Abstract
Context: After arterial occlusion, diametrical growth of pre-existing natural bypasses around the obstruction, i.e. arteriogenesis, is the body’s main coping mechanism. We have shown before that continuous infusion of chemokine (C-X-C motif) ligand 1 (CXCL1) promotes arteriogenesis in a rodent hind limb ischemia model.
Objective: For clinical translation of these positive results, we developed a new administration strategy of local and sustained delivery. Here, we investigate the therapeutic potential of CXCL1 in a drug delivery system based on microspheres.
Materials and methods: We generated poly(ester amide) (PEA) microspheres loaded with CXCL1 and evaluated them in vitro for cellular toxicity and chemokine release characteristics. In vivo, murine femoral arteries were ligated and CXCL1 was administered either intra-arterially via osmopump or intramuscularly encapsulated in biodegradable microspheres. Perfusion recovery was measured with Laser-Doppler.
Results: The developed microspheres were not cytotoxic and displayed a sustained chemokine release up to 28 d in vitro. The amount of released CXCL1 was 100-fold higher than levels in native ligated hind limb. Also, the CXCL1-loaded microspheres significantly enhanced perfusion recovery at day 7 after ligation compared with both saline and non-loaded conditions (55.4 ± 5.0% CXCL1-loaded microspheres versus 43.1 ± 4.5% non-loaded microspheres; n = 8–9; p < 0.05). On day 21 after ligation, the CXCL1-loaded microspheres performed even better than continuous CXCL1 administration (102.1 ± 4.4% CXCL1-loaded microspheres versus 85.7 ± 4.8% CXCL1 osmopump; n = 9; p < 0.05).
Conclusion: Our results demonstrate a proof of concept that sustained, local delivery of CXCL1 encapsulated in PEA microspheres provides a new tool to stimulate arteriogenesis in vivo.
Introduction
Obstructive cardiovascular diseases, such as ischemic heart disease, cerebrovascular accident, and peripheral artery disease, are one of the major causes of morbidity and mortality in industrialized countries (Hackam & Anand, Citation2003; De Backer et al., Citation2004). In these conditions, atherosclerosis leads to stenosis or total occlusion of conductance arteries, resulting in decreased blood flow and subsequent downstream ischemia (Ouriel, Citation2001; Levi et al., Citation2002). In response, new blood vessels grow, i.e. angiogenesis, and pre-existing collateral arteries develop into functional bypassing arteries, i.e. arteriogenesis. Arteriogenesis is an efficient adaptive mechanism for the survival of ischemic limbs or internal organs, e.g. the heart and the brain, because of its ability to permanently restore the blood supply by conducting relatively large blood flows (Wolf et al., Citation1998; Scholz et al., Citation2002; Helisch & Schaper, Citation2003). While angiogenesis is induced by hypoxia distal to the arterial occlusion (Deindl & Schaper, Citation2005), arteriogenesis is stimulated by the increment in shear stress in the pre-existing collateral arteries that bypass the occlusion (Jain, Citation2003; Heil et al., Citation2006). Increased shear stress results in the induction of expression of cytokines and chemokines in endothelial cells, which are presented at the luminal endothelial surface in order to recruit monocytes from the blood into the peri-collateral space. Subsequently, monocytes differentiate into macrophages and expedite arteriogenesis (Hoefer et al., Citation2005; Keeley et al., Citation2008).
Several chemokines have been described to promote arteriogenesis. Among them, (C-X-C-motif) ligand 1 (CXCL1), formerly referred to as growth-related oncogene alpha (GROα), appears to act as a major player. The role of chemokine CXCL1 in arteriogenesis was investigated for the first time by Hagiwara et al. (Citation1998) almost 20 years ago. CXCL1 expression was upregulated in endothelial cells subjected to shear stress, resulting in an evident increase in the number of monocytes adhering to endothelial cells (Hagiwara et al., Citation1998). In accordance, we have previously shown that continuous CXCL1 infusion via a subcutaneously implanted osmopump significantly promotes arteriogenesis in a rodent ischemic hind limb model of peripheral artery disease by augmenting the number of macrophages around arteriogenic collaterals (Vries et al., Citation2015).
Notwithstanding these promising results, clinical translation of CXCL1-mediated therapeutic arteriogenesis remains difficult due to inadequate drug delivery systems. The use of osmopumps in patients would guarantee desirable continuous and sustained delivery, but is impractical for a number of reasons. First, CXCL1 administered via osmopump enters directly into the arterial blood stream, thus creating the possibility of systemic unwanted side effects in other anatomical regions than the developing collateral arteries. Second, the protein is quickly eliminated, causing a decrease in concentration delivered to target sites (Sinha & Trehan, Citation2003). Finally, implantation of osmopumps in patients would require multiple surgeries with their associated risks of complications such as wound infection or device rejection. Taken together, these arguments make osmopumps clinically unfeasible. Accordingly, issues regarding CXCL1 administration route and the requirement for sustained delivery prompted us to explore new administration strategies.
Recently, a variety of biodegradable microspheres have been developed and used as protein carriers, constituting an appealing new tool for drug delivery due to their potential for local, controlled, and long-term drug release. Among the wide range of microspheres available, members of the family of linear aliphatic polyesters like polyglycolide (PGA), polylactide (PLA), and their copolymers (PLGA) have been most extensively studied (Bala et al., Citation2004). However, their instability in terms of hydrolytic degradation prompted the development of hydrolytically stable poly(ester amide)s (PEA). These PEA polymer spheres represent a promising new family of microspheres with favorable properties regarding hydrolytic stability and biological compatibility in a variety of applications (Guo & Chu, Citation2009; Fonseca et al., Citation2014; Andres-Guerrero et al., Citation2015; Sun et al., Citation2015). Moreover, they degrade at a steady rate for at least 28 d in the presence of enzymes (Ghaffar et al., Citation2011).
