Abstract
Objective: To compare the chondrogenic ability of mesenchymal stem cells (MSCs) derived from different tissues in rabbits’ full-thickness articular cartilage defects. Methods: Sixty New Zealand white rabbits of ordinary grade with a body weight of 2.5∼3.5kg were selected for this study. Six were sacrificed for preparation of deminerized bone matrix (DBM) as scaffold. Fifty-four were used for cartilage defects model. Full-thickness cartilage defect of knee joint was created on trochlear groove at two sides of the femur with a diameter of 4 mm and thickness of 3 mm. All 54 rabbits were randomly divided into 6 groups and treated by autogeneic MSCs isolated from bone marrow, periosteum, synovium, adipose tissue and muscle, respectively. The 6th group was a control group with nothing plugged into the defects. Every three rabbits were killed at three time points, which were 4, 8, and 12 weeks after the operation in each group. The reparative tissue samples were evaluated grossly, histologically, immunohistochemically, and graded according to gross and histological scales 12 weeks postoperatively. We input the scores into SPSS 11.5 software and the analysis of variance (one-way-ANOVA) and student-newman-keuls (SNK-q) test were used to process statistical analysis and find out if the differences between each group had statistical significance. Results: Fifty-four rabbits are included in the final analysis. The defects are all repaired by hyaline-like tissue except the control group. The bone-marrow-MSCs produced much more cartilage matrix than that of other groups. Gross and histological grading scale indicates that the defects repaired by MSCs isolated from bone marrow are superior to that repaired by MSCs isolated from periosteum, synovium, adipose tissue, and muscle (p < 0.05). In adipose-MSCs and muscle-MSCs group, some defects are even repaired by fibrous tissue. Conclusion: Bone-marrow-MSCs have greater in vivo chondrogenic potential than periosteum-, synovium-, adipose- and muscle-MSCs.
INTRODUCTION
Mesenchymal stem cells (MSCs) are an attractive cell source for regenerative medicine because they can be harvested in a minimally invasive manner, and they are easily isolated and expanded, with multipotentiality including chondrogenesis [Citation1,Citation2,Citation3] Text prints in black and white in final version They have been widely used in the field of tissue engineering in recent years. We previously studied the ability of repairing full-thickness defects with mesenchymal stem cells (MSCs) derived from bone marrow and demonstrated that bone marrow MSCs had the satisfactory chondrogenic ability in vivo in rabbits [Citation4]. The result shows that the bone marrow MSCs are suitable for therapy of cartilage defects and should advance the clinical application of MSC-based cell therapy for cartilage regeneration. Some researchers also successfully repaired the cartilage defects and constructed the tendon as well as bone by MSCs derived from other mesenchymal tissues such as periosteum [Citation5,Citation6], synovium [Citation7], adipose [Citation8,Citation9], skeletal muscle [Citation10], umbilical cord blood [Citation11], peripheral blood [Citation12], and so on. There is no doubt that multipotent cell populations of mesenchymal derivation reside in many tissues. The differentiation potentials and functional implications vary widely among MSCs derived from different tissues [Citation13]. It is necessary to investigate the differentiation ability of mesenchymal stem cells (MSCs) derived from different tissues. In this study, we compared the in vivo the chondrogenic potential of rabbit MSCs isolated from bone marrow, periosteum, synovium, adipose tissue, and muscle, respectively.
MATERIALS AND METHODS
Animals
Sixty New Zealand white rabbits, ordinary grade, with a body weight of 2.5∼3.5 kg, were standard fed in separate cages. Six of them were used for preparing demineralized bone matrix (DBM) as scaffold. 54 of them were used for the animal model. The harvesting of tissues and all operations were performed under anesthesia induced by intramuscular injection of 25 mg/kg ketamine hydrochloride and intravenous injection of 45 mg/kg sodium pentobarbital [Citation14]. The experimental animals were sacrificed according to Regulations for the Administration of Affairs Concerning Experimental Animals published by the Ministry of Science and Technology in 1988 [Citation15]. The animal experimental protocols were approved by the scientific research institute of Guilin Medical University.
