Abstract
Convection-enhanced delivery (CED) of therapeutic agents is a promising local delivery technique that has been extensively studied as a treatment for CNS diseases over the last two decades. One continuing challenge of CED is accurate and consistent delivery of the agents to the target. The present study focused on a new type of therapeutic agent, NK012, a novel SN-38-loaded polymeric micelle. Local delivery profiles of NK012 and SN-38 were studied using rodent brain and intracranial rodent brain tumor models. First, the cytotoxicity of NK012 against glioma cell lines was determined in vitro. Proliferations of glioma cells were significantly reduced after exposure to NK012. Then, the distribution and local toxicity after CED delivery of NK012 and SN-38 were evaluated in vivo. Volume of distribution of NK012 after CED was much larger than that of SN-38. Histological examination revealed minimum brain tissue damage in rat brains after delivery of 40 µg NK012 but severe damage with SN-38 at the same dose. Subsequently, the efficacy of NK012 delivered via CED was tested in 9L and U87MG rodent orthotopic brain tumor models. CED of NK012 displayed excellent efficacy in the 9L and U87MG orthotopic brain tumor models. Furthermore, NK012 and gadolinium diamide were co-delivered via CED to monitor the NK012 distribution using MRI. Volume of NK012 distribution evaluated by histology and MRI showed excellent agreement. CED of NK012 represents an effective treatment option for malignant gliomas. MRI-guided CED of NK012 has potential for clinical application.
Introduction
Malignant brain tumors, such as glioblastoma, remain the most difficult neoplasms to treat despite intensive multimodal treatment including surgical resection, radiation therapy and systemic chemotherapy. Systemic chemotherapy requires high doses of many anticancer drugs because of poor penetration of the blood–brain barrier (Groothuis, Citation2000; Huynh et al., Citation2006). Consequently, systemic side effects are the limiting factor in chemotherapeutic protocols for patients with brain tumor, and emphasize the need for efficient, specific methods of delivery in the treatment of human glioblastoma.
Convection-enhanced delivery (CED) circumvents the blood–brain barrier by delivering agents directly into the tumor and surrounding parenchyma based on continuous positive-pressure infusion (Bobo et al., Citation1994). CED can achieve large volumes of distribution, as the diffusive spread is not limited by concentration gradients (Chen et al., Citation1999). Importantly, CED provides direct access to the tumor bed, thus resulting in high local concentrations of drug with minimal systemic absorption (Yun et al., Citation2013). Currently, CED has been clinically tested in the treatment of neurodegenerative diseases, such as Parkinson’s disease (Gill et al., Citation2003; Eberling et al., Citation2008), and neuro-oncology (Mardor et al., Citation2001; Kunwar, Citation2003). The administration of therapeutic agents via CED is not without its challenges, such as accurate and consistent delivery of the agent (Sawamura et al., Citation1999). Thus, our studies have focused on the design of new therapeutic agents delivered by CED that enable reproducible delivery profile and will improve the prognosis without causing dose-limiting systemic or neurological toxicity.
NK012 is a novel SN-38-loaded polymeric micelle constructed in an aqueous medium by self-assembly of an amphiphilic block copolymer, poly(ethylene glycol)–poly(amino acid) (SN-38) (Koizumi et al., Citation2006). NK012 is categorized as a drug delivery system and several preclinical and clinical studies have shown that the formulation appears to accumulate selectively and persists for a long time in solid tumor tissues due to the enhanced permeability and retention effect (Matsumura, Citation2011). Systemically administered NK012 has antitumor activity within brain tumor (Kuroda et al., Citation2009, Citation2010), but no optimal methods for initial local brain delivery have been established.
The present study compared the distribution profile and therapeutic effect of NK012 delivered by CED on rodent orthotopic brain tumor models, and found that NK012 via CED significantly prolonged survival in rodent brain tumor models.