By using an optimized water/oil/water (W1/O/W2) emulsification protocol to encapsulate proteins (Freitas et al., Citation2005), we succeeded in producing PEA microspheres containing CXCL1 recombinant protein. Here, we show that these PEA microspheres display a sustained and slow release of the encapsulated CXCL1 protein. Furthermore, in a mouse hind limb ischemia model, injection of CXCL1-loaded microspheres significantly improved vascular reperfusion following femoral artery ligation compared with non-loaded spheres. Hence, CXCL1-loaded PEA microspheres could provide an attractive new tool to improve vascular perfusion recovery in patients affected by peripheral artery disease.
Material and methods
Particle formulation
Microspheres were prepared via a standard W1/O/W2 emulsification protocol as described before (Freitas et al., Citation2005). Briefly, 0.75 mL of the first water phase, comprising 200 μg CXCL1, was added to 4.41 mL of 8.5% (w/w) solution of PEA III Ac Bz in dichloromethane. The mixture was then emulsified via vortex at 2500 RPM for 30 s. Next, the primary water-in-oil emulsion was injected into the second water phase, i.e. 150 mL deionized water with 1% polyvinyl alcohol (PVA) and 5% sodium chloride, in a glass beaker using a 21 Gauge needle. This secondary emulsification was carried out with an ultra-turrax device at 4000 RPM for 3 min to produce the W1/O/W2 emulsion. The mixture was stirred overnight with a magnetic stirrer under nitrogen flow to remove residual dichloromethane. Subsequently, the particles were washed three times with a Tween-20 solution to prevent particle aggregation and lyophilized. All procedures were done at room temperature and medium was always removed via decantation. In a separate experiment, bovine serum albumin (BSA) conjugated with a fluorescein isothiocyanate (FITC) label was encapsulated to visualize the interior protein-containing domains in the microspheres. Fluorescent and scanning electron microscopy images were made of the microspheres. Size distribution was determined by measuring the diameter of all particles in a 450 × 450 μm SEM field of view with 570× magnification.
Animal model
Sixty male adult C57Black6/J mice (Charles River, Wilmington, MA) were randomly assigned to one of the four groups. After ligation with electrocoagulation of the left femoral artery, animals continually received recombinant CXCL1 at 50 ng/h via an Alzet osmotic pump (Alzet, Cupertino, CA; model 1004) or were subjected to injection in the adductor muscle of 150 μL of either saline solution, 5.0 mg/mL CXCL1-loaded microspheres, or 5.0 mg/mL unloaded microspheres.
Hind limb ischemia was induced as described before (Hellingman et al., Citation2010; Bastiaansen et al., Citation2013). Briefly, animals were sedated with an intraperitoneal injection of 5 mg/kg Dormicum, 0.5 mg/kg Domitor, and 0.05 mg/kg Fentanyl. This cocktail of anesthetics gives complete narcosis for at least 1 h and can be quickly antagonized with Antisedan 2.5 mg/kg and Anexate 0.5 mg/kg. All surgical procedures were performed under aseptic conditions. A longitudinal incision was made in the skin overlying the middle portion of the ventral left hind limb of the mouse. The femoral artery was dissected for several millimeters in length from the femoral nerve and the femoral vein. The artery was ligated proximal to the superficial epigastric artery with the use of an electrocoagulator. After electrocoagulation, the skin was closed with a continued suture. Next, a longitudinal incision was made in the skin just above the knee of the left hind limb. The femoral artery was dissected again for several millimeters in length from the femoral nerve and femoral vein. The artery was ligated distally from the bifurication of the saphenous artery and the popliteal artery with an electrocoagulator. Osmotic pumps were implanted in the abdomen of the animals when needed and their catheter was inserted at the branching off of the deep femoral artery. After recovery, the animals were housed in pairs with free access to water and chow and were allowed to move freely. Motor dysfunction was monitored for the first 3 d and before every Laser Doppler imaging measurement and scored by two independent researchers. Euthanasia was performed by exsanguination under anesthesia for all animals. Experiments were conducted according to the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health and were approved by the institutional Ethics Committee on Experimental Animal Welfare.
Laser Doppler imaging (LDI)
Mice were placed for at least 10 min in a climate-controlled chamber with a set temperature of 37 °C to achieve maximal vasodilation in the hind paws. Consequently, relative blood flow to the paw was measured with a Moor LDI2-HIR high-resolution Laser Doppler imaging system (Moor Instruments, Millwey, UK) and analyzed using moorLDI software (Moore Instruments, Millwey, UK) as a functional read-out for the arteriogenic response. Mice were anesthetized with 3–4% isoflurane and anesthesia was maintained with 1–2% isoflurane. Measurements were performed before and immediately after surgery, and on postoperative days 3, 7, 14 and 21. The readings of three individual measurements per mouse were averaged for each time point. Perfusion recovery of the occluded hind limb was calculated as a percentage compared to the non-occluded hind limb and results were analyzed with repeated measures analysis of variance (rANOVA).