Collection of Tissue and Isolation of Cells
Bone marrow was abstracted from inferior extremity metaphysis of femur with an 18-gauge needle. Periosteum-derived mesenchymal stem cells isolated from periosteum of tibia. Synovium with subsynovial tissue was harvested from the knee joint. Adipose tissue was harvested from the perinephric fat tissue intraperitoneally. Muscle was harvested from the anterior tibial muscle. Digested cells were filtered through a 70-μm nylon filter (Becton Dickinson, Franklin Lakes, NJ, USA), and the remaining tissues were discarded. Percoll density gradient centrifuge was used in vitro. Isochoric Perciol separating medium was mixed into the marrow and tissue suspension; then the mixture was centrifugated by 3000r/min for 20 minutes, drawing flocc layer and adding D-Hank's solution to 10ml, centrifuging by 1500r/min for another 10 minutes, abandoning clear supernatant, adding DMEM medium 10ml (containing 10% fetal bovine serum, 100 U/ml penicillin, 100 μg/ml streptomycin, and 250 ng/ml amphotericin B) and incubating at 37°C with 5% humidified CO2. After 3–4 days, the medium was changed to remove non-adherent cells and then cultured for 14 days. When these cells overgrew into a monolayer, they were trypsinized for 3 minutes, boasted into suspension, and serial subcultivated.
Table 1. Main reagent and equipment
Preparation of DBM
Six New Zealand rabbits were executed for preparing cancellous bone at metaphysis of extremities and vertebras, frozen for 72 hours in an ice cabinet with a temperature of −80°C, rejecting the muscles and periosteums, decalcifying for 72 hours, defatting for 24 hours in ether and 95% alcohol, rinsing and soaking by aqua destillata sterilis until the soak becomes neutral. Until now, the cancellated bone looked like a white sponge. It could be deformed and self-recovered to its original shape. The sponge-shaped bone disacidified in distilled water for 24 hours, naturally arescented and was sheared to a cylinder of 4 mm diameter and 3 mm thickness, then sterilized by epoxyethane and reserved under the condition of 4°C.
Preparation of MSCs/DBM Composite
The second filial generation of MSCs was diluted to 3.5 × 105 by culture fluid and seeded onto allogenic demineralized bone matrix (DBM). TGF-beta1 was incorporated into a scaffold. The MSC/DBM complex was cultured in an incubation box for 72 hours under the condition of 5% CO2 and 37.3°C, letting the cells absorb to DBM completely, and then they were transplanted into full-thickness defects on intercondylar fossa.
Preparation of Animal Model
The remaining 54 were divided into 6 groups. All of the rabbits’ two knees (n=108) were used for models. Under the condition of anesthesia and asepsis, an internal longitudinal longitude incision was cut layer by layer until the articular cavity was exposed. Then we laterally dislocated the patella for the exposure weight-bearing surface of the femoral trochlea. A full-thickness articular cartilage defect with a size of 4 mm in diameter and 3 mm in depth was created by hand drilling with a bit, which completely destroyed the inferior bone lamella of cartilage. After rinsing the tissue debris and blood clot with saline solution in the defect, the composite of MSCs/DBM was used to completely fill the defect. All the animals were immobilized for two weeks postoperatively. Gross and histological observation were performed on the cartilage defects at 4, 8, and 12 weeks postoperatively. Immunohistochemistry staining of type II collagen observation of MSC/DBM management was made 12 weeks postoperatively. Gross and histological observation was graded according to O'Driscoll's standard [Citation16].
Gross and Histological Scoring for Articular Cartilage Defects at 12 Weeks
The gross and histological scoring was made by three authors independently. The average score was used as the ultimate score for each sample. O'Driscoll's standard was used as shown in .
Table 2. Gross grading scale fsor articular cartilage defects
Statistical Analysis
Experimental data were demonstrated as ![]() . We input the scores into SPSS statistical package version 11.5 (SPSS Inc., Chicago, IL, USA). Variance (one-way-ANOVA) and student-newman-keuls (SNK-q) test were used in this analysis.
. We input the scores into SPSS statistical package version 11.5 (SPSS Inc., Chicago, IL, USA). Variance (one-way-ANOVA) and student-newman-keuls (SNK-q) test were used in this analysis.
RESULTS
Quantitative analysis of the experimental animal: 54 rabbits were included in the final analysis. All animals had a good motion and the synovial fluid in knee joint was clear. There was no conglutination in the articular cavity, no hyperplasia of synovium, no corpus liberum, no osteophyma, and it was worn out around the defects.