Materials and methods
Drugs and cell lines
SN-38 was purchased from Sigma-Aldrich Co. (St. Louis, MO) and was dissolved in dimethyl sulfoxide (Sigma-Aldrich Co.) at 40 mg/ml as stock solution. NK012 was provided by Nippon Kayaku Co., Ltd. (Tokyo, Japan). Infusion solution was prepared with 5% glucose solution. The human glioblastoma cell line U87MG, rat gliosarcoma cell line 9L and glioblastoma cell line F98 and mouse glioma cell line GL261 (American Type Culture Collection, Rockville, MD) were cultured in essential medium containing 10% fetal bovine serum (Invitrogen, Carlsbad, CA) and 1% penicillin/streptomycin (Invitrogen) at 37 °C in the presence of 5% CO2.
In vitro cytotoxicity assay
In vitro cell proliferation was measured by the water soluble tetrazolium (WST) assay. Briefly, cells (5 × 103 cells/well) were plated in 96-well plates in triplicate, allowed to attach for 24 h, and then the growth medium was changed to new medium containing various concentrations of NK012. After incubation for 72 h, 10% of WST-8 working solution (Cell Counting Kit-8®; Dojindo Molecular Technologies, Inc., Kumamoto, Japan) was added to each well followed by incubation for 1 h. The absorbance at 450 nm was then measured in a 96-well spectrophotometric plate reader (SpectraMax 190; Molecular Devices, Sunnyvale, CA). Cell viability was measured in triplicate and was repeated three times. Data were averaged and normalized against the non-treated controls to generate dose–response curves. The number of living cells was calculated using the following formula:
Animals
Twelve-week-old male Fischer 344 rats were purchased from Japan SLC, Inc. (Hamamatsu, Shizuoka, Japan). Eight-week-old male athymic nude rats (F344/NJcl-rnu/rnu) were purchased from CLEA Japan, Inc. (Tokyo, Japan). Protocols used in the animal studies were approved by the Institute for Animal Experimentation of Tohoku University Graduate School of Medicine.
Intracranial tumor implantation
Fischer 344 rats were used for the 9L model, and F344/NJcl-rnu/rnu (nude) rats were used for the U87MG model. The 9L and U87MG cells were obtained as described previously (Saito et al., Citation2004a,Citationb). Briefly, 9L and U87MG cells were harvested using trypsin-EDTA following washing once with complete medium, and resuspended in cold phosphate-buffered saline (PBS) for implantation. A cell suspension containing 1 × 104 (9L) or 2 × 105 (U87MG) cells per 10 μl of PBS was used for implantation into the striatum region of rat brains. Under deep isoflurane anesthesia, rats were placed in a small animal stereotactic frame (David Kopf Instruments, Tujunga, CA). A sagittal incision was made through the skin to expose the cranium, and a burr hole was made in the skull at 0.5 mm anterior and 3 mm lateral from bregma using a small dental drill. Five microliters of cell suspension were injected at a depth of 4.5 mm from the brain surface. After a wait of 2 min, another 5 μl were injected at a depth of 4 mm. After a final wait of 2 min, the needle was removed, and the wound was closed with sutures.
Convection-enhanced delivery
Infusion was performed using the CED method as described previously (Saito et al., Citation2004a,Citationb; Kikuchi et al., Citation2008). Briefly, a reflux-free step-design infusion cannula connected to a 1-ml syringe mounted on a microinfusion pump (BeeHive; Bioanalytical System, West Lafayette, IN) was used to control the infusion rate. Under deep isoflurane anesthesia, the rats were placed in small-animal stereotactic frames (Narishige Co., Tokyo, Japan). A sagittal incision was made to expose the cranium followed by a burr hole in the skull positioned at 0.5 mm anterior and 3 mm lateral from the bregma using a small dental drill. The following ascending infusion rates were applied to achieve 20 μl total infusion volume: 0.2 μl/min for 15 min, 0.5 μl/min for 10 min and 0.8 μl/min for 15 min.
