Abstract:
Crosslinking of ultrapure hemoglobin, crystalline catalase, and superoxide dismutase resulted in a soluble nanodimensional complex of polyhemoglobin-catalase-superoxide dismutase. A less expensive and more convenient way is to crosslink bovine stroma-free hemolysate (stroma-free hemolysate) that already contains hemoglobin, catalase, and superoxide dismutase into polyhemoglobin with catalase and superoxide dismutase activities (stroma-free polyhemolysate) [Citation]. The objective of the present study is to evaluate the immunological properties of this stroma-free polyhemolysate. Each of three groups of rats received weekly subcutaneous injections of one of the stroma-free polyhemolysate, stroma-free hemolysate, and saline for four weeks. One week after the four cycles of weekly immunization, serum and plasma were collected for C3a complement activation tests and Ouchterlony antibody-antigen precipitation tests, respectively. Results show that stroma-free polyhemolysate retained significant antioxidant enzyme activity. The C3a complement activation test and Ouchterlony test show that four weekly subcutaneous injections of bovine stroma-free polyhemolysate did not result in any immunological reaction in rats when tested this way.
INTRODUCTION
Red blood cell substitutes are oxygen carriers designed to temporarily replace transfused blood. The potential benefits of a RBC substitute include universal compatibility, immediate availability, freedom from disease transmission, and long-term storage. Various methods were utilized to make hemoglobin a useful and safe oxygen carrier. Among them, the basic principle of diacid [Citation4] or glutaraldehyde cross-linked poly-hemoglobin (poly-Hb) [Citation5] has been developed into hemoglobin-based oxygen carriers that have been tested clinically in patients [Citation20, Citation25, Citation30]. In certain clinical situations, such as in sustained severe hemorrhagic shock, stroke, and myocardial infarction, the use of poly-Hb might result in ischemia reperfusion injuries [Citation2, Citation6, Citation8, Citation13]. Ischemia occurs when there is a lack of oxygen supply to an organ or tissue, which leads to the accumulation of xanthine and hypoxanthine and also promotes the conversion of enzyme xanthine dehydrogenase to its oxidase form. When reperfused with oxygen carrier, xanthine oxidase converts xanthine and hypoxanthine into superoxide and other reactive oxygen species. This can lead to reperfusion injuries [Citation13]. Recently, a nanobiotechnological method has been used to prepare a soluble complex of polyHb-catalase-superoxidase dismutase (PolyHb-CAT-SOD) containing higher concentration of these antioxidase enzymes than those normally present in red blood cells [Citation7, Citation8, Citation9, Citation13] . Animal studies show that PolyHb-CAT-SOD significantly decreases oxygen radical production and ischemia reperfusion injuries [Citation7, Citation8, Citation9, Citation12, Citation29, Citation31].
This PolyHb-SOD-CAT was prepared from ultrapure hemoglobin, catalase (CAT), and superoxide dismutase (SOD) [Citation13]. The high costs of these purified enzymes and Hb are not practical for large-scale manufacturing. The original use of stroma-free content of red blood cells (stroma-free hemolysate) for preparing different types of blood substitutes [Citation4, Citation5] would be more practical. Our recent analysis of glutaraldehyde cross-linking of stroma-free hemolysate shows good retention of hemoglobin, SOD and CAT activity [Citation21]. However, the immunological properties of this preparation need to be analyzed. The objective of the present study is therefore to evaluate the immunological properties of bovine stroma-free polyhemolysate prepared this way when injected subcutaneously into rats. C3a complement activation tests and Ouchterlony tests show that four weekly subcutaneous injections of bovine stroma-free polyhemolysate did not result in any immunological reaction in rats when tested this way.
