Abstract
The present work describes the formulation and evaluation of the gastroretentive system of Itopride hydrochloride. In this research, we have formulated floating hydrogel-based microspheres employing calcium carbonate (CaCO3) as a gas forming agent dispersed in alginate matrix. In vitro characterizations such as drug content, particle size, and drug release were carried out. GI motility was determined by administration of charcoal meal to rats. Results demonstrated that prepared microspheres were spherical in shape with smooth surface, good loading efficiency, and excellent buoyancy. The gastro retentive dosage form of itiopride demonstrated significant antacid, anti-ulcer, and anti-GERD activity after 12 hours in comparison with the conventional dosage form.
INTRODUCTION
Gastroesophageal reflux disease (GERD) is a common, chronic, relapsing condition characterized by the regurgitation of stomach contents passing up into the esophagus with or without histological changes. However, the excessive reflux of gastric acid induces some complications such as esophagitis, esophageal stenosis, cancer, or Barrett's esophagus. Pathogenesis of GERD includes lower esophageal sphincter (LES) dysfunction, clearing capacity of refluxed materials, delayed gastric emptying, and abnormal resistance of esophageal mucosa to gastric acid, but motor dysfunction is regarded as the most important factor in general [Citation1,Citation2]. The goals of drug treatment of GERD include relief of symptom to improve quality of life and healing of mucosal lesions. Drugs commonly used include antacids, antisecretory agents like H2 recetpor antagonists, proton pump inhibitors, and prokinetic drugs [Citation3].
Itopride hydrochloride is a prokinetic agent. It improves gastrointestinal motility by dual mechanism. First, by virtue of dopamine D2 antagonism it enhances the release of acetylcholine in the myenteric plexus, and secondly it inhibits the enzyme acetylcholinesterase and thereby prevents the hydrolysis of acetyl choline. It also has an anti-emetic action [Citation4]. Gastroretentive drug delivery is an approach to prolong gastric residence time, thereby targeting site-specific drug release in the upper gastrointestinal tract (GIT) for local or systemic effects. Gastroretentive dosage forms can remain in the gastric region for long periods and hence significantly prolong the gastric retention time (GRT) of itopride hydrochloride [Citation5,Citation6]. Several approaches are currently used to develop GRDF. These include floating drug delivery systems, swelling and expanding systems, polymeric bioadhesive systems, modified-shape systems, high density systems, and other delayed gastric emptying devices [Citation7]. Among all sodium alginate basedhydrogels, preparations have attracted a lot of attention in the pharmaceutical and medical fields for their favorable biological properties such as biodegradability [Citation8] and biocompatibility [Citation9].
Sodium alginate precipitates an ingestion in the acid conditions of the stomach to form a gel of alginic acid. The gas-producing substance, bicarbonate, forms carbon dioxide with the gastric contents [Citation10]. The gas bubbles are entrapped by alginate gel and the resulting viscous foam (gas dispersion) rises to the surface of the stomach content and float. Calcium carbonate may be added in order to aid the gelation of the alginate. In acidic conditions the salt dissociates to release divalent calcium cations, which interact with the alginate to form an “egg box” structure, thereby considerably increasing the viscosity of the system [Citation11]. In the present investigation we developed an extended and controlled release formulation of Itopride hydrochloride using sodium alginate.
MATERIALS AND METHODS
Materials
Itopride hydrochloride was purchased from Themis Labs, Mumbai. Sodium alginate, hydroxypropyl methyl cellulose (HPMC), and calcium carbonate were purchased from SD Fine Chem Limited, Mumbai. All other chemicals used were of analytical grade.
Preparation of Microspheres [Citation12]
0.09 gm of itopride was dissolved in 5ml distilled water. The solution was dispersed in 30 ml alginate solution (3% w/v) containing HPMC in a ratio (alginate: HPMC = 9:1, w/w). The gas forming agent such as CaCO3 was added in two different concentrations (0.25% and 0.5% w/w). The above dispersion was added drop-wise via a 26-gauge hypodermic needle fitted with a 10 ml syringe into of 1 % w/v solution of gelling agents (CaCl2) containing 10% (v/v) acetic acid. The mixture was stirred using magnetics for 10 min to improve the mechanical strength of the microspheres and was allowed to complete the reaction. The resultant hydrogel beads were collected, washed twice with distilled water, air dried for 12 hours, and subsequently freeze dried to obtain discrete microspheres.