Evaluation of NK012 and SN-38 distribution in rat brain tissue by fluorescence microscopy
Normal Fischer 344 rats (three rats in each group) received CED using NK012 and free SN-38 (20 µl solution containing 2 mg/ml SN-38 equivalent), and were euthanized immediately after CED. Serial coronal tissue sections (20 μm thickness) were prepared using Tissue-Tek Cryo3 (Sakura Finetek USA, Inc., Torrance, CA), and frozen sections were examined under a fluorescence microscope, BIOREVO BZ9000 (Keyence, Osaka, Japan), at an excitation wavelength of 377 nm and an emission wavelength 447 nm to evaluate the distribution of NK012 within the tissues. Image data were recorded using BZ-II Analyzer 1.10 software (Keyence).
Toxicity evaluation of NK012
Four healthy male Fisher 344 rats weighting approximately 250 g received CED using NK012 (20 µl solution containing 2.0 mg/ml free SN-38 equivalent). Rats were monitored daily for survival and general health, including alertness, grooming, feeding, excreta, skin, fur, mucous, membrane conditions, ambulation, breathing and posture. Rats were deeply anesthetized with diethyl ether, and transcardially perfused with 0.9% saline followed by cold 4% paraformaldehyde 14 days after CED treatment. The brains were removed, post-fixed overnight in the same fixative at 4 °C, subjected to paraffin sectioning (4 µm), and histologically examined with hematoxylin and eosin staining.
Survival study against the 9L and U87MG orthotopic brain tumor models
Twenty rats with implanted 9L tumor cells were randomly divided into three groups on day 5 after tumor cell implantation and treated as follows: (1) CED of PBS (n = 7) as a control, (2) CED of free SN-38 (n = 7) and (3) CED of NK012 (n = 6). Fourteen rats with implanted U87MG tumor cells were randomly divided into two groups on day 5 after tumor cell implantation and treated as follows: (1) CED of PBS (n = 7) as a control and (2) CED of NK012 (n = 7). CED infusion consisted of 20 µl of 5% glucose in the control groups, 40 µg of NK012 in 20 µl of 5% glucose in the NK012 groups, and 40 µg of free SN-38 in a solution of 50% dimethyl sulfoxide in 20 µl of 5% glucose in the SN-38 group. All other rats were monitored daily for survival. Survival was compared between the treatment groups using a log-rank test. Estimated survival was expressed as a Kaplan–Meier curve.
Gadolinium (Gd) co-infusion to visualize NK012 distribution
Six rats received 20 µl of Gd-diethylenetriaminepenta-acetic acid (5 mM) co-infused with 2 mg/ml of NK012 into the striatum. MRI was performed approximately 1 h after the infusion using a 7.0-tesla PharmaScan System (Bruker Biospin, Karlsruhe, Germany) with a 38-mm diameter birdcage coil. Each rat was initially anesthetized with 4% isoflurane and an air/oxygen mixture (7:3). The isoflurane level was lowered to 2.0 ± 0.5% during the MRI. Throughout the MRI, the animal's body temperature was kept at 36 ± 1 °C with a feedback-controlled warm air system (SA Instruments, Stony Brook, NY). The respiration and rectal temperatures were continuously monitored with a small animal monitoring system (SA Instruments). To investigate the relationship between distribution volume of NK012 and Gd at the infusion site, the MR image-based distribution volumes of Gd were calculated with OsiriX (OsiriX Foundation, Geneva, Switzerland) using threshold-based segmentation to grow three-dimensional regions of interest (ROIs) with human-entered seed points. The lower threshold employed was increased by 60% of the maximum on the contralateral side. The “Grow Region (2D/3D segmentation)” tool in the “ROI” dropdown menu allowed automatic outlining of the Gd distribution.
