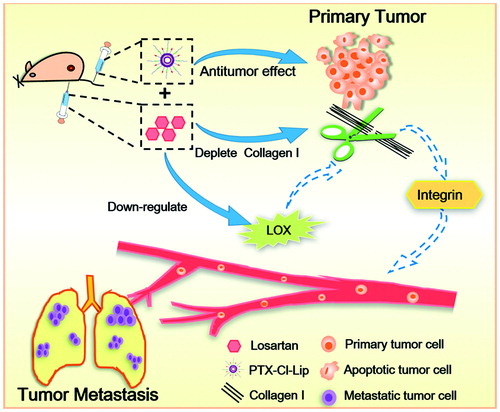
Abstract
Tumor metastasis would seriously impair the efficacy of chemotherapy. Our previous studies showed losartan combined with paclitaxel-loaded pH-sensitive cleavable liposomes (PTX-Cl-Lip) facilitated paclitaxel accumulation and led to enhanced antitumor efficacy in 4T1 bearing mice. Since losartan could inhibit the level of collagen I which was related to tumor metastasis, this strategy was further applied to suppress tumor metastasis this time. Our in vivo anti-metastatic study manifested losartan could lower the colonies occupied in lungs by 76.4% compared with that of saline group. When losartan and PTX-Cl-Lip were combined, anti-metastatic efficiency reached to 88.2%, which was the best among all the groups. In vitro 3D tumor spheroids studies proved losartan could significantly suppress the invasion of tumor cells. Losartan plus PTX-Cl-Lip could further weaken the metastasis of tumor cells. Mechanism study showed the declination of collagen I level via losartan was caused by inhibition of active transforming growth factor-β1. Western-blot study showed losartan could decrease the level of lysyl oxidase, then inhibit the cross-linking of collagen I, finally weakened the cell signaling transmit via integrin and the metastasis of tumor cells was restrained. All above studies illustrated this combined tactic could achieve favorable effect on suppression of lung tumor metastasis.
Introduction
Recent statistics displayed breast cancer was the most common cancer among females (DeSantis et al., Citation2014; Cao et al., Citation2015). The majority death (nearly 90%) of breast cancer patients was caused by tumor metastasis to distant organs rather than the malignant growth of primary tumors (Mei et al., Citation2014; Xu et al., Citation2014; Zhang et al., Citation2014). Although the chemotherapy to breast cancer had got certain effect to the primary tumor (Yang et al., Citation2013; Lu et al., Citation2015), most of these strategies could not inhibit the metastasis of tumor cells. Typical metastatic tropism of breast cancer contained lung, brain, liver and bone (Gupta & Massague, Citation2006; Valastyan & Weinberg, Citation2011). It was reported that 60–70% breast cancer patients had suffered from the pain caused by lung metastasis (Cao et al., Citation2015). Therefore, a strategy which not only inhibit tumor growth but also could suppress tumor metastasis was desperately needed, especially suppression of lung metastasis.
Tumor metastasis was a complicated cell-biological procedure which included invasion of tumor cells to the distant areas of tumors, intravasation into lumina of lymphatic or blood vessels, survival and arrest in the blood stream, extravasation from blood vessels and colonization in the distant organs (Steeg, Citation2006; Valastyan & Weinberg, Citation2011; Ryu et al., Citation2013). Any cutoff of these steps could suppress the metastasis of tumor cells. However, it could also be predicted that prevention of the first step which meant invasion of tumor cells could address the root cause of tumor metastasis.
The invasion of tumor cells which were separated from the original microenvironment was depended on breaching the surrounding extracellular matrix (ECM) and stromal cell layers (Valastyan & Weinberg, Citation2011). Collagen was the main component of ECM which accounted for nearly 90% of ECM (Gilkes et al., Citation2014). As the main subtype of collagen, collagen I turned to be highly strengthened, cross-linked and remodeled as the progression of tumors (Gilkes et al., Citation2013). Lysyl oxidase (LOX) was one kind of extracellular amine oxidase which played a crucial role in catalyzing the cross-linking of collagen I (Cox et al., Citation2013). Meanwhile, LOX was relevant to the expression of collagen I. In the late stage of tumors, the hypoxia microenvironment of tumors could stimulate the generation of LOX, and then promote the cross-linking of existing collagen I (Erler et al., Citation2006). Remodeled collagen I could interact with the integrin receptors including αvβ3 so that the invasion of breast cancer cells be facilitated (Levental et al., Citation2009; Hozumi et al., Citation2015). Therefore, interdiction of this pathway could be considered as a favorable method to suppress the tumor metastasis in breast cancers.
According to our previous studies, losartan combined with paclitaxel-loaded pH-sensitive cleavable liposomes (PTX-Cl-Lip) was utilized to enhance the accumulation of paclitaxel (PTX) in tumor tissues (Zhang et al., Citation2015a). In this combined strategy, losartan was pre-injected to deplete collagen I which could obstruct drug penetration in tumor area, and then PTX-Cl-Lip was used to facilitate the delivery of PTX. PTX-Cl-Lip was constructed by a short PEG2K anchored R8 and a long pH-sensitive PEG5K linked by hydrazone bond. The long PEG derivative could shield R8 when PTX-Cl-Lip circulated in blood circulation. Once the liposomes accumulated to the tumor area by EPR enhanced permeability and retention (EPR) effect, the mild acid environment of tumor site could hydrolyze the hydrazone bond and then exposed R8 which medicated the PTX-loaded liposomes entered into the tumor cells to exert antitumor efficacy.