Gross Observation
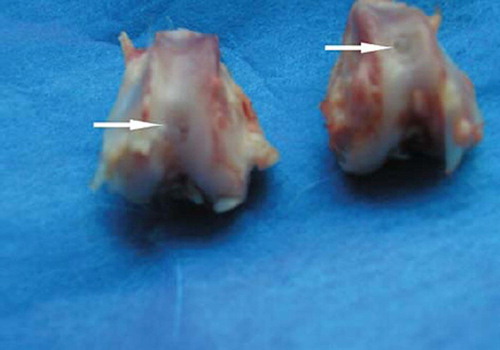
The defects of bone-marrow-derived -MSCs group were filled with translucent salmon pink tissue 4 weeks postoperatively. There were blurs in the boundary and it was slick on the surface of the defects, which were similar to normal cartilage tissue 8 weeks postoperatively; the repairing tissues were closer to the surrounding cartilage in color and luster 12 weeks postoperatively (). Periosteum-, synovium-, adipose tissue-, and muscle-derived-MSCs group were also filled with a hyaline-like cartilage, but it was inferior to that of the bone marrow-MSCs group. The control group was filled with oyster white tissue and fibrous tissue, some of which looked eclipsed on the surface ().
Table 3. Gross scores for articular cartilage defects at 12 weeks ![]() (O'Driscoll's scores, n = 6)
(O'Driscoll's scores, n = 6)
Histological Observation
Full-thickness cartilage defects treated with bone-marrow-derived-MSCs are repaired with hyaline-like cartilage tissue and are significantly better than those treated with periosteum-, synovium-, adipose tissue-, muscle-MSCs group as well as control group. Repaired tissues treated with bone marrow-MSCs appeared to have better cell arrangement, subchondral bone remodeling, and integration with surrounding cartilage. There were many more cells and cartilage lacuna in the bone marrow-MSCs group. They were fusiform-shaped in the surface layer, spherical or ellipse in deep lamella. Most of them are spherical hyaline cartilage and integrated with surrounding cartilage and had a normal thickness (). The histological score of the bone-marrow-derived-MSCs group is significantly better than that of periosteum-, synovium-, adipose tissue-, and muscle-MSCs group (). There are fewer cells and cartilage lacuna in periosteum-, synovium-, adipose tissue-, and the muscle-MSCs group and control group. Calcification cartilaginea can be found in some areas. Fibrous tissues in the defect area are much thicker and not well integrated with surrounding cartilage.
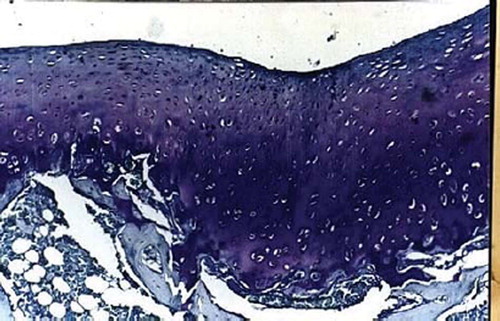
Figures 2. Sagittal sections of cartilage defects transplanted with MSCs derived from bone marrow (1) at 12 weeks (toluidine blue staining × 400).
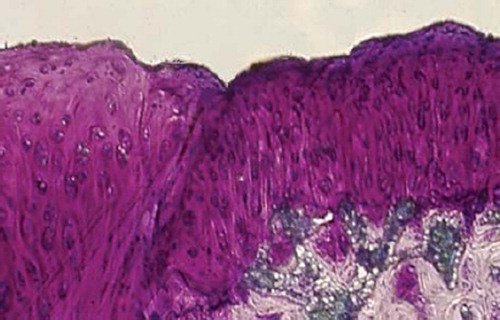
Figures 3. Sagittal sections of cartilage defects transplanted with MSCs derived from periostenum (2) at 12 weeks (toluidine blue staining × 400).
Figures 4. Sagittal sections of cartilage defects transplanted with MSCs derived from syanovium (3) at 12 weeks (toluidine blue staining × 400).