For histological evaluation of NK012 distribution, the rats were euthanized immediately after each MRI session. The brains were harvested, freshly frozen using ice-cold isopentane, and cut into serial coronal sections (20 µm) using a Tissue-Tek Cryo3 (Sakura Finetek USA, Inc.). The fluorescent signal generated by SN-38 was visualized with a fluorescence microscope, BIOREVO BZ9000 (Keyence), at an excitation wavelength of 377 nm and an emission wavelength of 447 nm to evaluate the distribution of NK012. Image data were recorded with BZ-II Analyzer 1.10 software (Keyence). The volume of the NK012 distribution was analyzed using ImageJ software (National Institute of Health, Bethesda, MD). The volume of NK012 distribution, as determined by the fluorescence microscopy images, was compared with the volume of Gd distribution as detected by 7.0-tesla MRI.
Statistical analysis
Statistical analysis was performed using GraphPad Prism 5 for Windows (GraphPad Software, Inc., San Diego, CA). All experiments were repeated three times and the differences for two sample comparisons were determined by Student's t-test. Survival analyses were carried out using the Kaplan–Meier curves and the log-rank test. Significance was determined at p < 0.05.
Results
Efficacy of NK012 in vitro
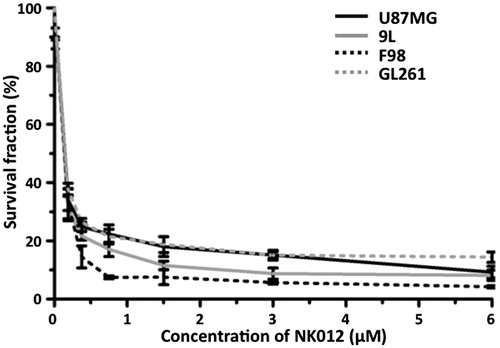
The WST assay showed that proliferation of the glioma cell lines, U87MG, 9L, F98 and GL261, was potently inhibited by NK012. The half maximal inhibitory concentrations of NK012 were 0.154, 0.196, 0.184 and 0.1511 µM for U87MG, 9L, F98 and GL261 cell lines, respectively ().
Figure 1. In vitro growth inhibitory activity of NK012 in human, rat and mouse glioma cells. U87MG, 9L, F98 and GL261 cells were placed in 96-well plates and treated for 72 h with various NK012 (0–6 µM) concentrations. After incubation for 72 h, 10% of CCK-8 regent was added to each well followed by incubation for 1 h at 37 °C, and then absorbance at 450 nm was measured in a 96-well spectrophotometric plate reader. Cell viability was measured in triplicate and was repeated three times. Data were averaged and normalized against the non-treated controls to generate dose–response curves.
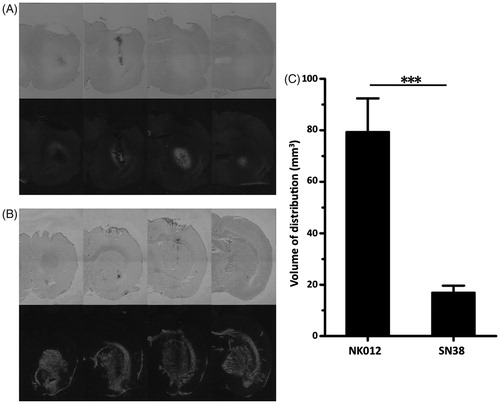
Distribution of NK012 and SN-38 in normal rat brain
The distribution of free SN-38 () was quite restricted after CED into normal rat striatum, whereas the distribution of NK012 () was diffuse and extensive. The mean volumes of distribution of free SN-38 and NK012 were 17.00 ± 2.65 and 79.39 ± 12.99 mm3, respectively (p < 0.0001, ). Note that increased local brain damage was found in brains that received free SN-38.