Since losartan in this combined strategy could dramatically deplete the tumor collagen I (Diop-Frimpong et al., Citation2011) and increase the oxygen intensity of tumor tissues as well (Chauhan et al., Citation2013), it could be deduced that application of losartan might also down-regulate the expression of LOX, which indicating inhibition of the cross-linking and expression of collagen I. By this way, the activation of integrin could be depressed and finally invasion of tumor cells was restrained. At the same time, as the drug frequently used for anti-hypertension in clinic, losartan was inexpensive and had high safety which meant the side effects caused by losartan was limited compared with others. Thus, losartan had a high possibility to be accepted by patients. In addition, PTX-Cl-Lip which was constructed by pH-sensitive PEG derivative PEG5K-Hydrzone-PE and R8 utilized passive targeting (Danhier et al., Citation2010), low pH in tumor area (Mo et al., Citation2013; Zhang et al., Citation2013) and high cell-penetrating ability by R8 (Yin et al., Citation2013). PTX-Cl-Lip could inhibit the growth of primary tumor. As inhibition of primary tumors could suppress tumor metastasis to a certain degree (Zhang et al., Citation2015b), utilization of PTX-Cl-Lip might had the same effect.
Overall, losartan in combination with PTX-Cl-Lip was applied to studies about tumor metastasis this time (). It was expected the antitumor effect caused by PTX loaded in PTX-Cl-Lip and inhibition of collagen I and LOX caused by losartan could all contribute to the suppression of colonization of tumor cell in lungs and lead to a satisfactory outcome. 4T1 breast cancer cells were selected due to the high metastatic ability of themselves (Gao et al., Citation2011). First of all, relevant in vivo studies were carried out to test the anti-metastasis effect of this strategy. Then 3D tumor spheroids were chosen to investigate the invasion of tumor cells after treating with losartan and PTX-Cl-Lip. Finally, the possible pathway and mechanism of losartan on depletion of collagen I were also detected.
Materials and methods
Materials
Methoxy (Polyethylene glysol)-5000 propionaldehyde (PEG5K-CHO) was purchased from Jenkem Technology Co. Ltd. (Beijing, China). 3-(2-Pyridyldithio) propionylhydrazide (PDPH) was purchased from Thermo Fisher Scientific Inc. (Waltham, MA). 1, 2-Dipalmitoyl-sn-glycero-3-phosphothioethanol (PE-SH), 1,2-distearoyl-sn-glycero-3-phosphoethalamine-N-[maleimide(polyethylene glycol)-2000] (DSPE-PEG2K-Mal) were purchased from Avanti Polar Lipids (Alabaster, AL). Soybean phospholipid (SPC) was purchased from Shanghai Advanced Vehicle Technology Co. Ltd. (Shanghai, China). Cholesterol was purchased from Chengdu Kelong Chemical Company (Chengdu, China). R8 peptide with a terminal cysteine (Cys-RRRRRRRR) was purchased from Chengdu Kai Jie Biopharmaceutical Co. Ltd. (Chengdu, China). Paclitaxel (PTX) was purchased from AP Pharmaceutical Co. Ltd. (Chongqing, China). Losartan was purchased from Meilun Biotech Co. Ltd. (Dalian, China). Matrigel and Rat Collagen I were purchased from BD Bioscience (Franklin, NJ). Transforming growth factor-β1 (TGF-β1) Elisa kit was purchased from Elabscience Biotechnology Co. Ltd. (Wuhan, China). Rabbit Collagen I antibody, mouse LOX (lysis oxidase) antibody and mouse integrin (β3) antibody were purchased from Proteintech (Chicago, IL). Cell culture plastic plates were purchased from Wuxi NEST Biotechnology Co. (Wuxi, China). Other chemicals were purchased from relevant commercial companies.
Preparation and characterization of liposomes
DSPE-PEG2K-R8 and PEG5K-Hydrazone-PE were synthesized by our previous studies (Zhang et al., Citation2015a). Liposomes were prepared by thin film hydration method. Briefly, pH-sensitive cleavable liposomes (Cl-Lip) were prepared by cholesterol, SPC, DSPE-PEG2K-R8 and PEG5K-Hydrazone-PE with the molar ratio of 35:57:0.8:8. These compositions were dissolved in chloroform and evaporated to form lipid films, then the films were further kept in vacuum overnight. All films were hydrated with PBS (pH 8.0) at 37 °C and sonicated at 4 °C for 100 s to form liposomes. As to PTX-loaded liposomes (PTX-Cl-Lip), a certain concentration of PTX was added before evaporation. The mean size and zeta potential of Cl-Lip and PTX-Cl-Lip were determined using Malvern Zetasizer Nano ZS90 (Worcestershire, UK).
Cell culture
4T1 cells were obtained from Chinese Academy of Sciences (Shanghai, China). Cells were cultured in high sugar-DMEM containing 10% (v/v) fetal bovine serum (FBS), 1% (v/v) 100 U/ml streptomycin and 1% (v/v) 100 U/ml penicillin. The cells were cultured under 37 °C in a humidified 5% CO2 incubator.
Animals
Female Balb/c mice (4–6 weeks old, 18–22 g) were purchased from the West China Animal Center of Sichuan University (Chengdu, China). All the animal procedures were carried out under the protocol approved by the Experiment Animal Administrative Committee of Sichuan University.
In vivo antitumor and anti-metastasis study
106 4T1 cells were injected into the left flank area of mice to establish tumor models. When the tumors reached to an average volume of 200 mm3, the mice were divided into six groups: saline, free losartan, free PTX, free losartan plus free PTX, PTX-Cl-Lip and free losartan plus PTX-Cl-Lip. The groups which needed losartan were injected with losartan 40 mg/kg/day for 2 weeks through peritoneal cavity. Other groups were injected with same volume of saline at the same period. Free PTX and PTX-Cl-Lip were injected with the dosage of 5 mg/kg every 2 days for 7 times via tail vein. Body weight and tumor volume of these mice were measured every 2 days after treatment began. The tumor volume was calculated by using the formula: Volume= 0.5 × length × (width)2. On the 42nd day of the tumor model establishment, the mice were weighed and sacrificed. The lungs were separated and nodules in lungs were counted and photographed. Hematoxylin and eosin (HE) staining on the lungs and Masson staining on tumors were carried out according to the protocols provided by the manufacturers.