MATERIALS
Materials
The following materials were purchased from different sources as shown: fresh bovine blood (McGill University, MacDonald campus), NaCl (Fisher), Potassium Phosphate (Sigma), Toluene (Sigma), Sodium Phosphate (Sigma), 99% Lysine Monohydrochloride (Sigma), 25% Glutaraldehyde (Sigma), 1.5cm*98cm Chromatography Column (Fisher), Sepacryl S-300HR (Sigma), Tris Base (Sigma), 37% Hydrochloric Acid (Sigma), Catalase from bovine liver (Sigma), Superoxide Dismuatase from bovine erythrocyte (Sigma), 30% Brij 35 solution (Sigma), Drabkin (Sigma), Molecular Weight Marker Kit (29,000-700,000) (Sigma), UltraPure Water (Cayman Chemical), 100kDa Centrifugal Filter (Millipore), 14.1 g/dl Haemiglobincyanide Standard (J.T. Baker), Catalase Assay Kit (Sigma), Superoxide Dismutase Kit (R&D Systems), 3%(w/w) Hydrogen Peroxide (Sigma), Sodium Perborate Tetrahydrate (Sigma), 95-98% Sulfuric Acid (Sigma), 99% Potassium Permanganate (Sigma), Hepalean 1000U.S.P. units/ml (Organon), Pentobarbital Sodique (CEVA Sante Animal), Complement C3a des Arg ELISA Kits (Assay Design), Sodium Hydroxide (Sigma), 99.9% Sodium Azide (Sigma), Agar (Sigma).
Instruments
254/280nm UV Detector (Mandel Scientific), Chart Recorder (Mandel Scientific), Ultra Spectrophotometer 2100 PRO (BioChrom), Gen5 MicroPlate Reader (BioTek).
Animals
Male Sprague-Dawley rats (250-300 g) (Charles River) were housed in a 12-hour light cycle environment in the McIntyre Animal Facility and were provided with food and water ad libitum. The experimental protocol was approved by the animal resources center of McGill University and met the Canadian Council on Animal Care guidelines for animal use.
METHODS
Stroma-free Hemolysate Preparation
This follows that of Gu and Chang [Citation21] with slight variations. Fresh bovine blood with heparin (anticoagulant) was centrifuged at 4000×g for 60 minutes at 4°C. After removal and plasma supernatant and upper layer of cell pellet, the blood cells were washed four times with sterile, ice-cold 0.9% NaCl. The red blood cells were then suspended in twice the volume of potassium phosphate, 12.5 mM, pH 7.4, and allow to lyse for 30 min. Then 2 volumes of ice-cold reagent-grade toluene was used to removed stroma lipid. Cellular debris was then separated by centrifugation at 15000×g for 2 hours at 4°C. The sample was aliquoted and stored at −80°C.
Preparation of Stroma-free Polyhemolysate
Polymerization of stroma-free haemoglobin was prepared according to Gu and Chang [Citation21]. Polymerized 9.573 g/dL stroma-free hemolysate in 0.2mM sodium phosphate buffer, pH 7.4. Before crosslinking, 1.3 M of lysine was added at a molar ratio of 11.1:1 lysine/hemolysate. Crosslinking reaction began with the addition of glutaraldehyde (0.5 M) at a molar ratio of 16.1:1 glutaraldehyde/hemolysate by four equal aliquots over a period of 15 min. Crosslinking was continued for 24 hrs with constant stirring (140rpm) under aerobic conditions at 4°C. The reaction was stopped by adding an excess of 1.3 M lysine monohydrochloride at a molar ratio of 1118:1 lysine/stroma-free hemolysate. Shaking was continued at 150rpm at 4°C for 1h. Finally, the solution was centrifuged at 16,000×g for 1 hr and then stored at 280°C to wait for the next step of purification.
Purification of Stroma-free Polyhemolysate
A Sephacryl-300 HR column (1.5cm*98 cm, Vtotal=560 ml) was prepared and equilibrated with 0.1M Tris-HCl and 0.15M NaCl pH 7.4 elution buffer. Samples (every 1.5ml) were passed through the column at a flow rate of 36 ml/hour. Standard catalase and tetrameric Hb peaks were determined by UV detector at 280nm (sensitivity “1”). This was to establish the exclusion for unlinked red blood cell enzymes such as catalase (250kDa), superoxide dismutase (32.5 kDa), and free tetrameric Hb (64kDa) by peak identification using UV detector at 280nm and at room temperature. Based on this, only stroma-free polyhemolysate with molecular weights higher than 250kDa was collected, thereby excluding all free enzymes and tetrameric Hb. The stroma-free polyhemolysate samples were pooled and concentrated using a 100kDa ultracentrifugal filter. The Drabkin’s method was used to analyze the hemolysate concentration of purified stroma-free polyhemolysate. After this aliquots were stored at −80°C.