Evaluation of Microspheres
Size and shape
a) SEM studies: The surface and cross-section of microspheres were studied using a scanning electron microscope (Jeol, JSM-T330A, Japan). Microspheres were mounted directly onto the sample stub and coated with platinum film.
b) Particle size distribution: Size and size distribution of the alginate microspheres were measured by optical microscopy. The particle size distributions of the microspheres were determined and the mean particle size of microspheres was calculated.
Drug content analysis. The drug content of alginate microspheres was determined by crushing 50 mg formulation in 100 ml purified water followed by agitation with a magnetic stirrer for 12 h to dissolve the polymer. The solution was then gently heated for two hours to extract the drugs completely, filtered, and the resulting solution was analyzed by UV spectrophotometer (Shimadzu Corporation, Japan) for itopride at 258 nm.
Floating properties [Citation13]. Floating properties of wet and dry microspheres were evaluated in a 500 ml beaker filled with 250 ml of simulated gastric fluid (pH 1.2) without pepsin. Fifty microspheres were placed in the media and maintained at 370C ± 0.50C for 12 hrs. The floating and settled portion of beads were recovered separately. Buoyancy percentage was calculated as the ratio of the number of beads that remained floating and total number of beads taken.
In-vitro release studies [Citation14]. In vitro drug release from alginate microspheres was performed at 37°C in dissolution apparatus (Electrolab, TDT-06 USP XXXIII) at 100 rpm. Drug release from the microspheres was studied in simulated gastric fluid at regular time intervals, aliquots were withdrawn, and fresh medium was added to maintain the sink condition. Drug content of the beads was determined by UV/visible spectroscopy (Shimadzu Corporation, Japan) at 258 nm.
Pharmacological Screening
Anti-ulcer and anti-GERD activities. Male Wistar rats with a body weight of about 200 gm were used for the experiments. Rats were starved for 24 hours before the experiment but were allowed free access to water. Sodium alginate microspheres containing itopride was mixed with 1% CMC solution and marketed preparation of itopride were administered orally in rats with a standard dose of 9 mg/kg body weight. Experiments were carried out under light ether anesthesia. In rats duodenogastro-esophageal reflux was caused by the ligation of the fourth portion of the duodenum [Citation15,Citation16]. Two experiments were carried out at two different time intervals for each activity. The animals were divided into six groups of six animals in each group. The abdomen was opened and the fourth portion of the duodenum was ligated at two different time intervals after the administration of test drugs (i.e. 30 min, 6 hrs). The abdomen was then sutured. For each time interval 3 groups of animals were used, i.e. control, marketed preparation treated group, and formulation treated group. Four hours after the ligation (for both time intervals) the rats were sacrificed and the stomach and esophagus were removed and were dissected out. Both stomach and esophagus were examined for gross and microscopic mucosal injury; the presence of ulcers and scores was made according to the severity of hemorrhagic erosions in the acid secreting mucosa and ulcer scoring was accessed on a scale of 0-3.
Mean ulcer score for each animal is expressed as ulcer index. The protection was calculated using the following formula.
Antacid activities of itopride containing different formulations. In the GERD model the gastric contents were collected and centrifuged for 5 min at 3000 rpm. The supernatant was separated and its pH was checked with the help of the pen type of pH meter [Citation17].
G.I. Motility [Citation18,Citation19]
Male Wistar rats were used for this study. Animals were divided into six groups of 6 animals in each group. The animals were fasted for approximately six hours prior to the experiment. Charcoal meal was administered at two different time intervals after drug administration (i.e. 30 minutes and 6 hours). An aqueous suspension of 1% (w/v) sodium carboxy methyl cellulose and 10% (w/v) activated charcoal was administered intragastrally to conscious rats. 30 min after receiving the charcoal meals, rats were anesthetized and the small intestine was exposed. The length of the small intestine was measured by laying out the small intestine on a measuring tape and measuring the distance from the pyloric sphincter to the ileo-caecal valve. The distance traveled by the charcoal was measured from the pyloric sphincter to the most caudal edge of the charcoal. Values for gastrointestinal transit times were expressed as a percent of total intestinal length and were calculated in the following manner. The individual distance traveled by the charcoal in centimeters divided by the total length of the intestine in centimeters (pylorus to caecum) was evaluated for each rat. Mean values were calculated for each group and statistical comparisons were made between the treatment groups using parametric statistics (one way analysis of variance followed by Dunnet's‘t’ test.