Establishment of tumor spheroid models
The 3D tumor spheroids were prepared as follows: 2% (m/v) the molten agarose was coated on a 96-well plate. When the agarose was cooled down and frozen, 100 μl culture media containing 8 × 103 cells was added into every well of the plate. About 3 days later, the compact spheroids were selected and washed by PBS for further use.
Preparation of cell free gels
Collagen I gels were prepared according to previous studies (Nguyen-Ngoc et al., Citation2012; Guzman et al., Citation2014). Briefly, rat-tail Collagen I solution was freeze-dried to get a concentration of 8 mg/ml. Then the freeze-dried Collagen I was mixed with 10*DMEM and NaOH at the ratio of 1: 0.032: 0.1 (v/v). NaOH was added to the neutralized Collagen I to reach pH 7.0 to pH 7.5 (pink color). Sterile water was added to get a series concentrations of Collagen I. Finally, the prepared Collagen I gels were incubated under 4 °C for 2 h in advance to increase the viscosity and opacity. Matrigel was diluted by 1 × DMEM to before use under 4 °C.
Tumor spheroids immigration study
Two-hundred microliters of neutralized Collagen I and a washed tumor spheroid suspended in 10 μl culture medium were added in a chamber with a coverslip bottom. Then the chamber was incubated for 1 h under 37 °C for gelation. Then the chamber was incubated under 37 °C to observe the tumor cell invasion. In order to investigate the anti-invasion ability of losartan, blank liposomes and PTX-loaded liposomes, these drugs were added before the gelation of gels. The stored matrigel disposed according to the procedures mentioned above. The relative invasion distance was calculated as follows: firstly, the area of the tumor spheroids before and after treated with different agents was calculated by the Image J; secondly, the statistically equal diameter was calculated; thirdly, the relative invasion distance was calculated according to the equation:
Western blot and Elisa study
When the antitumor and anti-metastasis study was completed, tumor tissues in each group were chosen to extract the whole cell lysis according to the protocols presented in the assay kit. The protein samples were separated by different concentrations of SDS–PAGE gels, transferred by polyvinylidene difluoride membranes, incubated with different antibodies and developed by Immobilon Western HRP Substrate (Millipore) on a Bio-Rad ChemiDoc MP System (Hercules, CA).
For quantitative measurement of TGF-β1, the whole cell lysis was detected by the specialized Elisa kit bought from the manufacture (Elabscience, China). Since the assay could only quantify the active form of TGF-β1, the total TGF-β1 needed to be activated by 1 N HCl for 15 min at room temperature and then neutralized by 1 N NaOH for further measurement.
Statistical analysis
All data were presented as mean ± SD. Statistical analysis was performed by one-way ANOVA for multiple groups and statistical difference between two groups was performed by Student’s test. The p value < 0.05, < 0.01 and < 0.001 were considered indications of statistical difference.
Results
Characterization of liposomes
Since the size and zeta potential were the basic characterizations of the liposomes, these two properties were investigated in this study. According to the result shown in and , the mean size of PTX-Cl-Lip was 109.93 nm, which was higher than that of Cl-Lip (101.78 nm). The mean zeta potential of PTX-Cl-Lip and Cl-Lip was almost to −5 mV. The above-mentioned results indicated both the Cl-Lip and PTX-Cl-Lip could satisfy the basic requirements of passive targeting.
Figure 2. The basic characterizations of PTX-Cl-Lip. (A) The schematic diagram of PTX-Cl-Lip. (B) The size distribution of PTX-Cl-Lip. (C) The zeta potential distribution of PTX-Cl-Lip.
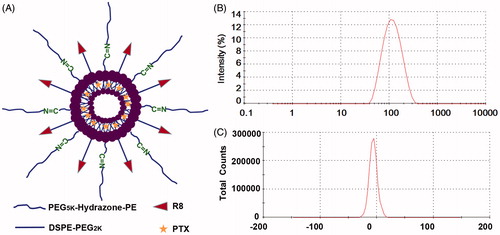
Table 1. The basic characterizations of Cl-Lip and PTX-Cl-Lip (mean ± SD, n= 3).
In vivo antitumor and anti-metastasis study
Due to the high lung metastatic ability of 4T1 bearing tumor models, 4T1 cells were widely used in many metastatic researches. According to the result shown in , the average body weight of mice at the end of treatment was around 20 g and there was no significant difference between all the groups, indicating that these preparations had no obvious toxicity to the mice.
Figure 3. The combination of losartan and PTX-Cl-Lip depressed the lung metastasis in 4T1 bearing mice. (A) Body weight of mice treated with different preparations during the treatment (mean ± SD, n = 6). (B) Tumor growth curves of mice receiving different preparations during the treatment (mean ± SD, n= 6). (C) The average number of nodules in lungs for each group at the end of treatment (mean ± SD, n= 6). (D) The photographs of typical lungs for each group at the end of treatment. The area surrounded by white circles represented metastatic nodules in lungs (E) HE staining of lungs after treatment. Arrows pointed to the metastatic nodules in lungs. Scale bars represented 100 μm. (F) Masson staining of tumors after treatment. Arrows pointed to the collagen I in tumors. Scale bars represented 50 μm.