Molecular Distribution of Stroma-free Polyhemolysate
The molecular weight distribution was recorded by a 280nm UV detector using the same gel filtration column at a recording velocity of 1mm/min and sensitivity “1.” We plotted the molecular weight peaks and calculated the area percentage of the two main peaks: a) the area of peak with molecular weight >66kDa; b) the area of peak with molecular weight ≤66kDa.
Measurements of CAT and SOD Activities
Three methods for catalase measurement were used in this paper: (1) UV 240nm spectrophotometeric method from the catalase assay kit (Sigma) measuring the rate of disappearance of H2O2; (2) UV 240nm spectrophotometeric method [Citation1] also monitor the decrease of H2O2 level; and (3) Perborate titration method [Citation17] to measure the decrease in perborate level. Superoxide dismutase activity was determined by Superoxide Dismutase kit (R&D Systems), which follows the inhibition of nitroblue tetrazolium (NBT) reduction using xanthine-xanthine oxidase used as a superoxide generator.
Rat Immunization
Three groups of Sprague Dawley rats were immunized over four weeks by weekly subcutaneous injections. (1) Group (P): four Sprague Dawley rats were subcutaneously injected with 0.1ml of 1g/dl purified Stroma-free polyhemolysate solution for four weeks. (2) Group (B): Four Sprague Dawley rats were injected subcutaneously with 0.1ml of 0.1M Tris-HCl and 0.15M NaCl, pH 7.4 buffer. (3) Group (S): four Sprague Dawley rats were injected subcutaneously with 0.1ml of 1g/dl free stroma-free hemolysate for four weeks.
Serum and Plasma Preparation
At week five, four rats of each group (P, B and S) were anaesthetized (intraperitoneal injection of 5mg of pentobarbital per 100 gram body weight) and blood was collected by intra-cardiac puncture method. (1) Plasma for complement activation studies: blood samples (one rat in each group) were quickly mixed with 10% (blood volume) heparin and centrifuged at 5500g for 20 min at 2°C to obtain plasma. (2) Serum for Ouchterlony Double Diffusion Study: Collected blood samples were allowed to clot. After coagulation, the samples (three rats in each group) were centrifuged at 3,000g for 20 min at 4°C to obtain serum. The (1) plasma samples and (2) serum samples were aliquoted as groups of P, B, and S and stored at −80°C for the following studies.
C3a Complement Activation Test
Chang and Lister [Citation10, Citation11] devised a simple screening method for blood substitutes using complement activation. Blood substitutes were added directly to plasma samples and increase in C3a was measured to see if there was any complement activation. Since then, this has been used for screening test of the purity and immunological properties of hemolysate-based oxygen carrier in both research and industrial production [Citation6]. The detailed method is as described [Citation6].
Ouchterlony Double Diffusion Method
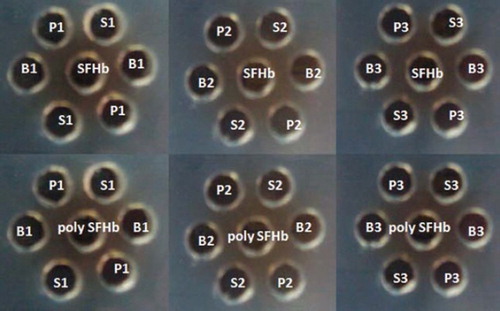
This Ouchterlony double diffusion method [Citation16] was slightly used with slight modification. (1) Antibody-antigen reaction with free hemolysate, catalase, and superoxide dismutase:first, 50μl of stroma-free hemolysate (1g/dl) was placed into each of the central wells of an 8% agarose gel plate (contains 2% sodium azide). Then 50μl of serum from group S1 (stroma-free hemolysate injected), group P1 (purified stroma-free polyhemolysate injected) and group B1 (buffer injected) into two diagonally peripheral wells in duplicates, respectively (). The same tests were carried out using serum from rats S2, S3, B2, B3 and P2, P3 in the same way. (2) Antibody-antigen reaction with polymerized polyHbStroma-free hemolysate (PolyHb-catalase-superoxide dismutase): stroma-free polyhemolysate is placed into the central well. Then 50μl of serum from group S1 (stroma-free hemolysate injected), group P1 (purified stroma-free polyhemolysate injected) and group B1 (buffer injected) into two diagonally peripheral wells in duplicates, respectively (). The same tests were carried out using serum from rats S2, S3, B2, B3 and P2, P3 in the same way. All plates were incubated at room temperature for four days. Digital photos of the plates were taken and analyzed for precipitation lines.