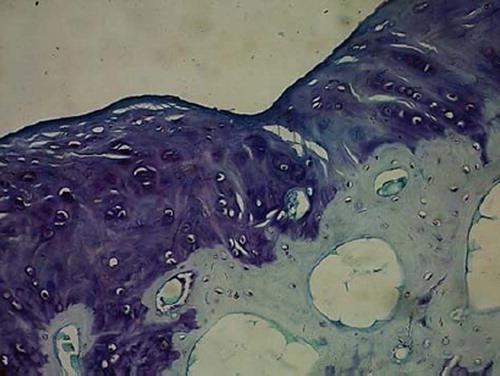
Figures 5. Sagittal sections of cartilage defects transplanted with MSCs derived from adipose (4) at 12 weeks (toluidine blue staining × 400).
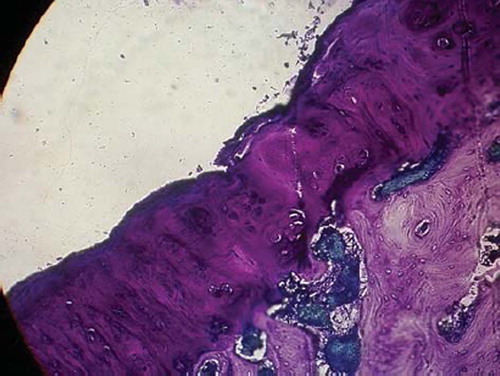
Figures 6. Sagittal sections of cartilage defects transplanted with MSCs derived from muscle (5) at 12 weeks (toluidine blue staining × 400).
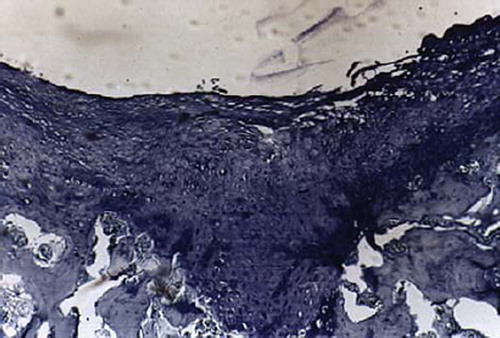
Figures 7. Sagittal sections of cartilage defects transplanted with MSCs derived from control group (6) at 12 weeks (toluidine blue staining × 400).
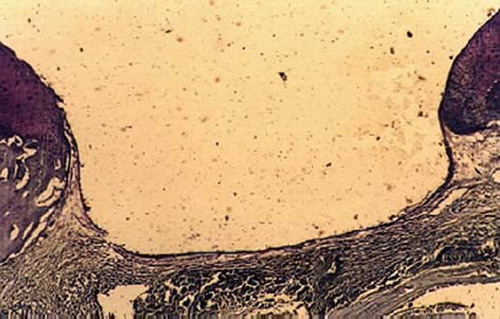
Table 4. Histological scores for articular cartilage defects at 12 weeks (O'Driscoll's scores, ![]() , n = 6)
, n = 6)
Immunohistochemistry Dyeing
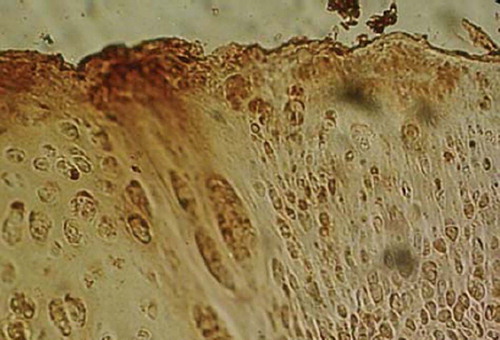
Collagen II in cytoplasm and extracellular matrix is strongly positive expressed in repaired tissue of bone-marrow-derived MSCs group (), weakly positive expressed in Periosteum-, synovium-, adipose tissue-, and muscle-MSCs group, and negatively expressed in control group.