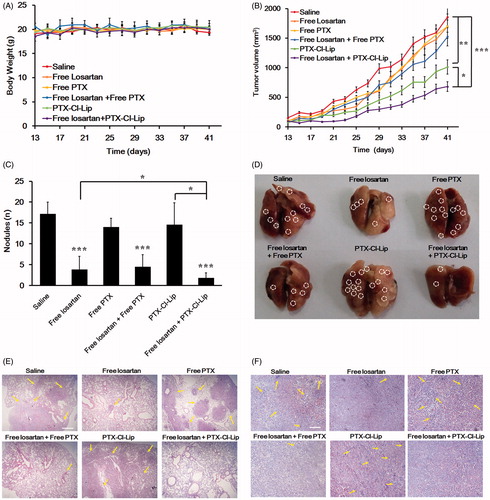
The result shown in manifested that losartan did not affect the growth rate of tumors, which was similar with that of the saline group. Similarly, free PTX and the combination of these two free drugs did not display obvious effect on tumor-size inhibition compared with saline group. The poor antitumor efficacy of PTX might due to the poor tumor targeting ability of the free drug and the low dosage we utilized compared with other dosages such as 15 mg/kg (Eldar-Boock et al., Citation2011). Thus, when the free PTX was in combination with losartan, sufficient free drug could not be accumulated in the tumor tissue and desirable antitumor efficacy did not result. As a comparison, PTX-Cl-Lip could inhibit the tumor growth rate by 45.4%, whereas losartan plus PTX-Cl-Lip posed the most distinguished therapeutic effect among all the groups, reaching an inhibition rate of 63.3% compared with the saline group.
showed that there were almost 17 nodules in lungs of the mice treated with saline, which was almost equal to that of free PTX group and PTX-Cl-Lip group. By contrast, losartan could sharply block the metastasis of tumor cells, as there were only 4 nodules occupied in lung tissues, which was decreased by 76.4% compared with the saline group. The combination of free losartan and free PTX could also depress the metastasis in lungs to same degree. Nevertheless, when losartan was in combination with PTX-Cl-Lip, it showed the highest anti-metastasis activity among all the groups we set. There were only 2 nodules dispreading in lung tissues, which was inhibited by 88.2% compared with the saline group. displayed the anti-metastasis ability of different preparations, as the area surrounded by white circles represented the nodules in lungs. The result of the images further proved that the combination of losartan and PTX-Cl-Lip could significantly depress the metastasis in lung.
HE staining of lungs confirmed that losartan could inhibit the metastasis of breast cancer cells in lungs, and the combination therapy of free losartan and PTX-Cl-Lip remarkably weakened the colonization of tumor cells in lungs (). In the Masson staining of tumor tissues, the lines in the tumor sections represented the network of collagen I in tumors (). The result manifested that losartan could deplete collagen I compared with saline, free PTX and PTX-Cl-Lip. When losartan was in combination with free PTX or PTX-Cl-Lip, the expression of collagen I was also declined.
Impact of collagen I on the invasion of 3D tumor spheroids
Now that losartan could depress collagen I in tumor tissues, relevant in vitro studies were also applied to further detect its anti-metastasis effect. As the tumor cells were surrounded and embedded in the network of collagen I, the monolayer cells could not effectively mimic the in vivo environment. It was considered that 3D multicellular tumor spheroids could be applied in order to investigate the effect of collagen I on the tumor cell invasion of tumor cells. BME (matrigel) was used as a contrast to collagen I gels. According to the results shown in , after tumor spheroids incubating with BME for 24 h, the tumor spheroids were integral and compact, and there were no significant difference with the tumor spheroids treated before BME was added.
Figure 4. The network of collagen I and application of losartan modulated the invasion of the tumor cells which formed 4T1 tumor spheriods. (A) Typical inverted microscope images on the invasion of 3D tumor spheriods in BME and different concentrations of collagen I gels. (B) Typical inverted microscope images on the invasion of 3D tumor spheriods in 4 mg/ml collagen I gels mixed with different dosages of losartan. (C) Relative invasion distance of tumor spheriods incubated at different collagen I gels compared with those without incubation calculated by Image J (mean ± SD, n= 5–8). (D) Relative invasion distance of tumor spheriods incubated with different dosages of losartan compared with those incubated with 4 mg/ml collagen I gels free from losartan calculated by Image J (mean ± SD, n = 5–8). Scale bars represented 100 μm. *represented p < 0.05.
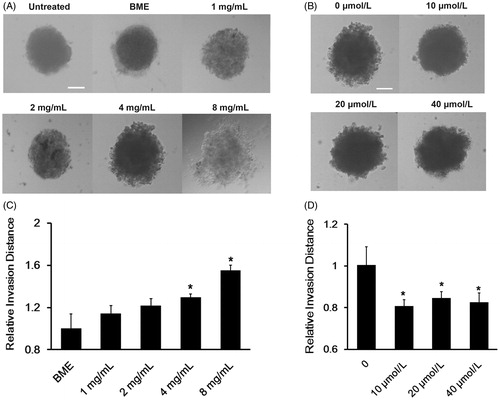
However, when the tumor spheroids were incubated with collagen I gels, the invasion ability of tumor cells could enhance as the concentration of collagen I increased. When the tumor spheroids were incubated with collagen I gel at the concentration of 4 mg/ml, the invasion of tumor cells could be easily observed. When the collagen I concentration reached to 8 mg/ml, the tumor spheroids were not compact any more as they turned to be loose and the tumor cells were scattered. After invasion distance calculating, the relative invasion distance of tumor spheroids incubated with 8 mg/ml and 4 mg/ml collagen I gels was almost 1.54- and 1.29-fold as that of BME. Therefore, the result above indicated that the network of collagen I could contribute to the invasion of tumor cells. As the tumor cell invasion ability could be easily detected in collagen I gels at the concentration of 4 mg/ml, the concentration was selected for further study.