RESULT
Development of Formulation and Evaluation
Sodium alginate microspheres containing itopride were prepared by precipitation followed by the cross-linking method using CaCl2 as a cross linking agent Spherical gel beads were formed instantaneously, in which intermolecular crosslinks were formed between the divalent calcium ions and the negatively charged carboxyl group of alginic acid. Various gas forming agents such as CaCO3 and NaHCO3 have been tried but a smoother surface was observed when CaCO3 was used. Hence in this research we have used CaCO3 as a gas-forming agent. Itopride is soluble in water and its solubility is not much affected by the pH.
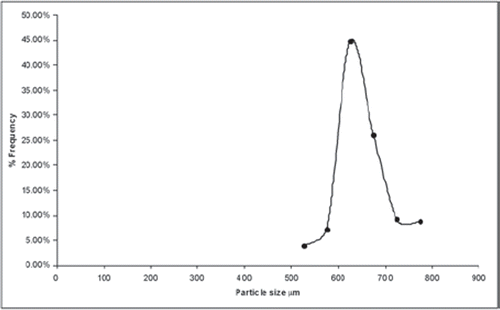
Particle Size and Size Distribution
Results of particle size and size distribution of prepared microspheres are mentioned in . It was observed that all particles formed were almost spherical with size range from 525 to 775 mm. it seems that the process variable did not greatly affectthe size and size distribution ().
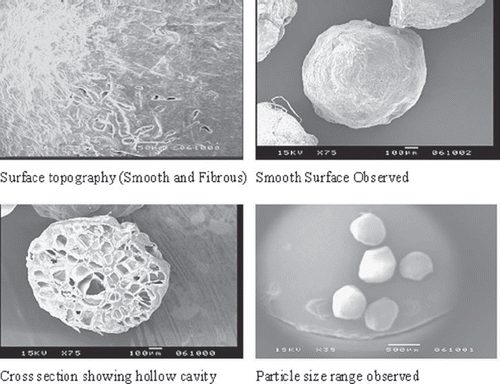
Morphological Analysis (Scanning Electron Microscopy)
The morphology, cross-section and particle size were analyzed by scanning electron microscopy using Jeol JSM-T330A and these photographs are shown in . The surfaces of the alginate microsphere obtained from CaCO3 as a gas-forming agent were found to be smooth and spherical in nature (). Cross-section indicates presence of air bubbles, which are formed during formulation and are responsible for floating.
Drug Content Analysis
The determination of itopride content is done by the UV spectrophotometric method at λmax of 258 nm. The itopride shows one peak at 258 nm and this peak was further used for quantitative analysis. Two different drug loadings were tried, i.e. 10% and 20%. Results of % drug content for various formulations are given in . It was observed that sodium alginate microspheres of itopride (10% loaded) had drug content in the range of 52% to 56% and sodium alginate microspheres of itopride (20% loaded) had drug content in the range of 52.5% to 61.25%. Content uniformity result indicated that as the theoretical loading of the drug increases the efficiency of loading also increases. This may be due to decrease in the leaching of the drug during formulation procedure.
Table 1. Drug content analysis of sodium alginate microspheres of itopride (10% & 20% drug loaded)
Floating Properties
The floating ability of prepared microspheres was evaluated in 500ml simulated gastric fluid. It was observed that gas-forming agent free microspheres sink uniformly in simulated fluid, and microspheres containing gas-forming agents in proportions ranging from 0.25 to 0.75%w/w demonstrated excellent floating ability (100% floating). The gas generated is trapped and protected within the gel formed by hydration of the polymer, thus decreasing the density of the microspheres. As the density of the microspheres falls below 1, the microspheres become buoyant. The wet microspheres had better floating ability than dry microspheres. Floating ability is directly related to gas content of the polymer matrix. Wet microspheres can contain a greater proportion of CO2 gas than dry ones and are thus more buoyant. Sodium alginate microspheres showed good gel strength, entrapping CO2 gas and imparting stable and persistent buoyancy.