Figure 2. Evaluation of distribution of NK012 in normal rats. (A and B) Distributions of free SN-38 (A) and NK012 (B) (0.2 mg/ml; 20 μl) infused by CED into the striatum of rat brains. Animals were euthanized immediately after infusion and serial sections were obtained using a cryostat (20 μm thickness; 1 mm interval). Bright-field images reveal the brain tissue (upper row), and the same sections visualized with a fluorescence microscope detect the fluorescence generated from SN-38 under ultraviolet radiation (lower row). Serial images obtained from representative rats are shown. (C) Mean volumes of distribution after infusion of NK012 and free SN-38. Columns show mean of determinations; bars, SD. ***p < 0.001.
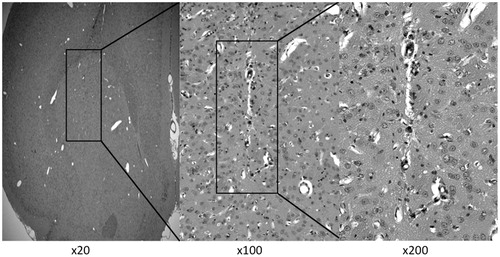
Toxicity of NK012 in normal rat brain
The rats that received CED infusion of 40 µg NK012 survived without neurological symptoms, and were euthanized on day 14 after CED. Histological examination revealed only slight tissue damage at the needle tract ().
Figure 3. Representative histological changes in brain tissues after CED of NK012 (2.0 mg/ml, 0.04 mg) infused locally into the intact striatum. Animals were euthanized 14 days after CED and brain sections (4 μm) were obtained for hematoxylin and eosin staining. Rats survived without neurological symptoms and negligible tissue damage.
Antitumor efficacy of NK012 delivered with CED in 9L and U87MG orthotopic brain tumor models
First, the survival of rats with the 9L orthotopic brain tumor model was tested. Rats in the control group that received PBS was all euthanized at 19–31 days after tumor cell implantation due to neurological symptoms indicative of tumor progression (). Median survival for this group was 29 days. Rats in the SN-38 group that received 0.04 mg SN-38 by CED were euthanized at 24–30 days after implantation due to tumor-related symptoms (median survival 28 days; p = 0.2846, log-rank test). Rats in the NK012 group that received 0.04 mg NK012 with CED survived for 27–44 days. Median survival for this group was 42 days (p = 0.0063 compared to the control group, p = 0.0045 compared to the SN-38 group, log-rank test).
Figure 4. Effect of CED of NK012 on the rodent orthotopic brain tumor models. (A) Outcome for rats bearing 9L tumors with single CED infusions of 5% glucose, free SN-38 and NK012. Five days after tumor implantation within the brain, rats were treated with 2 mg/ml of each agent. Median survivals for these groups were 29, 28 and 42 days, respectively. Statistically significant differences were observed between the control and NK012 groups (p = 0.0063, log-rank test) and between the free SN-38 and NK012 groups (p = 0.0045, log-rank test). (B) Animals implanted with U87MG tumor cells received NK012 on day 5 after tumor implantation. Median survivals for this group were 21 days for the control group and 28 days for the NK012 group. CED treatment with NK012 resulted in a significant survival benefit compared to the control (p = 0.0003, log-rank test).
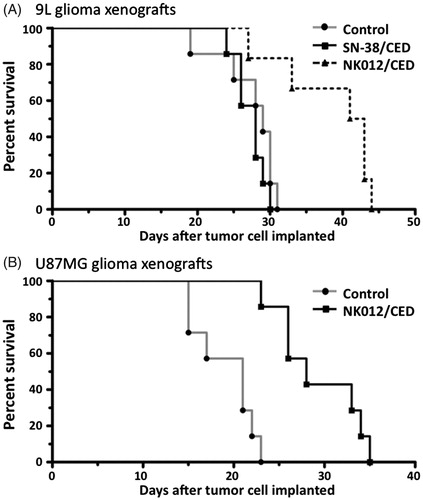
Subsequently, survival of rats with the U87MG brain tumor xenograft was tested. Rats in the control group were euthanized 15–23 days after implantation due to neurological symptoms indicative of tumor progression (median survival 21 days; ). Rats in the NK012 group were euthanized 23–33 days after tumor cell implantation (median survival 28 days). CED treatment with NK012 resulted in a significant survival benefit compared to the control (p = 0.0003, log-rank test).