Impact of losartan on depression of 3D tumor spheroids’ invasion
When these tumor spheroids were co-incubated with collagen I gels treated with different concentrations of losartan, the results from showed that 10 μmol/l losartan could inhibit the invasion of these tumor spheroids. As the dosage of losartan increased, the depression ability had not been dramatically improved. This might because the losartan we utilized in our research was enough to digest the collagen I surrounding tumor cells. All the tumor spheroids were similar after treating losartan at 10 μmol/l for 24 h. The statistic result displayed that losartan could decrease the relative invasion distance by 25.3% compared with the tumor spheroids treated only with collagen I gels at the concentration of 4 mg/kg. Thus, the in vitro study indicated that losartan could inhibit the immigration of tumor cells. In order to guarantee the high safety of losartan, the dosage of losartan at the concentration of 10 μmol/l was chosen for further study.
Effect of losartan and PTX-Cl-Lip on 3D tumor spheroids’ invasion
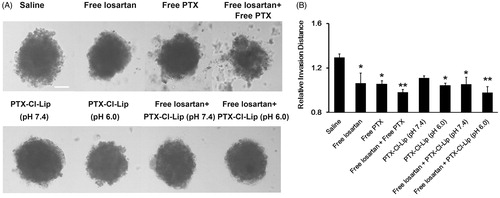
Apart from investigation of losartan on the invasion of tumor spheriods, the combined application of losartan and PTX-Cl-Lip on tumor spheriods was also explored. When these tumor spheriods were incubated with 2 μg/ml free PTX, it could be observed that there were many cell fragments (). Once losartan and free PTX were added and incubated for 24 h, the fragments proportion was further enhanced. Furthermore, PTX-Cl-Lip pre-incubated in pH 7.4 also showed the anti-invasion ability to some extent, but it did not have significant difference with the saline group according to the statistic result. By contrast, PTX-Cl-Lip pre-incubated in pH 6.0 showed a much more obvious anti-invasion ability over the liposomes pre-incubated in pH 7.4. It could also be observed that in spite of different pre-incubation conditions of PTX-Cl-Lip, as long as it was combined with losartan, it would lead to an adorable anti-invasion ability on tumor spheroids. Based on the statistic result, it could be concluded that the losartan plus either PTX or PTX-Cl-Lip pre-incubated in pH 6.0 group led to the best anti-invasion effect on tumor spheroids among the groups above (almost equal to 25%), indicating the combined strategy would bring about remarkable anti-metastasis impact.
Figure 5. The combination of losartan and different PTX preparations modulated the invasion of tumor spheroids. (A) Typical inverted microscope images on the invasion of 3D tumor spheriods treated with different preparations. Scale bars represented 100 μm. (B) Relative invasion distance of tumor spheriods incubated with different preparations compared with those untreated by Image J (mean ± SD, n= 5–8). * and **represented p < 0.05 and p < 0.01, respectively.
Immune analysis for relative protein expression in tumor tissues
Having noted that losartan and the combination therapy could depress the invasion of tumor cells and suppress the metastasis of tumor cells, to analyze the pathway which might be blocked to inhibit the meatastasis of tumor cells were deeply needed. Thus, we utilized the Western-blot assay to investigate which pathway might be cut off.
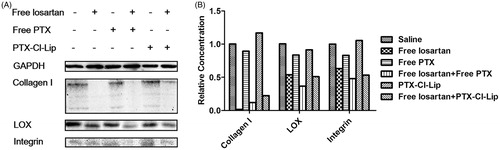
According to the results shown in , after losartan was administrated, the level of collagen I was declined compared with the saline group. The level of LOX (lysis oxidase) and Intergrin were also reduced. On the contrary, free PTX and PTX-Cl-Lip could not down-regulate the level of collagen I, neither did the level of LOX nor Integrin. Despite some fuctuations, when losatan was in combination with free PTX or PTX-Cl-Lip, the level of these three kinds of proteins was significantly decreased compared with the saline group. Therefore, the down-regluation of collagen I, LOX and Integrin were attributed to the administration of losartan and not to rather than those PTX preparations.As these proteins were positively related to the metastasis of tumor cells, the results also showed that losartan and the combination therapy could inhibit the metastasis of tumor cells.
Detection of TGF-β1 in tumor tissues
Since the immunoblotting analysis dedicated that it was losartan which depleted collagen I in tumors, we then set out to explore the underlying mechanism. As the angiotensin receptor antagonist, it was accepted that losartan modulated the expression of TGF-β1 to exert the anti-hypertension effect (Kalluri & Han, Citation2008). It was also reported that TGF-β1 could also promote the metastasis of tumor cells (Wan et al., Citation2013). Based on this background, we extracted the whole cell lysis of tumor tissues and quantify the TGF-β1 level by Elisa assay.
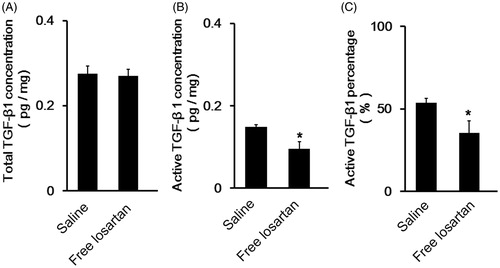
However, the results shown in did not indicate that losartan could inhibit the total TGF-β1 concentration in tumor tissues. We then quantify the level of activated TGF-β1. Further study showed that losartan could inhibit the activation of TGF-β1 (), which made the active TGF-β1 percentage reduced from 53.8% to 35.4%. As a consequence, it could be concluded that losartan inhibited the activation of TGF-β1 to modulate the level of collagen I.
Figure 7. The detection of the activation of TGF-β1 in tumor tissues after administration of losartan. (A) The level of total TGF-β1 concentration in tumor tissues of 4T1 bearing breast cancer models (mean ± SD, n= 3). (B) The level of active TGF-β1 concentration in tumor tissues of 4T1 bearing breast cancer models (mean ± SD, n= 3). (C) The percentage of active TGF-β1 account for total TGF-β1 in tumor tissues (mean ± SD, n= 3).