In Vitro Release Study
The in vitro drug release study was conducted in 0.1N HCl for 12 hrs. About 92.38% of drug release was observed at 12 hr for 10% drug loaded formulation and 98.42% of drug release was observed at 12 hrs for 20% drug loaded formulation. Percentage cumulative drug released vs. time are shown . There is no burst effect observed for both 10% and 20% drug loaded microspheres and release pattern for both 10% and 20% loaded are almost similar. All the microspheres produced have 100% buoyancy for 12 hrs and are expected to release all the drugs in the stomach. We have not studied release of the drug in a 7.4 pH buffer solution. About 98% of the drug was released at 12 hrs. This indicates that there is no drug loss due to formulation.
Table 2. In vitro release studies of microspheres of itopride (10%) & (20%) loaded in sodium alginate
PHARMACOLOGICAL SCREENING
Sodium alginate microspheres containing 20% of itopride were screened for anti-ulcer, anti-GERD and GI motility in rats.
Anti-ulcer and Anti-GERD Activities
Anti-ulcer and anti-GERD activities were performed by pylorus ligation (fourth portion of duodenum). Two experiments at two different time intervals were carried out for these activities (i.e. 30 min and 6 hours). The ulcer index, % ulcer protection in both stomach and esophagus, was calculated using the formula [Citation20,Citation21]. For the above data statistical analysis ANOVA followed by Dunnett's t test was performed. The mean standard error (SE) and p-value are calculated. After ligation of fourth portion of the duodenum ulcers were formed both in the stomach and esophagus. The effect of different itopride formulation on the ulcer index of stomach and esophagus for pylorus ligation 30 min and six hours after drug administration is shown in . Percentage ulcer protection in stomach for pylorus ligation 30 min was found to be 10.42, 56.01 and 45.62 after administration of control, marketed preparation of itopride and sodium alginate microspheres, respectively. Similarly, the percentage ulcer protection in the esophagus was observed at 3.167, 42.12, and 28.95 respectively. The effect of different itopride formulation on the ulcer index of the stomach and esophagus for pylorus ligation six hours after drug administration is summarized in . The results indicated that the sodium alginate microspheres containing itopride showed more ulcer protection than the marketed preparation.
Table 3. Results of anti-ulcer activity of different itopride formulations in pylorus ligated rat model
Antacid Activity
The gastric juice was collected in the pylorus ligated model (two different time intervals) and its acid volume, pH, free acidity, and total acidity were estimated. Results of pH, free acidity, and total acidity of control, marketed preparation and sodium alginate microspheres of itopride treated group are summarized in . The results indicated that sodium alginate microspheres containing itopride showed decreased acid volume, free acidity, total acidity, and increased pH when compared with the marketed preparation.
Table 4. Results of antacid activity of different itopride formulations in pylorus ligated model (after 6 hrs. of drug administration) in rats
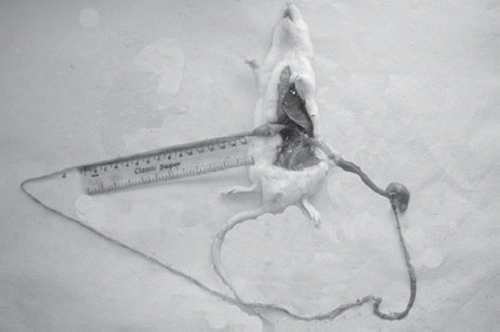
G.I. Motility (Intestinal Transit of Charcoal Meal in Rats)
The gastrointestinal motility was determined after administration of charcoal meal to the rats. Two experiments were carried out at two different time intervals, i.e. 30 min and six hours. The results of length traveled by charcoal after 30 min of administration, in centimeter and percentage transit for control, marketed preparation of itopride treated group and sodium alginate microspheres of itopride treated group are summarized in . The photograph of intestine traveled by charcoal is also shown in . It was observed that the marketed preparation of itopride showed an increase in GI motility in the 30 min model whereas in the 6 hr model sodium alginate microspheres showed an increase in GI motility.