Endothelial cell proliferation assay
The possibly cytotoxic effects of PEA microspheres in and by themselves were investigated by assessing human microvascular endothelial cell (HMVEC) proliferation. Per well, 2.0 × 103 cells were plated in a 96-well tissue culture plate and cultured for 4 d in endothelial cell growth medium EGM-2MV with 0.5% fetal bovine serum for control conditions. In experimental conditions, 1.0 mg/mL PEA microspheres without any protein encapsulated was dispersed in the EGM-2MV growth medium. CellTiter 96® Aqueous One solution Cell Proliferation Assay (Promega, San Luis Obispo, CA) was added directly to culture wells and incubated for 2 h with subsequent recording of absorbance at 490 nm on a Victor-3 fluorescent micro plate reader (Perkin Elmer, Waltham, MA). The number of cells was averaged over four independent wells for each condition and time point.
Protein release assay
About 1.0 mg of CXCL1-loaded microspheres was diluted in 1.0 mL of endothelial cell growth medium EGM-2MV with 0.5% fetal bovine serum (Lonza, Verviers, Belgium). The medium containing the microspheres was placed in an incubator at 37 °C for 28 d. At scheduled time intervals (i.e. 6 h, 1 d, 2 d, 3 d, 7 d, 10 d, 14 d, 17 d, 22 d, 24 d, and 28 d), the medium was collected and replaced by equal volumes of fresh medium. Samples were centrifuged at 20 000 × g at 4 °C for 10 min to eliminate debris. Finally, the medium samples were analyzed for CXCL1 content via ELISA (R&D Systems, Minneapolis, MN) by determining the optical density at 470 nm on a Victor-3 fluorescent microplate reader (Perkin Elmer, Waltham, MA). The experiments were repeated three times.
Endogenous protein production assay
Total protein was isolated from 50 mg of murine muscle tissue from both the hind limb subjected to femoral artery ligation and the contralateral hind limb of two mice. Ligation was performed as described in the animal model section, albeit without implantation of an osmopump or injection of microspheres. Proteins were isolated using radioimmune precipitation assay (RIPA) lysis buffer supplemented with PMSF, protease inhibitor mixture, and sodium orthovanadate according to the protocol of the manufacturer (Santa Cruz Biotechnology, Dallas, TX). Tissue lysates were subjected to CXCL1 ELISA as described above.
Statistical analysis
Data are expressed as mean ± SEM. Statistical analysis was performed using GraphPad Prism (GraphPad Software, Inc., La Jolla, CA). Analysis of variance (ANOVA) or repeated measures ANOVA were used to compare groups. p values below 0.05 were considered statistically significant. To test for normality of microsphere distribution, D'Agostino & Pearson omnibus normality test was used and a p value above 0.05 was considered proof of a normal distribution.
Results
PEA microspheres are characterized by spherical internal protein-containing domains, high loading efficiency, and Gaussian size distribution
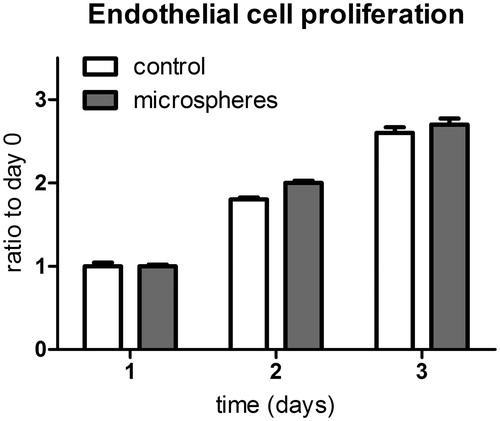
The basic building block of our microspheres was a poly(ester amide) (). Utilizing an optimized water/oil/water (W1/O/W2) emulsification protocol to encapsulate proteins, we succeeded in producing PEA microspheres with CXCL1 recombinant protein encapsulated (). To visualize the interior structure of the spheres, we also produced microspheres containing FITC-labeled BSA. Fluorescent microscopy revealed relatively small spherical domains within which the protein of interest is contained (). A loading efficiency of > 85% was achieved.
Figure 1. PEA microspheres were prepared via an emulsification protocol. (A) Chemical structure of the poly(ester amide) (PEA) used as a building block for our PEA microspheres. (B) Schematic illustration of the formulation of PEA microspheres employing a water-in-oil-in-water (W1/O/W2) technique. (C) Optical and fluorescent microscopy images of PEA microspheres loaded with FITC-conjugated BSA (magnification 200×). A loading efficiency of > 85% was achieved.
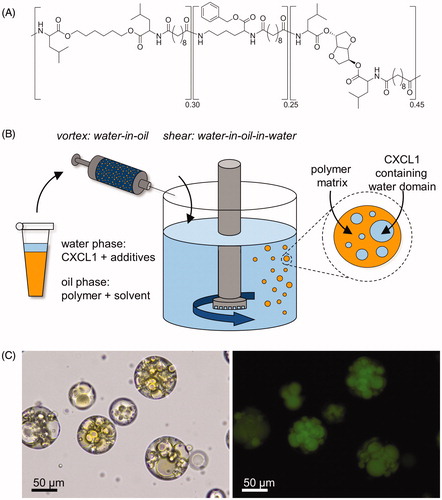
To assess the surface and also size distribution of the microspheres, scanning electron microscopy images of the spheres were taken and microsphere diameters were measured (). The smooth microsphere surface is studded with pores. Moreover, the quantification of microsphere size shows a normal distribution (p = ns) with a mean of 57.2 μm and a standard deviation of 17.7 μm. The coefficient of variation is 30.9% ().