Correlation between volume of distribution and histological analysis
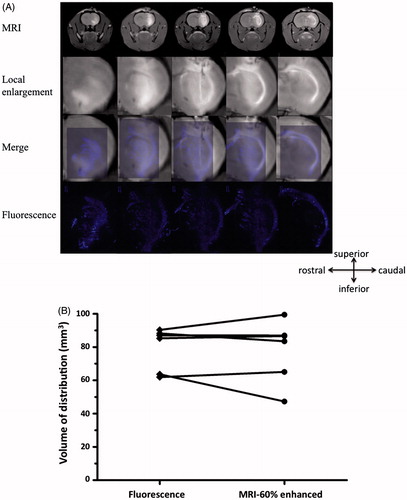
The tissue distribution of NK012 was consistent, so we subsequently compared the distribution to that of co-infused Gd. The volume of NK012 distribution in normal rat brain was determined by histological detection of fluorescence generated from SN-38. To assess the feasibility using MRI to monitor NK012 distribution in the rodent brain during CED, a Gd and NK012 mixture was infused with CED in six rats. Robust and clearly defined distributions of Gd were observed at each infusion site with T1-weighted MRI obtained immediately after infusion. Distribution volumes of Gd were automatically measured with OsiriX, an open source DICOM (Digital Imaging and Communications in Medicine) reader, using an imaging workstation. T1-weighted MRI demonstrated a close correlation between contrast-enhancement and NK012 distribution determined by histological detection of fluorescence generated from SN-38 ().
Figure 5. (A) Superimposed fluorescence and MR images of Gd co-delivered to the rat brain using CED. Correlation of MR images and histological fluorescence images showed the accuracy of the MRI monitoring method. Animals euthanized immediately after real-time MRI were processed for histological detection of fluorescence generated from NK012 that was co-infused with Gd. The fluorescence areas overlapped with the Gd distribution detected by MRI. (B) Volume calculations done with MRI data and histological fluorescence data further confirmed this finding.
Discussion
CED is a promising method for delivering therapeutic agents specifically into the targeted region of the CNS, but the PRECISE randomized phase III clinical trial employing CED failed to meet its clinical end points (Kunwar et al., Citation2010). Although drug distribution was not assessed in that study, a retrospective analysis using BrainLAB iPlan® Flow software (Feldkirchen, Germany) to estimate the expected drug distribution reported that the estimated coverage of the relevant target volumes, defined as the 2-cm penumbra of the resection cavity, was as low as 20.1% on average (Sampson et al., Citation2010). Therefore, poor drug distribution is a possible explanation for the failure of the PRECISE trial.
Distribution volume is a key property that affects the anti-tumor efficacy of therapeutic agents delivered by CED. However, locally applied agents with different physical and chemical properties have demonstrated different distribution volumes during CED into rodent brains (Saito et al., Citation2006). Therefore, chemotherapeutic agents that are tested with CED should be formulated specifically to achieve the required distribution. The distribution characteristics of individual agents delivered by CED are difficult to evaluate, but we previously demonstrated the effectiveness of drug carriers such as liposomes and micelles. We have already demonstrated the efficacy of doxorubicin delivered in liposome or micelle formulation in the rodent brain tumor model (Kikuchi et al., Citation2008; Inoue et al., Citation2009). Further investigations will search for clinically promising agents to be delivered by CED and for clinically relevant methods of delivery.