Table 5. Effects of different itopride formulations on gastrointestinal motility in rat model
DISCUSSION
Treatment of GERD is done with many classes of drugs, including antacids, H2 receptor antagonists, proton pump inhibitors, anti-secretaries, and prokinetic drugs. Controlled drug delivery systems offer several advantages over conventional dosage forms such as reduction in drug plasma level fluctuations, reduction in adverse side effects, improvement in tolerability and patient compliances. In the present study, we have used floating drug delivery systems using sodium alginate hydrogel to increase the gastric retention time. Sodium alginate microspheres were prepared by precipitation followed by the cross-linking method using CaCl2 as a cross linking agent. Various gas-forming agents such as CaCO3 and NaHCO3 have been tried but smoother surface was observed with CaCO3. Itopride is soluble in water and its solubility is not much affected by the pH. Prepared microspheres were smooth surface with spherical in shape and the size range of 525 to 775 μm. It was observed that all the formulation parameters did not greatly affect the particle size, but buoyancy and drug content were varied. Two different drug loadings were tried, i.e. 10% and 20%. Content uniformity results indicated that as the theoretical loading of the drug increases the efficiency of loading also increases. This may be due to decrease in leaching of drug during formulation process.
It was observed that the microspheres containing the gas-forming agents in proportions ranging from 0.25 to 0.75%w/w demonstrated excellent floating ability (100% floating). The gas generated is trapped and protected within the gel, formed by hydration of polymer, thus decreasing the density of the microspheres. As the density of the microspheres falls below 1, the microspheres become buoyant. The wet microspheres had better floating ability than dry microspheres. Floating ability is directly related to gas content of the polymer matrix. Wet microspheres contain a greater proportion of CO2 gas than dry ones and are thus more buoyant. Sodium alginate microspheres with good gel strength entrapped CO2 gas and imparted stable and persistent buoyancy. In vitro release studies were conducted in 0.1N HCl for 12 hrs. All the microspheres produced 100% buoyancy for 12 hrs and were expected to release all the drugs in the stomach. We have not studied release of the drug in 7.4 pH buffer solution.
About 98% of the drug was released after 12 hrs. This indicates that there is no drug loss during formulation development. Sodium alginate microspheres containing 20% itopride were evaluated for anti-ulcer, anti-GERD, and GI motility in rats. GERD is effectively produced in rats by ligation of the fourth portion of the duodenum for four hours and six hours. It was observed that, four hours after the ligation animals were sacrificed, both the stomach and esophagus were opened and ulcers were found both in the stomach and esophagus. This indicates that at this time point the pressure generated due to accumulation of the stomach content is increased and pushed the content into the esophagus, thereby producing ulcers. The entry of stomach content into the esophagus is due to the relaxation of LES. Hence administration of itopride increases the contractions of LES, thereby preventing the entry of stomach contents into the esophagus and decreasing the ulceration in the esophagus; thus it shows anti-GERD properties. The comparison of anti-GERD activity of conventional dosage form available in the market and floating delivery system containing itopride was performed. Thirty minutes prior to the ligation both formulations showed significant anti-GERD activity, whereas six hours prior to the ligation floating delivery systems containing itopride produced significant anti-GERD activity. Similarly, results of GIT motility also support the anti-GERD properties of sodium alginate microspheres. Administration of conventional dosage form shows GIT motility about 74% in the 30 min model whereas it is only 43% for sodium alginate microspheres due to less availability of the drug at this time point (<10%).
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
REFERENCES
- Goyal, R.K. (1998). Diseases of the esophagus. Fauci, A.S., Braunwald, E., Isselbacher, K.J.. Harrison's Principles of Internal Medicine 14th, McGraw Hill, New York, 1592–93.
- Kim, Y.S., Kim, T.H., Choi, C.S., Shon, Y.W., Kim, S.W., Seo, G.S., . (2005). Effect of itopride, a new prokinetic, in patients with mild GERD: A pilot study. World J. Gastroenterol 11(27): 4210–14.