Figure 2. Scanning electron microscopic visualization and size distribution of PEA microspheres. (A) A microsphere ensemble and (B) the surface of a single PEA microsphere, showing evident surface pores (magnification 570× and 2350×, respectively). (C) Size distribution histogram, displaying a normal distribution with a mean of 57.2 μm and a standard deviation of 17.7 μm. D'Agostino & Pearson omnibus normality test; p = ns.
PEA microspheres do not hamper HMVEC proliferation
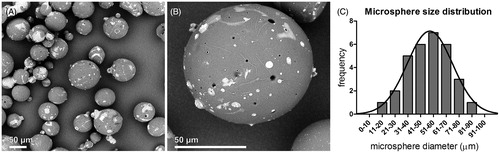
To investigate the possible toxic effects of the PEA microspheres on cell viability and proliferation capacity, HMVECs were cultured for 4 d in the presence or absence of microspheres (). The proliferation rate of HMVECs was not affected by the presence of PEA microspheres and did not differ from cells cultured in medium without microspheres (NS), indicating that the microspheres or their break down products are neither cytotoxic nor disruptive to cellular proliferation.
CXCL1-loaded microspheres show a sustained chemokine release
To evaluate the in vitro drug release of CXCL1 containing PEA microspheres, 1.0 mg of CXCL1-loaded microspheres was dispersed into 1.0 mL of medium and placed at 37 °C for 28 d (). The encapsulated protein is released in two distinct phases. In the first days, there is a substantial burst release, followed by a more steady increase in total amount of released protein. The maximum amount of released protein amounted to 6.0 ng/mL.
Figure 4. Microsphere release versus endogenous production of CXCL1. (A) Cumulative CXCL1 release from PEA microspheres over the course of 28 d, showing a marked burst release of the incorporated chemokine followed by a period of sustained long-term protein dispensation. CXCL1 release from microspheres is a 100-fold higher than endogenous CXCL1 production in ligated hind limbs. n = 3. (B) Endogenous CXCL1 production in ligated and contralateral murine hind limbs, displaying increase of CXCL1 production in the ligated leg compared with the non-ligated leg. n = 2; ANOVA; *p < 0.05 compared with thigh musculature of the ligated leg.
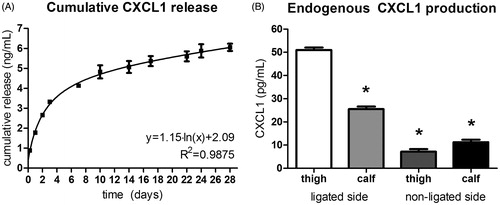
To correlate the chemokine release with in vivo levels of CXCL1 protein, adductor muscle tissue from both ligated and contralateral hind limb was processed for protein measurements (). CXCL1 production in the thigh muscles of the ligated hind limb was 51 pg/mL, while in the control hind limb, only 7.0 pg/mL of CXCL1 was present. The in vitro CXCL1 release thus is a 100- to a 1000-fold higher than endogenous CXCL1 production.
CXCL1-loaded microspheres improved perfusion recovery
To investigate the effect of CXCL1 loaded microspheres on perfusion recovery in vivo, mice were infused with CXCL1 recombinant protein or injected with saline solution, empty microspheres or CXCL1-loaded microspheres in the adductor muscle following femoral artery ligation (). A difference in the function of the treated hind limbs versus the contralateral hind limbs was only seen at day 1 after operation. However, this effect was also seen in the placebo group and thus we attribute it to the ligation itself and not to the injection of microspheres.
Figure 5. Perfusion recovery after femoral artery ligation in mice treated with either saline, continuous CXCL1 infusion, non-loaded microspheres, or CXCL1-loaded microspheres. Continuous CXCL1 infusion and CXCL1-loaded microsphere injection enhance perfusion recovery at day 7 compared with both saline and empty microsphere conditions. CXCL1-loaded microspheres show enhanced perfusion recovery compared with all other groups at day 21 after ligation. n = 8–9; rANOVA; *p < 0.05.
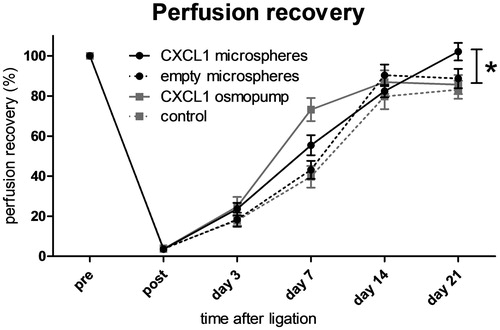
Mice that received empty microspheres did not show any difference in vascular perfusion recovery when compared to control mice treated with saline (43.1 ± 4.5% non-loaded microspheres versus 39.7 ± 5.4% control; n = 8–9; p = ns). Mice infused with CXCL1 via osmopump as well as mice injected with CXCL1-loaded PEA microspheres showed significantly improved perfusion recovery at day 7 following femoral artery occlusion as compared with mice injected with saline or non-loaded microspheres. (39.7 ± 5.4% control versus 73.2 ± 5.8% CXCL1 osmopump; n = 9; p < 0.05; 43.1 ± 4.5% non-loaded microspheres versus 55.4 ± 5.0% CXCL1-loaded microspheres; n = 8–9; p < 0.05). Moreover, at day 21 after femoral artery ligation, CXCL1-loaded microspheres resulted in significantly better perfusion recovery compared with all other groups (85.7 ± 4.8% CXCL1 osmopump versus 102.1 ± 4.4% CXCL1-loaded microspheres; n = 9; p < 0.05). Taken together, these data signify that locally injected CXCL1 releasing microspheres promote arteriogenesis, an effect that is even greater than continuous CXCL1 infusion on day 21 after ligation.