The anticancer plant alkaloid 7-ethyl-10-hydroxy-camptothecine (SN-38) is a broad spectrum anticancer camptothecin analogue which acts as a DNA topoisomerase I inhibitor. Irinotecan hydrochloride (CPT-11), a prodrug of SN-38, has shown some antitumor activities in patients with recurrent glioblastoma, with response rates of 0–17% in several trials (Friedman et al., Citation1999; Chamberlain, Citation2002; Cloughesy et al., Citation2003; Prados et al., Citation2006). The activity of CPT-11 depends on the conversion ratio of CPT-11 to SN-38. Thus, direct use of SN-38 might be effective for glioblastoma treatment, but clinical application has been limited because SN-38 is water-insoluble with high toxicity, so cannot be administered intravenously.
The present study demonstrated that SN-38 infused by CED caused severe local tissue damage and distribution was quite restricted around the infusion site (). In contrast, NK012 was consistently and extensively distributed by CED (). Locally delivered NK012 had superior antitumor activity in the 9L orthotopic brain tumor model compared with SN-38, and the Kaplan–Meier analysis revealed a statistically significant difference compared with the SN-38 group (p = 0.0045). In addition, histological examination revealed minimal brain tissue damage in rat brains that received 40 µg NK012, the same SN-38 dose that caused severe damage with free administration. Recently, we demonstrated that concentration rather than dose is the controlling factor in local tissue toxicity after extensive delivery with CED (Zhang et al., Citation2013). Our present findings suggest that a formulation of NK012 containing 2 mg/ml SN-38 or less is safe for CED application.
The present study established a method using MRI to monitor the CED of NK012 in the brain, to explore the clinically relevant applications of this CED-based therapy. A close correlation between the distributions of Gd and NK012 was observed. Therefore, the distribution of NK012 can be monitored by co-delivery of Gd during CED and may have important implications in ensuring effective delivery of therapeutic agents into brain targets.
The present study provides experimental evidence that NK012 can be efficiently administered into the brain by CED, with resultant activity against glioblastoma cells at concentrations lower than those causing dose-limiting systemic or neurological toxicity to the normal brain. The distribution of NK012 could be monitored by MRI of co-delivered Gd. Such NK012 administration by CED is a promising therapeutic approach to treat patients with glioblastoma.
Acknowledgements
We thank Mr. Takeshi Nakanishi at Nippon Kayaku Co., Ltd. for providing the NK012.
Declaration of interest
The authors declare that they have no conflict of interest. This work was supported in part by Grants-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology in Japan (#26293319 to R.S.).
References
- Bobo RH, Laske DW, Akbasak A, et al. (1994). Convection-enhanced delivery of macromolecules in the brain. Proc Natl Acad Sci USA 91:2076–80
- Chamberlain MC. (2002). Salvage chemotherapy with CPT-11 for recurrent glioblastoma multiforme. J Neurooncol 56:183–8
- Chen MY, Lonser RR, Morrison PF, et al. (1999). Variables affecting convection-enhanced delivery to the striatum: a systematic examination of rate of infusion, cannula size, infusate concentration, and tissue-cannula sealing time. J Neurosurg 90:315–20
- Cloughesy TF, Filka E, Kuhn J, et al. (2003). Two studies evaluating irinotecan treatment for recurrent malignant glioma using an every-3-week regimen. Cancer 97:2381–6
- Eberling JL, Jagust WJ, Christine CW, et al. (2008). Results from a phase I safety trial of hAADC gene therapy for Parkinson disease. Neurology 70:1980–3
- Friedman HS, Petros WP, Friedman AH, et al. (1999). Irinotecan therapy in adults with recurrent or progressive malignant glioma. J Clin Oncol 17:1516–25