Discussion
Tumor metastasis was the direct cause of the death of patients with cancers, therefore the depression of tumor metastasis could remarkably guarantee the survival of cancer patients. Since collagen I was closely relevant to the tumor metastasis, depletion of collagen I might also affect the metastasis of tumor cells. In this study, we applied losartan to digest collagen I in order to inhibit the tumor metastasis. Meanwhile, PTX-Cl-Lip rather than PTX-loaded PEGylation liposomes was applied as PTX-Cl-Lip had better antitumor effect (Zhang et al., Citation2015a). Since inhibition of primary tumors could suppress tumor metastasis o some extend, better anti-metastasis effect was expected after the combination of the two preparations.
First of all, the basic characterizations of PTX-Cl-Lip were investigated, and the results shown in and showed the size of PTX-Cl-Lip which was ∼109.33 nm could meet the basic need of passive targeting (Zong et al., Citation2014). The negative zeta potential of PTX-Cl-Lip was due to the shielding effect of PEG5K-Hydrzone-PE and the PBS (pH 8.0) with high pH we used to hydrate. PBS with pH 8.0 was utilized to reduce the percentage of hydrolyzed PEG5K-Hydrazone-PE during procedure of storage. This kind of method was also utilized in other researches (Sawant et al., Citation2006). In addition, the negative zeta potential of PTX-Cl-Lip indicated the liposomes would weaken the reaction with plasma proteins, so the antitumor efficacy of these liposomes could be guaranteed (Tang et al., Citation2014).
displayed losartan combined with PTX-Cl-Lip could dramatically suppress lung metastasis of tumor cells, but relevant experiments were still needed to verify this kind of effect. It was well known that the network of collagen I wrapped tumor cells, so traditional 2D models including the wound healing experiment (Mei et al., Citation2014) could not stimulate tumor micro-environment well. While 3D tumor spheroids could better reflect the effect of collagen I in the invasion of tumor cells, therefore they were utilized in our study. At the same time, the application of serum free culture media was to avoid the growth of the cells which formed the tumor spheroids.
The first step of tumor metastasis was to escape from the original tumor microenvironment. As for the tumor spheroids, the fibriliar structure would promote the invasion of tumor cells. Since BME (matrigel) was the component of membrane basement, it was used to stimulate the tumor normal epithelial environment (Kleinman & Martin, Citation2005). Collagen I gels were used to mimic the matrix stromal surrounding the tumor cells. According to , the tumor spheroids incubated with BME for 24 h did not obviously invaded to the distant areas. However, when the tumor spheroids were transferred into collagen I gels, it could be seen that 4 mg/ml collagen I gels enabled the tumor cells immigrate to the distant areas. When the tumor spheroids were incubated with collagen I gels at the concentration of 8 mg/ml, they could protrude into the collagen I gels. At the same time, the morphology of tumor cells also changed, even the protrusions could be seen. This result showed the collagen I could indeed promote the immigration of tumor cells to distant areas, which was consistent with previous studies (Nguyen-Ngoc et al., Citation2012).
According to the previous studies, losartan could deplete the concentration of collagen I, therefore when tumor spheroids were co-cultured with losartan, the invasion of tumor cells was depressed (). Due to the cytotoxicity of PTX, the fragments of tumor cells could be seen when the tumor spheroids were co-cultured with PTX (). Then when losartan was in combination with free PTX or PTX-Cl-Lip which was pre-incubated in pH 6.0, the invasion distance was further declined. Our pervious study showed the PEG5K-Hydrazone-PE had a favorable shielding and de-shielding ability to R8 under different pH conditions (Zhang et al., Citation2015a), this character enabled PTX-Cl-Lip had a pH-sensitive property. PTX-loaded PTX-Cl-Lip could not efficiently enter tumor cells when it was pre-incubated in pH 7.4 due to the shielding effect of PEG5K-Hydrazone-PE, so the invasion in this group had no significant difference with that of the control group. By contrast, PEG5K-Hydrazone-PE could be hydrolyzed and R8 could be exposed to mediate the cellular uptake of PTX-Cl-Lip under pH 6.0, the PTX loaded in liposomes pre-incubated in pH 6.0 might enter into tumor cells by endocytosis (Liu et al., Citation2014), and this uptake pathway would restrict the accumulation of PTX in cells. However, the anti-invasion ability of PTX-Cl-Lip (pH 6.0) was lower than that of free PTX. This was because free PTX could penetrate into tumor cells by passive infusion (Shao et al., Citation2014). Similar result was attained when these two preparations were combined with losartan, indicating combined therapy could lead to better anti-metastasis effect than the monotherapy.
Apart from the anti-metastasis ability of losartan and the cytotoxicity of PTX and its preparations to primary tumor cells, the signal pathway through which losartan depressed the metastasis was also detected. The results shown in illustrated collagen I was widespread in 4T1 breast tumor models. Despite some fluctuations in collagen I expression of all the groups which were caused by individual difference, when the mice were injected with losartan, the level of collagen I was significantly declined. This was in consistent with previous studies (Chauhan et al., Citation2013). As the network of collagen could aggravate the hypoxia in tumor areas, it would up-regulate the level of LOX. Excessive level of LOX could promote the cross-linking and generation of collagen I, then activated integrin to modulate cell signaling, finally the metastasis of tumor cells would be triggered (Levental et al., Citation2009). Previous studies showed losartan could lower the expression of hypoxia inducible factor α1 (HIF-α1) (Zhang et al., Citation2015a), which would regulate relevant products to induce the level of LOX. Therefore, when the mice were injected with losartan, the level of collagen I, LOX and integrin were all remarkably declined. There were also studies demonstrating the hypoxia microenvironment could increase the expression of prolyl hydroxylase which could promote the deposition of collagen to enhance the invasion of tumor cells to lungs (Gilkes et al., Citation2013), and collagen I could upregulate N-cadherin to promote tumor metastasis (Shintani et al., Citation2006). Thus, losartan might exert these anti-metastasis effect in these ways.