Discussion
Over the last years, major efforts have been made to improve treatment and clinical outcome in patients with arterial occlusive diseases, i.e. peripheral artery disease, coronary heart disease, and cerebrovascular disease (Olin & Sealove, Citation2010). Despite progress, many patients suffering from arterial occlusive diseases do not benefit sufficiently from current therapies such as exercise, antiplatelet therapy, angioplasty, and bypass surgery (Mangiafico & Fiore, Citation2009). These patients would benefit greatly from adjunctive approaches such as molecular stimulation of both angiogenesis and arteriogenesis (Cao et al., Citation2005; Fischer et al., Citation2006; Semenza, Citation2007).
CXCL1 is a potential stimulator of neovascularization. CXCL1 and family members CXCL2 and CXCL3 function as specialized monocyte arrest chemokines in inflammation (Smith et al., Citation2005). In arteriogenesis, CXCL1 production by endothelial cells is induced by increases in shear stress (Hagiwara et al., Citation1998). We have reported before that CXCL1 is likely presented on the luminal side of arteriogenic shear stress primed endothelium, thus providing an adhesion address for pro-arteriogenic monocytes. Indeed, CXCL1 improves the arteriogenic response when administered directly into the collateral vasculature (Vries et al., Citation2015). In accordance, here we show that continuous systemic administration of CXCL1 via osmopump infusion improved perfusion recovery at 7 d in a mouse hind limb ischemia model.
An important issue in the development of effective molecular therapeutic neovascularization strategies is the duration and location of delivery of the compound (Cao et al., Citation2005; Roy et al., Citation2011). Cytokines and chemokines often act as survival factors for the endothelium and other cell types, but exposure to a certain growth factor likely needs to be sustained to prevent early apoptosis of target cells and bring about the desired effect (Benjamin et al., Citation1999). Moreover, cytokines and chemokines perform a wide range of functions and thus systemic administration might have unwanted side effects (van Royen et al., Citation2003). Therefore, sustained as well as local delivery has been the goal in most therapeutic growth factor applications (Cao et al., Citation2005).
Several types of controlled drug release systems have been investigated (Roy et al., Citation2011; Mitragotri et al., Citation2014). Among them, microspheres present significant advantages as a drug delivery platform, especially for proteins. First, it is possible to control the release of the protein of interest over periods of hours to days (Mitragotri et al., Citation2014). This offers the opportunity to predesign drug release profiles according to the patient’s need. Additionally, the protein, encapsulated within the microspheres, is protected against enzymatic degradation. Finally, the microspheres are easy to deliver, and depending on the size and the composition, can be administered in different ways, i.e. subcutaneously, intramuscularly, intravenously, or orally (Mitragotri et al., Citation2014).
In the present study, we developed and tested a polymer-based drug delivery platform that provides local and sustained delivery of CXCL1 for promoting arteriogenesis in vivo. We opted for a water/oil/water emulsification technique for PEA microsphere preparation, because of the favorable characteristics of PEA regarding degradability and compatibility and the ease of production through emulsification (Ghaffar et al., Citation2011).
The SEM appearance and size distribution of our PEA microspheres was comparable with previous reports (Tarvainen et al., Citation2002; Vera et al., Citation2006). A wide range of cell types remains viable in the presence of polymers of different compositions in vitro (Deng et al., Citation2011; Knight et al., Citation2011) and PEA-coated suture material reduces inflammation in vivo (Hernandez et al., Citation2015). Our PEA microspheres indeed did not hamper endothelial cell proliferation and accordingly did not display any cytotoxic effects. Furthermore, the core material of the microspheres was designed to be compatible with the selected therapeutic protein. Loaded microspheres displayed a slow and sustained release of the encapsulated CXCL1 protein, comparable in release pattern to other microspheres (Lei et al., Citation2015). The amount of protein released reached a maximum of 6 ng/mL over a period of 28 d, an amount 100- to a 1000-fold higher than the endogenous CXCL1 production in ischemic and non-ischemic hind limbs, respectively. Moreover, the maximum amount of released protein could potentially be increased by poly(ethylyne glycol) modification of CXCL1, as has been shown for other proteins (Diwan & Park, Citation2003; Pai et al., Citation2009).
Proteins are still biologically active after they have been processed and released from our PEA microspheres, as evidenced by the ability of vascular endothelial growth factor (VEGF) to phosphorylate VEGFR2 in human endothelial cells after microsphere release (see Appendices A and B). Other proteins, such as fibroblast growth factor 9 (FGF9) and vaccination antigens, have also been proven to retain their biological activity in PEA-based carriers (dos Santos et al., Citation2009; Said et al., Citation2014). As further proof of CXCL1 activity after release, we observed significant in vivo evidence of microsphere released CXCL1. Specifically, CXCL1-loaded microspheres injected in the vicinity of the arteriogenic collaterals promoted vascular reperfusion in mice subjected to femoral artery ligation. The effects seen with CXCL1-loaded microspheres were better than the results of unloaded microspheres and placebo at day 7 and even superior to systemically administered CXCL1 at day 21 after femoral artery ligation. We, therefore, postulate that CXCL1 is released from microspheres into the peri-collateral space. Transport from the peri-collateral space to the endothelium through transcytosis has been shown to occur and depends on Duffy antigen receptor for chemokines (DARC) (Lee et al., Citation2003; Pruenster et al., Citation2009). In this fashion, the chemokine is ultimately presented on the luminal side of the endothelial cell layer as a target for pro-arteriogenic monocytes to enhance arteriogenesis.