- Gill SS, Patel NK, Hotton GR, et al. (2003). Direct brain infusion of glial cell line-derived neurotrophic factor in Parkinson disease. Nat Med 9:589–95
- Groothuis DR. (2000). The blood–brain and blood–tumor barriers: a review of strategies for increasing drug delivery. Neuro Oncol 2:45–59
- Huynh GH, Deen DF, Szoka FC Jr. (2006). Barriers to carrier mediated drug and gene delivery to brain tumors. J Control Release 110:236–59
- Inoue T, Yamashita Y, Nishihara M, et al. (2009). Therapeutic efficacy of a polymeric micellar doxorubicin infused by convection-enhanced delivery against intracranial 9L brain tumor models. Neuro Oncol 11:151–7
- Kikuchi T, Saito R, Sugiyama S, et al. (2008). Convection-enhanced delivery of polyethylene glycol-coated liposomal doxorubicin: characterization and efficacy in rat intracranial glioma models. J Neurosurg 109:867–73
- Koizumi F, Kitagawa M, Negishi T, et al. (2006). Novel SN-38-incorporating polymeric micelles, NK012, eradicate vascular endothelial growth factor-secreting bulky tumors. Cancer Res 66:10048–56
- Kunwar S. (2003). Convection enhanced delivery of IL13-PE38QQR for treatment of recurrent malignant glioma: presentation of interim findings from ongoing phase 1 studies. Acta Neurochir Suppl 88:105–11
- Kunwar S, Chang S, Westphal M, et al., PRECISE Study Group. (2010). Phase III randomized trial of CED of IL13-PE38QQR vs Gliadel wafers for recurrent glioblastoma. Neuro Oncol 12:871–81
- Kuroda J, Kuratsu J, Yasunaga M, et al. (2009). Potent antitumor effect of SN-38-incorporating polymeric micelle, NK012, against malignant glioma. Int J Cancer 124:2505–11
- Kuroda J, Kuratsu J, Yasunaga M, et al. (2010). Antitumor effect of NK012, a 7-ethyl-10-hydroxycamptothecin-incorporating polymeric micelle, on U87MG orthotopic glioblastoma in mice compared with irinotecan hydrochloride in combination with bevacizumab. Clin Cancer Res 16:521–9
- Mardor Y, Roth Y, Lidar Z, et al. (2001). Monitoring response to convection-enhanced taxol delivery in brain tumor patients using diffusion-weighted magnetic resonance imaging. Cancer Res 61:4971–3
- Matsumura Y. (2011). Preclinical and clinical studies of NK012, an SN-38-incorporating polymeric micelles, which is designed based on EPR effect. Adv Drug Deliv Rev 63:184–92
- Prados MD, Lamborn K, Yung WK, et al., North American Brain Tumor Consortium. (2006). A phase 2 trial of irinotecan (CPT-11) in patients with recurrent malignant glioma: a North American Brain Tumor Consortium study. Neuro Oncol 8:189–93
- Saito R, Bringas JR, McKnight TR, et al. (2004). Distribution of liposomes into brain and rat brain tumor models by convection-enhanced delivery monitored with magnetic resonance imaging. Cancer Res 64:2572–9
- Saito R, Bringas JR, Panner A, et al. (2004). Convection-enhanced delivery of tumor necrosis factor-related apoptosis-inducing ligand with systemic administration of temozolomide prolongs survival in an intracranial glioblastoma xenograft model. Cancer Res 64:6858–62
- Saito R, Krauze MT, Noble CO, et al. (2006). Tissue affinity of the infusate affects the distribution volume during convection-enhanced delivery into rodent brains: implications for local drug delivery. J Neurosci Methods 154:225–32
- Sampson JH, Archer G, Pedain C, et al., PRECISE Trial Investigators. (2010). Poor drug distribution as a possible explanation for the results of the PRECISE trial. J Neurosurg 113:301–9
- Sawamura Y, Shirato H, de Tribolet N. (1999). Recent advances in the treatment of central nervous system germ cell tumors. Adv Tech Stand Neurosurg 25:141–59
- Yun J, Rothrock RJ, Canoll P, Bruce JN. (2013). Convection-enhanced delivery for targeted delivery of antiglioma agents: the translational experience. J Drug Deliv 2013:107573
- Zhang R, Saito R, Mano Y, et al. (2013). Concentration rather than dose defines the local brain toxicity of agents that are effectively distributed by convection-enhanced delivery. J Neurosci Methods 222:131–7