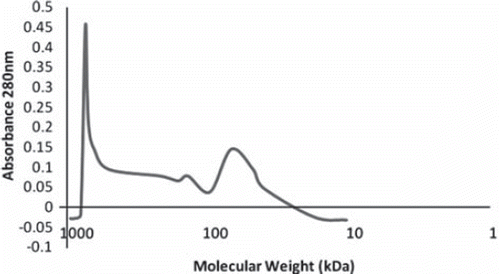
Figure 1. Molecular distribution of crosslinked bovine stroma-free polyhemolysate. The elution profile of stroma-free polyhemolysate was obtained by running 1 ml of stroma-free polyhemolysate on Sepacryl S-300 1.5398cm column with 0.1 M Tris-Hcl, 0.15M NaCl pH 7.5 buffer at elution speed at 36 ml/hr.
RESULTS AND DISCUSSIONS
Molecular Weight Distribution for Stroma-free Polyhemolysate
In order to judge the degree of polymerization, Sepacryl S-300 gel column chromatography was performed. The molecular weight distribution for stroma-free polyhemolysate showed three main components: the low molecular weight range (molecular weights less than 100 kDa containing tetrameric hemoglobin and demeric hemoglobin), the intermediate range (with molecular weights between 100 kDa and 600 kDa), and the high range (with molecular weights higher than 600 kDa) (). After 24 hrs of cross-linking, the stroma-free polyhemolysate sample yielded 20% of polymer with molecular weight higher than 600 kDa, 53% of polymer with molecular weight between 100 kDa and 600 kDa, and 27% of sample with molecular weight lower than 100kDa. In total, there was about 73% stroma-free polyhemolysate with molecular weight higher than 100kDa.
A size exclusion column [Citation9] was used to remove those with molecular weight lower than 250kDa (). This removed the following molecules: dimeric hemoglobin (33kDa), tetrameric hemoglobin (66kDa), free red blood cell enzymes such as cytochrome (12kDa), pyrimidine 5′-nucleotidase (28kDa), carbonic anhydrase (29kDa), superoxide dismutase (32.5 kDa), pyruvate kinase (57kDa), purine nucleoside phosphorylase (81kDa), glucose 6-phosphate dehydrogenase (240kDa) and catalase (240kDa) [Citation14, Citation15, Citation22, Citation23, Citation24, Citation32, Citation34]. The resulting stroma-free polyhemolysate is then used in the immunological studies.
Figure 2. Ouchterlony double diffusion test for determining antigen specific antibody activation. Equal amounts of stroma-free hemolysate (SFHb) (1g/dl) were placed into the central well of the 8% agarose gel plate (containing 2% sodium azide) and the same amount of serum from rat S1 (stroma-free hemolysate - SFHb injected) (positive control), rat P1 (purified stroma-free polyhemolysate - SFPolyHb injected) (test) and rat B1 (buffer injected) (negative control) were placed into two diagonal wells in duplicates, respectively (). The serum from rats S2, S3, P2, P3 and B2, B3 were repeated in the same way. We replaced stroma-free hemolysate - SFHb with stroma-free polyhemolysate into the central well and repeated the same procedure as above to determine anti-stroma-free polyhemolysate specific antibody production. All plates were incubated at room temperature in a humid environment for four days.
Dismutase Activity of Stroma-free Polyhemolysate
Two of the three methods were used. The result from the first method based on the detection kits shows interference by the high concentration of the colored hemoglobin. The second method [Citation1] was more precise for measuring catalase activity in the presence of high hemoglobin concentration. This method uses test samples as a blank to minimize hemoglobin interferences. Furthermore, the result was similar to the data measured by the titration method of Feinstein [Citation17] using NaBO3 as substrate that does not depend on color changes. The result from the Aebi method of measurement is shown in . The superoxide dismutase activities were determined by SOD inhibition kits (R&D). Since stroma-free polyhemolysate collected was only a portion of origin polymerized sample (molecular weight higher 250kDa), the SOD activity of stroma-free polyhemolysate was lower than that of stroma-free hemolysate. Stroma-free polyhemolysate containing higher concentrations of catalase or superoxide dismutase can be prepared by adding more enzymes extracted from red blood cell stroma-free hemolysate [Citation21].