DISCUSSION
Cartilage, as tissue with the exclusively mechanical tasks of reducing relative load transfer by enlarging contact area and providing damping and gliding, has a high sensitivity to mechanical injuries. These injuries and their consequences are relevant, especially in the weight-bearing joints of the lower limbs, such as the knee or ankle joints. Cartilage defects > 1 cm2 are symptomatic with pain, joint effusion, limited range of motion, or even blockage of movement. The hyaline cartilage actually only has a very small potential of self-repair. The aim of all therapeutic procedures to treat patients with damaged articular cartilage is to reconstruct the integrity of the articular cartilage surface in order to enable them to live an unrestricted painless professional and private life. There are different approaches to surgically treat cartilage damage: small defects (< 1 cm2) may be treated with a certain success through drilling, abrasion arthroplasty or microfracturing techniques [Citation17,Citation18], which have the same concept of achieving subchondral stimulation of healing capacity, but from there only fibrocartilage scars can be expected. In the case of larger lesions with profound influence on joint mechanics, transplantation technique [Citation19], e.g., osteochondral transplantations have been established.
All these methods mentioned earlier are not very satisfactory for repairing cartilage. It is the tissue engineering that brought hope for us to regenerate the cartilage. These strategies usually fall into three categories [Citation20]: (1) cell-based therapy; (2) the use of biomaterials (scaffolds) alone; or (3) the use of scaffolds seeded with cells. The term “mesenchymal stem cells” (MSCs) is commonly applied to plastic-adherent cell preparations isolated from bone marrow or other tissues that are positive for several antigens such as CD105, CD73, and CD90, which lack expression of hematopoetic antigens and which are able to differentiate at least into osteoblasts, adipocytes, and chondroblasts under specific in vitro differentiating conditions [Citation21,Citation22]. The shortage of organ donors for regenerative medicine has stimulated research on stem cells as a potential resource for cell-based therapy. Stem cells have been used widely for regenerative medicine applications. The development of innovative methods to generate stem cells from different sources suggests that there may be new alternatives for cell-based therapies.
MSCs can be derived from several tissues, such as bone marrow [Citation23], adipose [Citation24], periosteum and synovial [Citation25], et al. MSCs used for tissue engineering are obtained from a small biopsy of tissue, which is dissociated in culture. The resulting cell population is expanded, seeded onto a matrix, and implanted back into the host. The source of donor tissue can be allogenic (donor derived) or autologous (the host's cells), but autologous cells are preferred because they are not rejected by the immune system, and the use of immunosuppressant drugs is avoided. For this reason, we selected the autologous stem cells as seed cells to repair the full thickness cartilage defects in this study. To determine a suitable cell source, Hideya Yoshimura [Citation26] isolated rat MSCs from bone marrow, synovium, periosteum, adipose, and muscle and compared their properties for yield, expansion, and multipotentiality. The results show that colony number per nucleated cells derived from synovium was 100-fold higher than that for cells derived from bone marrow. With regard to expansion potential, synovium-derived cells were the highest in colony-forming efficiency, fold increase and growth kinetics, an in vitro chondrogenesis assay demonstrated that the pellets derived from synovium were heavier, because of their greater production of cartilage matrix, than those from other tissues, indicating their superiority in chondrogenesis. Synovium-derived cells retained their chondrogenic potential after a few passages. The Oil Red-O positive colony rate assay demonstrated higher adipogenic potential in synovium- and adipose-derived cells. Alkaline phosphatase activity was greater in periosteum- and muscle-derived cells during calcification. The yield and proliferation potential of rat MSCs from solid tissues was much better than those from bone marrow. In particular, synovium-derived cells had the greatest potential for both proliferation and chondrogenesis, indicating their usefulness for cartilage study in a rat model. Some similar research [Citation27] shows that when rabbit MSCs are compared in vitro, synovium and bone-marrow-derived cells show much more chondrogenic potential than adipose- and muscle-derived cells. The in vivo study has also demonstrated that synovium-derived cells have much more chondrogenic potential than adipose- and muscle-derived cells [Citation28]. With regard to proliferation potential, our study shows that bone-marrow-derived derived cells have much higher proliferation potential than synovium-derived cells, which indicates that bone-marrow-derived cells are a superior cell source for cartilage regeneration. Our study indicates that it is not the cells from synovium but the synovial fluid and cavity environment which lead to successful chondrogenic repair. A study [Citation29] investigated the possible effects of the normal joint cavity environment on chondrocytic differentiation of bone-marrow-derived mesenchymal stem cells (MSCs). To investigate the possible factors involved in chondrocytic differentiation of MSCs further, the author co-cultured sheep MSCs with the main components of the normal joint cavity, viz. synovial fluid or synovial cells, in vitro. After 1 or 2 weeks of co-culture, the MSCs in both co-culture systems expressed markers of chondrogenesis. These results suggest that synovial fluid and synovium from normal joint cavity are important for the chondrocytic differentiation of adult bone-marrow-derived MSCs.