However, the down-regulation of relevant proteins by PTX and PTX-Cl-Lip could not be seen. This might be because of the mechanism of PTX, which exerted cell cytotoxicity by stabilizing microtubules in the procedure of caryomitosis (Shao et al., Citation2014). Therefore, it could be deduced that when these two drugs were combined, the level of collagen I, LOX and integrin would not be further down-regulated.
Furthermore, the mechanism by which losartan inhibited collagen I was also investigated. Since the activation of TGF-β1 could enhance the production and reduce degradation of ECM components including collagen I (Anscher, Citation2010; Pang et al., Citation2013), the concentration of active TGF-β1 was measured. illustrated losartan could decrease the percentage of active TGF-β1 by 18.4%. Therefore, losartan could inhibit the production of collagen I by depress the activation of active TGF-β1. TGF-β had been proved to promote the metastasis by induction of mesenchymal transition (EMT) of tumors in their late stages (Pickup et al., Citation2013). However, displayed that losartan did not affect the production of TGF-β1. Thus, losartan would not affect the metastasis of tumor cells in this way.
Based on the above results, as to the in vivo anti-metastasis study, because of the anti-metastasis ability of losartan. The nodules in lung were significantly decreased compared with the saline group (). Forty mg/kg/day in the in vivo anti-metastasis study was selected according to our previous study, the losartan under this dosage could significantly deplete collagen I (Chauhan et al., Citation2013; Zhang et al., Citation2015a). Unfortunately, PTX did not exert any anti-metastasis effect. When losartan was combined with PTX, the anti-metastasis ability had not further enhanced. By contrast, many colonies were still occupied in the lungs of PTX-Cl-Lip group. However, our study showed PTX-Cl-Lip had an antitumor ability to some extent and the antitumor efficacy could be further improved when PTX-Cl-Lip was combined with losartan (). The better antitumor ability after PTX-Cl-Lip combined with losartan was due to the depletion of collagen I by losartan which would facilitate the tumor accumulation of PTX-Cl-Lip at tumor sites, and this result was consistent with our previous studies (Zhang et al., Citation2015a). Thus, because of the inhibition of primary tumors by the combined tactic and anti-metastasis effect of losartan, there were the least number of metastatic colonies in lungs of free losartan plus PTX-Cl-Lip group compared with others. Similarly, the HE staining showed the same result (). Masson staining also manifested that losartan could depress collagen ().
Compared with other studies focused on suppression of tumor metastasis, the combined strategy we utilized could inhibit metastasis by controlling of primary tumor and invading of tumor cells to blood vessels. By this way, we could restrain the metastasis from the original steps of tumor development, and then considerable effect could be achieved.
Conclusion
To sum up, our study showed that losartan could decrease the level of collagen I in tumors, which was caused by down-regulating of transformation growth factor-β1 (TGF-β1). Losartan could inhibit the activation of LOX in turn to inhibit the generation and cross-linking of collagen I and then weaken the cell signaling transmitted by integrin. By this way, the tumor metastasis was significantly suppressed. When it was combined with PTX-Cl-Lip, the anti-metastasis effect could be further improved by inhibition of the primary tumor caused by the combined therapy and anti-metastasis effect caused by losartan. Therefore, the strategy we utilized not only could inhibit the growth of primary tumor but also could suppress the lung metastasis of breast cancer cells, which meant this strategy might be a powerful tactics in cancer treatment.
Declaration of interest
The work was funded by the National Basic Research Program of China (973 program, 2013CB932504) and the National Natural Science Foundation of China (81373337).The authors report no conflicts of interest.
References
- Anscher MS. (2010). Targeting the TGF-beta1 pathway to prevent normal tissue injury after cancer therapy. Oncologist 15:350–9
- Cao H, Zhang Z, Zhao S, et al (2015). Hydrophobic interaction mediating self-assembled nanoparticles of succinobucol suppress lung metastasis of breast cancer by inhibition of VCAM-1 expression. J Control Release 205:162–71
- Chauhan VP, Martin JD, Liu H, et al (2013). Angiotensin inhibition enhances drug delivery and potentiates chemotherapy by decompressing tumour blood vessels. Nat Commun 4:2516
- Cox TR, Bird D, Baker AM, et al (2013). LOX-mediated collagen crosslinking is responsible for fibrosis-enhanced metastasis. Cancer Res 73:1721–32
- Danhier F, Feron O, Preat V. (2010). To exploit the tumor microenvironment: passive and active tumor targeting of nanocarriers for anti-cancer drug delivery. J Control Release 148:135–46
- DeSantis CE, Lin CC, Mariotto AB, et al (2014). Cancer treatment and survivorship statistics, 2014. CA Cancer J Clin 64:252–71
- Diop-Frimpong B, Chauhan VP, Krane S, et al (2011). Losartan inhibits collagen I synthesis and improves the distribution and efficacy of nanotherapeutics in tumors. Proc Natl Acad Sci USA 108:2909–14
- Eldar-Boock A, Miller K, Sanchis J, et al (2011). Integrin-assisted drug delivery of nano-scaled polymer therapeutics bearing paclitaxel. Biomaterials 32:3862–74