Table 1. Catalase activity of stroma-free hemolysate (SFHsate) and purified stroma-free polyhemolysate (SFPolyHsate)
Effects on C3a Compliment Activity
The effect of stroma-free hemolysate and stroma-free polyhemolysate on C3a levels were studied. As shown in , the amount of C3a des Arg in the control buffer immunized rat (0.42) was almost the same as the negative control (0.46). There was also no complement activation from the plasma of rats immunized with stroma-free hemolysate and stroma-free polyhemolysate (0.15 and 0.18) as compared to the negative control (0.30 and 0.25), respectively. There were no significant differences in C3a levels between the plasma incubated with stroma-free hemolysate, stroma-free polyhemolysate, and the saline control.
Table 2. The plasma C3a des Arg level of rats immunized with buffer, stroma-free hemolysate (SFHsate), or stroma-free polyhemolysate (SFPolyHsate); the saline in each test was used as negative control
C3a is a multifunctional, pro-inflammatory mediator and has the ability to mediate hypersensitivity, anaphylactic reactions, and specific antibody-antigen responses. Complement activation has been used for screening contaminations in blood substitutes [Citation9-11]. The activation of C3a is triggered by either classical or alternate complement pathways upon the hydrolyzation of protein C3. If any antibody is produced, the C3a will be activated and its stable form molecule, C3a des Arg, can be quantified by ELISA assay. As shown in , the amount of C3a des Arg in the buffer immunized rat (0.42) was almost the same as in the negative control (0.46). The C3a of stroma-free hemolysate and stroma-free polyhemolysate immunized rats (0.15 and 0.18) were lower than the negative control (0.30 and 0.25), respectively. The low amount of C3a des Arg indicated that fewer C3a was activated in all three groups of immunized rats. These results also demonstrated that fewer antigen specific antibodies, including specific anti-blood substitute antibody, were produced in rats. The low C3a activity demonstrated that no significant amount of antibody was produced.
Ouchterlony Double Diffusion Test
Ouchterlony’s method was used for analyzing specific antibodies using antisera collected from each immunized rat (stroma-free hemolysate, stroma-free polyhemolysate, and buffer immunized rat). The undiluted antisera were incubated for 4 days with 1g/dl stroma-free hemolysate in azide containing agar gel (). There did not appear to be an immunological response (i.e. no precipitin reaction in agar gel diffusion tests) between stroma-free hemolysate and antisera from all immunized rats. In order to test the immunological response between stroma-free polyhemolysate and antisera, another set of Ouchterlony tests were also performed. As shown in , there was no precipitin reaction between 1g/dl stroma-free polyhemolysate and undiluted antisera from all immunized rats after 4 days of incubation.
CONCLUSIONS
Crosslinking of ultrapure hemoglobin, crystalline catalase and superoxide dismutase resulted in a soluble nanodimensional complex of Polyhemoglobin-catalse-superoxide dismutase [Citation13]. This PolyHb-SOD-CAT has the ability to scavenge reactive oxygen species such as superoxide and hydrogen peroxide and has the ability to carry oxygen [Citation13]. Additionally, investigations show that the use of the PolyHb-SOD-CAT leads to less disruption of the blood-brain barrier and less damage to liver, kidney, and intestine when compared to other oxygen-carrying solutions [Citation31]. A less expensive and more convenient way is to follow the original use of red blood cell contents (stroma-free hemolysate) for the preparation of different types of hemoglobin-based blood substitutes [Citation4, Citation5]. In this way, crosslinking stroma-free hemolysate that already contains hemoglobin, catalase, and superoxide dismutase can result in the preparation of stroma-free polyhemolysate with catalase and superoxide dismutase activities (stroma-free polyhemolysate) [Citation21]. The present study using C3a complement activation and Ouchterlony antibody-antigen precipitation shows that four weekly subcutaneous injections of bovine stroma-free polyhemolysate into rats did not result in any immunological reaction. These results are promising, especially if they can be supported by further studies.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
REFERENCES
- Aebi, H. (1974). UV-assay of catalase. H.U. Bergmeyer. Methods of Enzymatic Analysis, vol. 2, Verlag Chemie, Weinheim, Germany, 673.
