Baglin TP, Weitz JI, Samama MM. (2012). Parenteral Anticoagulants: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 141(2 Suppl):e24S–e43S
- Grace JB. (2001). Thromboembolic disease. 2001. In: Shargel L, Mutnick AH, Souney PF, Swanson LN, eds. Comprehensive pharmacy review. 4th ed. Maryland, USA: Lippincott W&W, 754–67
- Greer IA. (2005). Venous thromboembolism and anticoagulant therapy in pregnancy. Gender Med 2:S10–17
- Hallan SS, Kaur P, Kaur V, et al. (2014). Lipid polymer hybrid as emerging tool in nanocarriers for oral drug delivery. Artif Cells Nanomed Biotechnol 19:1–16
- Hirsh J, Anand SS, Halperin JL, Fuster V. (2001). Guide to anticoagulant therapy, Heparin: a statement for healthcare professionals from the American Heart Association. Circulation 103:2994–3018
- Hirsh J, van Aken WG, Gallus AS, et al. (1976). Heparin kinetics in venous thrombosis and pulmonary embolism. Circulation 53:691–5
- Hoffart V, Lamprecht A, Maincent P, et al. (2006). Oral bioavailability of a low molecular weight heparin using a polymeric delivery system. J Control Release 113:38–42
- Hoffart V, Ubrich N, Lamprecht A, et al. (2003). Microencapsulation of low molecular weight heparin into polymeric particles designed with biodegradable and nonbiodegradable polycationic polymers. Drug Deliv 10:1–7
- Hwang SR, Byun Y. (2014). Advances in oral macromolecular drug delivery. Expert Opin Drug Deliv 11:1955–67
- Jiao YY, Ubrich N, Marchand-Arvier M, et al. (2001). Preparation and in vitro evaluation of heparin-loaded polymeric NPs. Drug Deliv 8:135–41
- Kher A, Samama MM. (2005). Primary and secondary prophylaxis of venous thromboembolism with low-molecular-weight heparins: prolonged thromboprophylaxis, an alternative to vitamin K antagonists. J Thromb Haemost 3:473–81
- Köse GT, Arica MY, Hasirci V. (1998). Low-molecular-weight heparin-conjugated liposomes with improved stability and hemocompatibility. Drug Deliv 5:257–64
- Kreitz MR, Domm JA, Mathiowitz E. (1997). Controlled delivery of therapeutics from microporous membranes: II. In vitro degradation and release of heparin-loaded poly(D,L-lactide-co-glycolide). Biomaterials 18:1645–51
- Lamprecht A, Ubrich N, Maincent P. (2007). Oral low molecular weight heparin delivery by MPs from complex coacervation. Eur J Pharm Biopharm 67:632–8
- Lane DA. (1989). Heparin binding and neutralizing proteins. In: David A, Lindahl U, eds. Heparin: chemical and biological properties clinical applications lane. Boca Raton, FL: RC Press, 363–91
- Lanke SS, Gayakwad SG, Strom JG, D'souza MJ. (2009). Oral delivery of low molecular weight heparin microspheres prepared using biodegradable polymer matrix system. J Microencapsul 26:493–500
- Lever R, Clive P. (2002). Novel drug development opportunities for heparin. Nature Rev Drug Discov 1:140–8
- Loira-Pastoriza C, Sapin-Minet A, Diab R, et al. (2012). Low molecular weight heparin gels, based on NPs, for topical delivery. Int J Pharm 426:256–62
- Mahjub R, Shayesteh TH, Radmehr M, et al. (2014). Preparation and optimization of N-trimethyl-O-carboxymethyl chitosan NPs for delivery of low-molecular-weight heparin. Pharm Dev Technol 25:1–12
- Meissner Y, Ubrich N, El Ghazouani F, et al. (2007). Low molecular weight heparin loaded pH-sensitive MPs. Int J Pharm 335:147–53
- Molinari AC, Banov L, Bertamino M, et al. (2014). A practical approach to the use of low molecular weight heparins in VTE treatment and prophylaxis in children and newborns. Paediatr Hematol Oncol 32:1–10
- Moon JW, Song YK, Jee JP, et al. (2006). Effect of subconjunctivally injected, liposome-bound, low-molecular-weight heparin on the absorption rate of subconjunctival hemorrhage in rabbits. Invest Ophthalmol Vis Sci 47:3968–74
- Motlekar NA, Youan BB. (2006). The quest for non-invasive delivery of bioactive macromolecules: a focus on heparins. J Control Release 113:91–101
- Motlekar NA, Youan BB. (2008). Optimization of experimental parameters for the production of LMWH-loaded polymeric microspheres. Drug Des Devel Ther 2:39–47
- Mustafa F, Yang T, Khan MA, Ahsan F. (2004). Chain length-dependent effects of alkylmaltosides on nasal absorption of enoxaparin. J Pharm Sci 93:675–83
- Nitta SK, Numata K. (2013). Biopolymer-based NPs for drug/gene delivery and tissue engineering. Int J Mol Sci 14:1629–54