We used Laser Doppler imaging as a functional non-invasive read-out of arteriogenesis to compare intra-arterial infusion with microsphere delivery of CXCL1. Previous data demonstrated a proportional relationship between perfusion recovery and the arteriogenic response of the collateral vessels (Scholz et al., Citation2002). Moreover, LDI measurements correlate well with collateral diameter as analyzed by visual inspection as well as immunohistochemistry and vascular casting (Chandraratne et al., Citation2015; Heuslein et al., Citation2015; Teunissen et al., Citation2015).
Although we have not directly measured the levels of CXCL1 on the luminal side of the endothelium to optimize the different administration strategies, the effect of microsphere treatment was similar or even slightly better than the maximum effect seen with systemic CXCL1 infusion. Higher dosages are unlikely to be more efficacious. Similar to other cytokines and growth factors, CXCL1 has a reduced efficacy when given at supra-maximal dosages. Presumably, the cognate receptor for CXCL1 on monocytes, CXCR2, becomes phosphorylated and internalized when the circulating levels of its ligands are too high (Richardson et al., Citation1998,Citation2003). The microsphere administration furthermore is limited by the maximum amount of intramuscularly injectable microspheres (Shimizu, Citation2004). We opted for a rather large volume of maximally dissolved microspheres that did not induce motor dysfunction.
We have specifically chosen to employ a relatively mild form of hind limb ischemia, even though, in contrast to more severe models such as ligation in Balb/C mice or arterio-venous shunting (Scholz et al., Citation2002; Eitenmuller et al., Citation2006), our model has a strong tendency to self-correct. We suppose that this milder model reflects intermittent claudication, the disease state in which CXCL1 therapy would be most beneficial. In critical limb ischemia, stimulation of angiogenesis is the main therapeutic goal. However, in intermittent claudication, exercise training might be augmented by chemokine therapy to achieve collateral diametrical growth and enhance the body’s natural arteriogenic response (Nowak et al., Citation2012).
To our knowledge, this is the first time a PEA microsphere-based drug delivery system in the setting of arteriogenesis is described. This novel platform enables local and prolonged chemokine delivery and promotes arteriogenesis in vivo up to several weeks after arterial obstruction. PEA microspheres bring CXCL1 pro-arteriogenic therapy a step closer to translation into a clinical application in patients affected by intermittent claudication and possibly other arterial occlusive diseases, for it is a local, less invasive delivery system with ostensibly fewer side effects than continuous systemic administration. In addition, further insights into the mechanism of action of polymer-based microspheres could make this new drug delivery system possibly suitable for other applications beyond the scope of therapeutic stimulation of arteriogenesis.
Declaration of interest
The authors gratefully acknowledge the support of the PENT and the CTMM EMINENCE Programs (Grant no. 01C-204) of the Netherlands Ministry of Economic Affairs and the Netherlands Ministry of Education, Culture and Science. John Zupancich, Marcel Houben and George Mihov are full employees of Royal DSM.
Supplementary material available online
Supplemental_figure.tif
Download TIFF Image (2.8 MB)References
- Andres-Guerrero V, Zong M, Ramsay E, et al. (2015). Novel biodegradable polyesteramide microspheres for controlled drug delivery in ophthalmology. J Control Release 211:105–17
- Bala I, Hariharan S, Kumar MN. (2004). PLGA nanoparticles in drug delivery: the state of the art. Crit Rev Ther Drug Carrier Syst 21:387–422
- Bastiaansen AJ, Ewing MM, de Boer HC, et al. (2013). Lysine acetyltransferase PCAF is a key regulator of arteriogenesis. Arterioscler Thromb Vasc Biol 33:1902–10
- Benjamin LE, Golijanin D, Itin A, et al. (1999). Selective ablation of immature blood vessels in established human tumors follows vascular endothelial growth factor withdrawal. J Clin Invest 103:159–65
- Cao Y, Hong A, Schulten H, Post MJ. (2005). Update on therapeutic neovascularization. Cardiovasc Res 65:639–48
- Chandraratne S, von Bruehl ML, Pagel JI, et al. (2015). Critical role of platelet glycoproteinibα in arterial remodeling. Arterioscler Thromb Vasc Biol 35:589–97
- De Backer G, Ambrosioni E, Borch-Johnsen K, et al. (2004). European guidelines on cardiovascular disease prevention in clinical practice. Third joint task force of European and other societies on cardiovascular disease prevention in clinical practice (constituted by representatives of eight societies and by invited experts). Atherosclerosis 173:381–91
- Deindl E, Schaper W. (2005). The art of arteriogenesis. Cell Biochem Biophys 43:1–15
- Deng M, Wu J, Reinhart-King CA, Chu CC. (2011). Biodegradable functional poly(ester amide)s with pendant hydroxyl functional groups: synthesis, characterization, fabrication and in vitro cellular response. Acta Biomater 7:1504–15