- Alayash, A.I., D’Agnillo, F., Buehler, P.W. (2007). First-generation blood substitutes: what have we learned? Biochemical and physiological perspectives. Expert Opin. Biol. Ther. 7(5): 665–675.
- Burger, R., Zilow, G., Bader, A., Friedlein, A., Naser, W. (1988). The C terminus of the anaphylatoxin C3a generated upon complement activation represents a neoantigenic determinant with diagnostic potential. The Journal of Immunology 141(2): 553–558.
- Chang, T.M.S. (1964). Semipermeable microcapsules. Science 146(3643): 524.
- Chang, T.M.S. (1971). Stabilisation of enzymes by microencapsulation with a concentrated protein solution or by microencapsulation followed by cross-linking with glutaraldehyde. Biochem Biophys Res Common. 44(6): 1531–1536.
- Chang, T.M.S. (1997). Blood Substitutes: Principles, Methods, Products and Clinical Trials. vol. 1. Karger, Basel (full text available for free online viewing at www.artcell.mcgill.ca).
- Chang, T.M.S. (2005). Therapeutic applications of polymeric artificial cells. Nature Review: Drug Discovery 4: 221–235.
- Chang, T.M.S. (2006). Blood substitutes based on nanobiotechnology. TRENDS in Biotechnology 24(8): 372–377.
- Chang, T.M.S. (2007). Artificial Cells: Biotechnology, Nanomedicine, Regenerative Medicine, Blood Substitutes, Bioencapsulation, and Cell/Stem Cell Therapy, vol. 1 of Regenerative Medicine, Artificial Cells and Nanomedicine, World Scientific, Singapore.
- Chang, T.M.S., Lister, C.W. (1990). C3a complement activation in human plasma. Biomaterials, Artificial Cells, and Artificial Organs 18(5): 693–701.
- Chang, T.M.S., Lister, C.W. (1994). Assessment of blood substitutes: II. In-vitro complement activation of human plasma and blood for safety studies in research, development, industrial production and preclinical Aanalysis. Artificial Cells, Blood Substitutes and Biotechnology 22(2): 171–180.
- Chang, T. M. S., D’Agnillo, F., Yu, W.P., Razack, S. (2000). Two future generations of blood substitutes based on polyhemoglobin–SOD–catalase and nanoencapsulation. Advanced Drug Delivery Reviews 40: 213–218.
- D'Agnillo, F., Chang, T.M.S. (1998). Polyhemoglobin-superoxide dismutase-catalase as a blood substitute with antioxidant properties. Nature Biotechnology 16: 667–671.
- Domagk, Süleyman Gezici, Götz, F. (1979). Glucose-6-phosphate dehydrogenase from bovine erythrocytes: Kinetic properties and the electrophoretic comparison of its subunit structure with g6pdh from other sources. International Journal of Biochemistry 10(5): 427–432.
- D'Souza, A., Kurien, B.T., Rodgers, R., Shenoi, J., Kurono, S., Matsumoto, H., Hensley, K., Nath, S.K., Scofield, R.H. (2008). Detection of catalase as a major protein target of the lipid peroxidation product 4-HNE and the lack of its genetic association as a risk factor in SLE. BMC Medical Genetics 9(62): 1–8.
- Feinstein, R. N., Suter, H., Jaroslow, B.N. (1968). Blood catalase polymorphism: some immunological aspects. Science 159: 638–640.
- Feinstein, N. R. (1949). Perborate as sunstrate in a new assay of catalase. The Journal of Biological Chemistry 180(3): 1197–1202.
- Forman, H. J., Fridovich, I. (1973). On the stability of bovine superoxide dismutase. J. Biol. Chem. 248: 2645–2649.
- Freedman, S, Anderson, P., Epstein, D. (1985). Superoxide dismutase and catalase of calf trabecular meshwork. Invest Ophthalmol Vis Sci 26: 1330–1335.