- Oliveira SS, Oliveira FS, Gaitani CM, Marchetti JM. (2011). MPs as a strategy for low-molecular-weight heparin delivery. J Pharm Sci 100:1783–92
- Oyarzun-Ampuero FA, Brea J, Loza MI, et al. (2009). Chitosan-hyaluronic acid NPs loaded with heparin for the treatment of asthma. Int J Pharm 381:122–9
- Paliwal R, Paliwal SR, Agrawal GP, Vyas SP. (2011). Biomimetic solid lipid NPs for oral bioavailability enhancement of low molecular weight heparin and its lipid conjugates: in vitro and in vivo evaluation. Mol Pharm 8:1314–21
- Paliwal R, Paliwal SR, Agrawal GP, Vyas SP. (2012a). Recent advances in search of oral heparin therapeutics. Med Res Rev 32:388–409
- Paliwal R, Paliwal SR, Agrawal GP, Vyas SP. (2012b). Chitosan nanoconstructs for improved oral delivery of low molecular weight heparin: in vitro and in vivo evaluation. Int J Pharm 422:179–84
- Patel B, Gupta N, Ahsan F. (2014). Low-molecular-weight heparin (LMWH)-loaded large porous PEG-PLGA particles for the treatment of asthma. J Aerosol Med Pulm Drug Deliv 27:12–20
- Patel RP, Narkowicz C, Jacobson GA. (2009). Investigation of the effect of heating on the chemistry and antifactor Xa activity of enoxaparin. J Pharm Sci 98:1700–11
- Periyasamy N, Murugan S, Bharadhirajan P. (2013). Isolation and characterization of anticoagulant compound from marine mollusc Donax faba (Gmelin, 1791) from Thazhanguda, Southeast Coast of India. Afr J Biotechnol 12:5968–74
- Pineo G, Hull R, Marder V. (2004). Oral delivery of heparin: SNAC and related formulations. Best Pract Res Clin Haematol 17:153–60
- Qi Y, Zhao G, Liu D, et al. (2004). Delivery of therapeutic levels of heparin and low-molecular-weight heparin through a pulmonary route. Proc Natl Acad Sci USA 101:9867–72
- Rawat A, Majumder QH, Ahsan F. (2008). Inhalable large porous microspheres of low molecular weight heparin: in vitro and in vivo evaluation. J Control Release 128:224–32
- Reyes-Ortega F, Rodríguez G, Aguilar MR, et al. (2013). Encapsulation of low molecular weight heparin (bemiparin) into polymeric NPs obtained from cationic block copolymers: properties and cell activity. J Mater Chem B 1:850–60
- Saravanan R, Shanmugam A. (2010). Isolation and characterization of low molecular weight glycosaminoglycans from marine mollusc Amussium pleuronectus (linne) using chromatography. Appl Biochem Biotechnol 160:791–9
- Scala-Bertola J, Rabiskova M, Lecompte T, et al. (2009). Granules in the improvement of oral heparin bioavailability. Int J Pharm 374:12–16
- Schulman S. (2003). Care of patients receiving long-term anticoagulant therapy. N Engl J Med 349:675–83
- Song YK, Kim CK. (2006). Topical delivery of low-molecular-weight heparin with surface-charged flexible liposomes. Biomaterials 27:271–80
- Sun W, Mao S, Mei D, Kissel T. (2008). Self-assembled polyelectrolyte nanocomplexes between chitosan derivatives and enoxaparin. Eur J Pharm Biopharm 69:417–25
- Sun W, Mao S, Wang Y, et al. (2010). Bioadhesion and oral absorption of enoxaparin nanocomplexes. Int J Pharm 386:275–81
- Viehof A, Lamprecht A. (2013). Oral delivery of low molecular weight heparin by polyaminomethacrylate coacervates. Pharm Res 30:1990–8
- Walter RJ, Moores LK, Jimenez D. (2014). Pulmonary embolism: current and new treatment options. Curr Med Res Opin 30:1975–89
- Wang L, Li L, Sun Y, et al. (2013). Exploration of hydrophobic modification degree of chitosan-based nanocomplexes on the oral delivery of enoxaparin. Eur J Pharm Sci 50:263–71
- Weitz JI. (1997). Low-molecular-weight heparins. N Engl J Med 337:688–99
- Yang T, Hussain A, Paulson J, et al. (2004). Cyclodextrins in nasal delivery of low-molecular-weight heparins: in vivo and in vitro studies. Pharm Res 21:1127–36
- Yang T, Nyiawung D, Silber A, et al. (2012). Comparative studies on chitosan and polylactic-co-glycolic acid incorporated NPs of low molecular weight heparin. AAPS PharmSciTech 13:1309–18
- Yoo JW, Doshi N, Mitragotri S. (2011). Adaptive micro and NPs: temporal control over carrier properties to facilitate drug delivery. Adv Drug Deliv Rev 63:1247–56
- Young E, Prins M, Levine MN, Hirsh J. (1992). Heparin binding to plasma proteins, an important mechanism for heparin resistance. Thromb Haemost 67:639–43