- Sethu, B. (2003). Drug therapy of Gastroesophageal reflux disease (GERD): Focus on itopride hydrochloride. The Indian Pract. 56(12): 827–30.
- Kakrani, A.L., Madraki, R. (2002). Clinical evaluation of itopride hydrochloride in patients with non-ulcer dyspepsia and chronic gastritis. The Indian Pract. 55(7): 441–44.
- Klausner, E.A., Lavy, E., Stepensky, D., Friedman, M., Hoffman, A. (2002). Novel gastroretentive dosage forms: Evaluation of gastroretentivity and its effect on riboflavin absorption in dogs. Pharm Res. 19(10): 1516–23.
- Hoffman, A., Stepensky, D. (1999). Pharmacodynamic aspects of modes of drug administration for optimization of drug therapy. Crit Rev Ther Drug Carrier Syst 16(6): 571–639.
- Dave, B.S., Amin, A.F., Patel, M.M. (2004). Gastroretentive drug delivery system of ranitidine hydrochloride: Formulation and in-vitro evaluation. AAPS Pharm Sci Tech. 5(2): 1–6.
- Struszczyk, H., Wawro, D., Niekraszewicz, A. (1991). Biodegradability of chitosan fibres. Brine, C.J., Sandford, P.A., Zikakis, J.P., Advances in Chitin and Chitosan, Elsevier Applied Science, London, 580–585.
- Chandy, T., Sharma, C. (1990). Chitosan–as a biomaterial. Biomat Art Cells Art Org. 18: 1–24.
- Hirano, S., Seino, H., Akiyama, Y., Nonaka Y. (1990). Chitosan: a biocompatible material for oral and intravenous administrations. Gebelein, C.G., Dunn, R.L., Progress in Biomedical Polymers, Plenum, New York, 283–290.
- Choi, B.Y., Park, H.J., Hwang, S.J., Park, J.B. (2002). Preparation of alginate beads for floating drug delivery system: Effects of CO2 gas-forming agents. Int. J. Pharm. 239: 81–91.
- Johnson, F.A., Craig, D.Q.M., Mercer, A.D., Chauhan, S. (1997). The effects of alginate molecular structure and formulation variables on the physical characteristics of alginate raft systems. Int J Pharm 159: 35–42.
- Garg, S., Sharma, S. (2003). Gastroretentive drug delivery systems. Pharmatech, 160–66.
- Kulkarni, A.R., Soppimath, K.S., Aminabhavi, T.M. (2001). Chemically modified polyacrylamide-g-guargum based crosslinked anionic microgels as pH sensitive drug delivery systems. J Controlled Release 75: 331–45.
- Singh, B.N., Kim, K.H. (2000). Floating drug delivery systems: an approach to oral controlled drug delivery via gastric retention. J Control Release 63: 235–59.
- Joffe, S.N., Roberts, M.B., Taylor, W.H. (1980). Exogenous and endogenous acid and pepsin in the pathogenesis of duodenal ulcers in rat. Dig Dis Sci, 825–37.
- Maity, S., Chaudhari, T. (2003). Cytoprotection mediated anti-ulcer effect of tea root extract. Indian J Pharmacol 35: 213–19.
- Akhtar, H.A., Ahmed, U.K. (1995). Anti-ulcerogenic evaluation of methanolic extract of some indigenous medicinal plants of Pakistan in aspirin-ulcerated rats. J Ethnopharmacol. 46: 1–6.
- Mittelstadt, S.W., Hemenway, C.L., Spruell, R.D. (2005). Effects of fasting on evaluation of gastrointestinal transit with charcoal meal. J Pharmacol Toxicol Methods 52: 154–58.
- Lee, H.T., Seo, E.K., Chung, S.J., Shim, C.K. (2005). Prokinetic activity of an aqueous extract from dried immature fruit of Poncirus trifoliate (L.) Raf. J Ethnopharmacol 102: 131–36.
- Kulkarni, S.K. (1999). Handbook of Experimental Pharmacology, 2nd, Vallabh Prakashan, Delhi, 148–50.