- Diwan M, Park TG. (2003). Stabilization of recombinant interferon-alpha by pegylation for encapsulation in PLGA microspheres. Int J Pharm 252:111–22
- dos Santos DF, Nicolete R, de Souza PR, et al. (2009). Characterization and in vitro activities of cell-free antigens from Histoplasma capsulatum-loaded biodegradable microspheres. Eur J Pharm Sci 38:548–55
- Eitenmuller I, Volger O, Kluge A, et al. (2006). The range of adaptation by collateral vessels after femoral artery occlusion. Circ Res 99:656–62
- Fischer C, Schneider M, Carmeliet P. (2006). Principles and therapeutic implications of angiogenesis, vasculogenesis and arteriogenesis. Handb Exp Pharmacol 157–212
- Fonseca AC, Gil MH, Simoes PN. (2014). Biodegradable poly(ester amide)s – a remarkable opportunity for the biomedical area: review on the synthesis, characterization and applications. Prog Polym Sci 39:1291–311
- Freitas S, Merkle HP, Gander B. (2005). Microencapsulation by solvent extraction/evaporation: reviewing the state of the art of microsphere preparation process technology. J Control Release 102:313–32
- Ghaffar A, Draaisma GJ, Mihov G, et al. (2011). Monitoring the in vitro enzyme-mediated degradation of degradable poly(ester amide) for controlled drug delivery by LC-ToF-MS. Biomacromolecules 12:3243–51
- Guo K, Chu CC. (2009). Biodegradable and injectable paclitaxel-loaded poly(ester amide)s microspheres: fabrication and characterization. J Biomed Mater Res Part B – Appl Biomater 89B:491–500
- Hackam DG, Anand SS. (2003). Emerging risk factors for atherosclerotic vascular disease: a critical review of the evidence. JAMA 290:932–40
- Hagiwara H, Mitsumata M, Yamane T, et al. (1998). Laminar shear stress-induced GRO mRNA and protein expression in endothelial cells. Circulation 98:2584–90
- Heil M, Eitenmuller I, Schmitz-Rixen T, Schaper W. (2006). Arteriogenesis versus angiogenesis: similarities and differences. J Cell Mol Med 10:45–55
- Helisch A, Schaper W. (2003). Arteriogenesis: the development and growth of collateral arteries. Microcirculation 10:83–97
- Hellingman AA, Bastiaansen AJ, de Vries MR, et al. (2010). Variations in surgical procedures for hind limb ischaemia mouse models result in differences in collateral formation. Eur J Vasc Endovasc Surg 40:796–803
- Hernandez KA, Hooper RC, Boyko T, et al. (2015). Reduction of suture associated inflammation after 28 days using novel biocompatible pseudoprotein poly(ester amide) biomaterials. J Biomed Mater Res Part B Appl Biomater 103:457–63
- Heuslein JL, Meisner JK, Li X, et al. (2015). Mechanisms of amplified arteriogenesis in collateral artery segments exposed to reversed flow direction. Arterioscler Thromb Vasc Biol 35:2354–65
- Hoefer IE, Grundmann S, van Royen N, et al. (2005). Leukocyte subpopulations and arteriogenesis: specific role of monocytes, lymphocytes and granulocytes. Atherosclerosis 181:285–93
- Jain RK. (2003). Molecular regulation of vessel maturation. Nat Med 9:685–93
- Keeley EC, Mehrad B, Strieter RM. (2008). Chemokines as mediators of neovascularization. Arterioscler Thromb Vasc Biol 28:1928–36
- Knight DK, Gillies ER, Mequanint K. (2011). Strategies in functional poly(ester amide) syntheses to study human coronary artery smooth muscle cell interactions. Biomacromolecules 12:2475–87
- Lee JS, Frevert CW, Wurfel MM, et al. (2003). Duffy antigen facilitates movement of chemokine across the endothelium in vitro and promotes neutrophil transmigration in vitro and in vivo. J Immunol 170:5244–51
- Lei L, Wang S, Wu H, et al. (2015). Optimization of release pattern of FGF-2 and BMP-2 for osteogenic differentiation of low-population density hMSCs. J Biomed Mater Res A 103:252–61
- Levi F, Lucchini F, Negri E, La Vecchia C. (2002). Trends in mortality from cardiovascular and cerebrovascular diseases in Europe and other areas of the world. Heart 88:119–24
- Mangiafico RA, Fiore CE. (2009). Current management of intermittent claudication: the role of pharmacological and nonpharmacological symptom-directed therapies. Curr Vasc Pharmacol 7:394–413
- Mitragotri S, Burke PA, Langer R. (2014). Overcoming the challenges in administering biopharmaceuticals: formulation and delivery strategies. Nat Rev Drug Discov 13:655–72
- Nowak WN, Mika P, Nowobilski R, et al. (2012). Exercise training in intermittent claudication: effects on antioxidant genes, inflammatory mediators and proangiogenic progenitor cells. Thromb Haemost 108:824–31
- Olin JW, Sealove BA. (2010). Peripheral artery disease: current insight into the disease and its diagnosis and management. Mayo Clin Proc 85:678–92
- Ouriel K. (2001). Peripheral arterial disease. Lancet 358:1257–64
- Pai SS, Tilton RD, Przybycien TM. (2009). Poly(ethylene glycol)-modified proteins: implications for poly(lactide-co-glycolide)-based microsphere delivery. AAPS J 11:88–98
- Pruenster M, Mudde L, Bombosi P, et al. (2009). The Duffy antigen receptor for chemokines transports chemokines and supports their promigratory activity. Nat Immunol 10:101–8