- Gould, S. A. . (2002). The life-sustaining capacity of human polymerized Hb when red cells might be unavailable. J. Am. Coll. Surg. 195: 445–452.
- Gu, J., Chang, T.M.S. (2009). Extraction of erythrocyte enzymes for the preparation of polyhemoglobincatalase-superoxidase dismutase. Artificial Cells, Blood Substitutes, and Biotechnology 37: 69–77.
- Hanset, M., Ansay, R. (2009). Purine nucleoside phosphorylase (NP) of bovine erythrocytes: genetic control of electrophoretic variants. Animal Genetics 3(4): 219–227.
- Harkness, D.R., Villa, L., Berman, I., Wilson, J.B., Huisman, T.H.J. (1977). Biosynthetic and structural studies of hemoglobin in a patient with congenital dyserythropoietic anemia Type I. Hemoglobin 1(7): 663–677.
- Hultquist, R. H., Douglas, D. E. (1978). Evidence that two forms of bovine erythrocyte cytochrome b5 are identical to segments of microsomal cytochrome b5. Proc Natl Acad Sci U S A 75(7): 3118–3122.
- Jahr, J.S., Mackenzie, C., Pearce, L.B., Pitman, A., Greenburg, A.G. (2008). HBOC-201 as an alternative to blood transfusion: efficacy and safety evaluation in a multicenter phase III trial in elective orthopaedic surgery. J Trauma 64: 1484–97.
- Tian, J., Xu, Z., Smith, J.S., Hofherr, S.E., Barry, M.A., Byrnes, A.P. (2009). Adenovirus activates complement by distinctly different mechanisms in vitro and in vivo: indirect complement activation by virions in vivo. Journal of Virology 83(11): 5648–5658.
- McCord, J.M., Keele, Jr., B.B., Fridovich, I. (1971). An enzyme-based theory of obligate anaerobiosis: The physiological function of superoxide dismutase. Proc. Nat. Acad. Sci. USA 68(5): 1024–1027.
- Moore, E.E., Moore, F.A., Fabian, T.C., Bernard, A.C., Fulda, G.J., Hoyt, D.B., Duane, T.M., Weireter Jr., L.J., Gomez, G.A., Cipolle, M.D., Rodman Jr., G.H., Malangoni, M.A., Hides, G.A., Omert, L.A., Gould, S.A. (2009). Human polymerized hemoglobin for the treatment of hemorrhagic shock when blood is unavailable: The USA multicenter trial. J Am Coll Surg. 208: 1–13.
- Mun, K. C., Bae, J.H., Suh, S.I., Kim, Y.H., Lee, S.H., Kim, S.P., Kwon, T.K., Hwang, J.S. (2003). Effect of modified polyhemoglobin on the oxidative damage after ischemia-reperfusion in the liver. Transplantation Proceedings 33: 126–127.
- Pearce, L.B., Gawryl, M.S., Rentko, V.T., Moon-Massat, P.F., Rausch, C.W. (2006). HBOC-201 (Hb Glutamer-250 (Bovine), Hemopure): Clinical studies. Blood Substitutes, Winslow, R. Academic Press, San Diego, 437–4509.
- Powanda, D., Chang, T.M.S. (2002). Cross-linked polyhemoglobin-superoxide dismutase catalase supplies oxygen without causing blood brain barrier disruption or brain edema in a rat model of transient global brain ischemia-reperfusion. Artificial Cells, Blood Substitutes, and Biotechnology 30: 23–37.
- Simoni, R.D., Hill, R.L., Vaughan, M. (2002). The structure and function of hemoglobin: Gilbert Smithson Adair and the Adair equations. The Journal of Biological Chemistry 277: 20.
- Beydemir, S., Yilmaz, H., Ciftci, M., Bakan, E., Kufrevioglu, O.I. (2003). Purification of glucose 6-phosphate dehydrogenase from goose erythrocytes and kinetic properties. Turkish Journal of Veterinary & Animal Sciences 27(5): 1179–1185.
- Torrance, J.D., Whittaker, D., Beutler, E. (1977). Purification and properties of human erythrocyte pyrimidine 5′-nucleotidase. Proc Natl Acad Sci U S A 74(9): 3701–3704.