- Erler JT, Bennewith KL, Nicolau M, et al (2006). Lysyl oxidase is essential for hypoxia-induced metastasis. Nature 440:1222–6
- Gao ZG, Tian L, Hu J, et al (2011). Prevention of metastasis in a 4T1 murine breast cancer model by doxorubicin carried by folate conjugated pH sensitive polymeric micelles. J Control Release 152:84–9
- Gilkes DM, Chaturvedi P, Bajpai S, et al (2013). Collagen prolyl hydroxylases are essential for breast cancer metastasis. Cancer Res 73:3285–96
- Gilkes DM, Semenza GL, Wirtz D. (2014). Hypoxia and the extracellular matrix: drivers of tumour metastasis. Nat Rev Cancer 14:430–9
- Gupta GP, Massague J. (2006). Cancer metastasis: building a framework. Cell 127:679–95
- Guzman A, Ziperstein MJ, Kaufman LJ. (2014). The effect of fibrillar matrix architecture on tumor cell invasion of physically challenging environments. Biomaterials 35:6954–63
- Hozumi K, Fujimori C, Katagiri F, et al (2015). Suppression of cell adhesion through specific integrin crosstalk on mixed peptide-polysaccharide matrices. Biomaterials 37:73–81
- Kalluri R, Han Y. (2008). Targeting TGF-beta and the extracellular matrix in Marfan’s syndrome. Dev Cell 15:1–2
- Kleinman HK, Martin GR. (2005). Matrigel: basement membrane matrix with biological activity. Semin Cancer Biol 15:378–86
- Levental KR, Yu H, Kass L, et al (2009). Matrix crosslinking forces tumor progression by enhancing integrin signaling. Cell 139:891–906
- Liu Y, Ran R, Chen J, et al (2014). Paclitaxel loaded liposomes decorated with a multifunctional tandem peptide for glioma targeting. Biomaterials 35:4835–47
- Lu J, Liu C, Wang P, et al (2015). The self-assembling camptothecin-tocopherol prodrug: an effective approach for formulating camptothecin. Biomaterials 62:176–87
- Mei L, Liu Y, Zhang Q, et al (2014). Enhanced antitumor and anti-metastasis efficiency via combined treatment with CXCR4 antagonist and liposomal doxorubicin. J Control Release 196:324–31
- Mo R, Sun Q, Li N, et al (2013). Intracellular delivery and antitumor effects of pH-sensitive liposomes based on zwitterionic oligopeptide lipids. Biomaterials 34:2773–86
- Nguyen-Ngoc KV, Cheung KJ, Brenot A, et al (2012). ECM microenvironment regulates collective migration and local dissemination in normal and malignant mammary epithelium. Proc Natl Acad Sci USA 109:E2595–604
- Pang Y, Gara SK, Achyut BR, et al (2013). TGF-β signaling in myeloid cells is required for tumor metastasis. Cancer Discov 3:936–51
- Pickup MW, Laklai H, Acerbi I, et al (2013). Stromally derived lysyl oxidase promotes metastasis of transforming growth factor-β-deficient mouse mammary carcinomas. Cancer Res 73:5336–46
- Ryu S, McDonnell K, Choi H, et al (2013). Suppression of miRNA-708 by polycomb group promotes metastases by calcium-induced cell migration. Cancer Cell 23:63–76
- Sawant RM, Hurley JP, Salmaso S, et al (2006). “SMART” drug delivery systems: double-targeted pH-responsive pharmaceutical nanocarriers. Bioconjug Chem 17:943–9
- Shao K, Ding N, Huang S, et al (2014). Smart nanodevice combined tumor-specific vector with cellular microenvironment-triggered property for highly effective antiglioma therapy. ACS Nano 8:1191–203
- Shintani Y, Hollingsworth MA, Wheelock MJ, et al (2006). Collagen I promotes metastasis in pancreatic cancer by activating c-Jun NH(2)-terminal kinase 1 and up-regulating N-cadherin expression. Cancer Res 66:11745–53
- Steeg PS. (2006). Tumor metastasis: mechanistic insights and clinical challenges. Nat Med 12:895–904
- Tang J, Fu H, Kuang Q, et al (2014). Liposomes co-modified with cholesterol anchored cleavable PEG and octaarginines for tumor targeted drug delivery. J Drug Target 22:313–26
- Valastyan S, Weinberg RA. (2011). Tumor metastasis: molecular insights and evolving paradigms. Cell 147:275–92
- Wan L, Pantel K, Kang Y. (2013). Tumor metastasis: moving new biological insights into the clinic. Nat Med 19:1450–64
- Xu P, Yu H, Zhang Z, et al (2014). Hydrogen-bonded and reduction-responsive micelles loading atorvastatin for therapy of breast cancer metastasis. Biomaterials 35:7574–87
- Yang Y, Pan D, Luo K, et al (2013). Biodegradable and amphiphilic block copolymer-doxorubicin conjugate as polymeric nanoscale drug delivery vehicle for breast cancer therapy. Biomaterials 34:8430–43
- Yin Y, Wu X, Yang Z, et al (2013). The potential efficacy of R8-modified paclitaxel-loaded liposomes on pulmonary arterial hypertension. Pharm Res 30:2050–62
- Zhang J, Li X, Huang L. (2014). Non-viral nanocarriers for siRNA delivery in breast cancer. J Control Release 190:440–50
- Zhang L, Wang Y, Yang Y, et al (2015a). High tumor penetration of paclitaxel loaded pH sensitive cleavable liposomes by depletion of tumor collagen I in breast cancer. ACS Appl Mater Interfaces 7:9691–701
- Zhang Q, Tang J, Fu L, et al (2013). A pH-responsive α-helical cell penetrating peptide-mediated liposomal delivery system. Biomaterials 34:7980–93
- Zhang Q, Ran R, Zhang L, et al (2015b). Simultaneous delivery of therapeutic antagomirs with paclitaxel for the management of metastatic tumors by a pH-responsive anti-microbial peptide-mediated liposomal delivery system. J. Controlled Release 197:208–18
- Zong T, Mei L, Gao H, et al (2014). Synergistic dual-ligand doxorubicin liposomes improve targeting and therapeutic efficacy of brain glioma in animals. Mol Pharm 11:2346–57