- Richardson RM, Marjoram RJ, Barak LS, Snyderman R. (2003). Role of the cytoplasmic tails of CXCR1 and CXCR2 in mediating leukocyte migration, activation, and regulation. J Immunol 170:2904–11
- Richardson RM, Pridgen BC, Haribabu B, et al. (1998). Differential cross-regulation of the human chemokine receptors CXCR1 and CXCR2. Evidence for time-dependent signal generation. J Biol Chem 273:23830–6
- Roy RS, Roy B, Sengupta S. (2011). Emerging technologies for enabling proangiogenic therapy. Nanotechnology 22:494004
- Said SS, Pickering JG, Mequanint K. (2014). Controlled delivery of fibroblast growth factor-9 from biodegradable poly(ester amide) fibers for building functional neovasculature. Pharm Res 31:3335–47
- Scholz D, Ziegelhoeffer T, Helisch A, et al. (2002). Contribution of arteriogenesis and angiogenesis to postocclusive hindlimb perfusion in mice. J Mol Cell Cardiol 34:775–87
- Semenza GL. (2007). Vasculogenesis, angiogenesis, and arteriogenesis: mechanisms of blood vessel formation and remodeling. J Cell Biochem 102:840–7
- Shimizu S. (2004). Routes of administration. In: Hedrich H, ed. The laboratory mouse. London: Elsevier Academic Press
- Sinha VR, Trehan A. (2003). Biodegradable microspheres for protein delivery. J Control Release 90:261–80
- Smith DF, Galkina E, Ley K, Huo Y. (2005). GRO family chemokines are specialized for monocyte arrest from flow. Am J Physiol Heart Circ Physiol 289:H1976–84
- Sun H, Cheng R, Deng C, et al. (2015). Enzymatically and reductively degradable α-amino acid-based poly(ester amide)s: synthesis, cell compatibility, and intracellular anticancer drug delivery. Biomacromolecules 16:597–605
- Tarvainen T, Karjalainen T, Malin M, et al. (2002). Degradation of and drug release from a novel 2,2-bis(2-oxazoline) linked poly(lactic acid) polymer. J Control Release 81:251–61
- Teunissen PF, Boshuizen MC, Hollander MR, et al. (2015). MAb therapy against the IFN-alpha/beta receptor subunit 1 stimulates arteriogenesis in a murine hindlimb ischaemia model without enhancing atherosclerotic burden. Cardiovasc Res 107:255–66
- van Royen N, Hoefer I, Bottinger M, et al. (2003). Local monocyte chemoattractant protein-1 therapy increases collateral artery formation in apolipoprotein E-deficient mice but induces systemic monocytic CD11b expression, neointimal formation, and plaque progression. Circ Res 92:218–25
- Vera M, Puiggali J, Coudane J. (2006). Microspheres from new biodegradable poly(ester amide)s with different ratios of l- and d-alanine for controlled drug delivery. J Microencapsul 23:686–97
- Vries MH, Wagenaar A, Verbruggen SE, et al. (2015). CXCL1 promotes arteriogenesis through enhanced monocyte recruitment into the peri-collateral space. Angiogenesis 18:163–71
- Wolf C, Cai WJ, Vosschulte R, et al. (1998). Vascular remodeling and altered protein expression during growth of coronary collateral arteries. J Mol Cell Cardiol 30:2291–305
Appendix A
Protein activity assay
Besides CXCL1, recombinant vascular endothelial growth factor (VEGF) was successfully incorporated in the PEA microspheres. The biological activity of the protein incorporated into the microspheres was studied by determining the phosphorylation of the tyrosine 1175 of VEGFR2 following treatment of HMVECs with VEGF microsphere conditioned medium. Proteins were isolated as described above. The protein concentration was measured using micro BCA (Thermo Scientific Pierce, Waltham, MA). XT designation reducing agent and sample buffer (Bio-Rad, Hercules, CA) were added to cell lysates. Samples were heated at 95 °C for 5 min, loaded on a 4–12% gradient Criterion XT Bis-Tris gel, and were subsequently subjected to electrophoresis in MOPS running buffer (Bio-Rad, Hercules, CA) at 200 V for 55 min. Proteins were transferred to a PVDF membrane (Bio-Rad, Hercules, CA) at 30 V overnight using Tris-glycine transfer buffer. Non-specific binding of the antibodies was blocked by incubating with 3% milk powder in TBS supplemented with Tween 20 (TBS-T) for 1 h. The membranes were exposed to anti-VEGFR2 (55B11) or anti-pY1175 VEGFR2 (19A10) (Cell Signaling Technology, Danvers, MA), or β-actin (A5441) (Sigma Aldrich, St. Louis, MO) antibodies and were subsequently incubated with polyclonal goat anti-rabbit immunoglobulins/HRP (P0448) and polyclonal goat anti-mouse immunoglobulins/HRP (P0447) (Dako, Glostrup, Denmark). The detection was performed by using SuperSignal West Femto or Pico substrate (Thermo Scientific Pierce, Waltham, MA) and was visualized with the ChemiDoc XRS System (Bio-Rad, Hercules, CA).
Appendix B
Proteins released by PEA microspheres are still biologically active
To test whether proteins are still biologically active after they have been processed and released from our PEA microspheres, we utilized a VEGF activity assay using Western blotting (Supplemental figure). HMVECs incubated with VEGF microsphere conditioned medium displayed increased phosphorylation of tyrosine 1175 of VEGFR2 as compared with HMVECs treated with empty microsphere-conditioned medium and control conditions. Thus, biological activity of VEGF is preserved after processing and release from our PEA microspheres. β-actin is used as control.