In the early study on stem cells or chondrocyte implantation, the cells dedifferentiate and lose their ability to produce collagen type II, the major collagen component of normal hyaline cartilage. In most studies, cells seed onto/into scaffolds are a common mode for tissue regeneration. Before implantation the cells are transferred to the scaffold, which provides the cells with a three-dimensional framework for growth. In vitro studies have shown that growth in such an environment allows the cells to redifferentiate and resume synthesis of collagen type II [Citation30]. The results of M. Rudert's [Citation31] study support the importance of cell-based therapy approaches. There was a significant improvement of the histological results in osteochondral defect repair when cells were added to the implanted materials. But research conducted by S. Løken [Citation32] did not find any difference in the degree of filling when using scaffolds with MSC compared to scaffolds without cells as judged by morphometry. Cancellous DBM can be regarded as the natural cell scaffolds for cartilage tissue engineering [Citation33]. In this study, we used the diminerazed bone matrix (DBM) as scaffold. The results demonstrated it is the suitable condition of MSCs therapy for cartilage defects and should advance the clinical application. It allows for definitive control over the processes of cell isolation, proliferation, differentiation, and matrix production for a normally structured autologous transplant.
In this paper, we have only performed a comparative evaluation, not an absolute conclusion, and do not conclude that the quality of the best regenerated cartilage that we repaired by bone marrow MSCs is comparable with neighboring cartilage. Indeed, cartilage matrix production is just one of the parameters that could be used to evaluate cartilage quality, and mechanical testing will be required to assess and strengthen our results. However, histology often reflects biomechanical property [Citation34]. Therefore we consider that this paper provides valuable findings even without additional mechanical testing.
CONCLUSION
MSCs derived from bone marrow have the best chondrogenic ability. The finding should advance the clinical application of MSC-based cell therapy for cartilage regeneration.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
REFERENCES
- Koga, H., Muneta, T., Nagase, T., Nimura, A., Mochizuki, Y.J.J.T., Sekiya, I. (2008). Comparison of mesenchymal tissues-derived stem cells for in vivo chondrogenesis: suitable conditions for cell therapy of cartilage defects in rabbit. Cell Tissue Res 333: 207–215.
- Kuroda, R., Ishida, K., Matsumoto, T., Akisue, T., Fujioka, H., Mizuno, K., Ohgushi, H., Wakitani, S., Kurosaka, M. (2007) Treatment of a full-thickness articular cartilage defect in the femoral condyle of an athlete with autologous bone-marrow stromal cells. Osteoarthritis Cartilage 15(2): 226–31.
- Csaki, C., Matis, U., Mobasheri, A., Ye, H., Shakibaei, M. (2007). Chondrogenesis, osteogenesis and adipogenesis of canine mesenchymal stem cells: a biochemical, morphological and ultrastructural study. Histochem Cell Biol. 128(6): 507–20.
- Li, Q., Tang, J., Sun, Z., Wang, S., Liu, W. (2008). Repairing full-thickness articular cartilage defects with homograft of mesenchymal stem cells seeded onto cancellous demineralized bone matrix. Journal of Clinical Rehabilitative Tissue Engineering Research 12 (45): 8943–47.
- O'Driscoll, S.W., Keeley, F.W., Salter, R.B. (1988). Durability of regenerated articular cartilage produced by free autogenous periosteal grafts in major full-thickness defects in joint surfaces under the influence of continuous passive motion: A follow-up report at one year. J Bone Joint Surg [Am] 70: 595–606.
- Choi, Y.S., Noh, S.E., Lim, S.M., Lee, C.W., Kim, C.S., Im, M.W., Lee, M.H., Kim, D.I. (2008). Multipotency and growth characteristic of periosteum-derived progenitor cells for chondrogenic, osteogenic, and adipogenic differentiation. Biotechnol Lett. 30(4): 593–601.
- Ju, Y.J., Muneta, T., Yoshimura, H., Koga, H., Sekiya, I. (2008). Synovial mesenchymal stem cells accelerate early remodeling of tendon-bone healing. Cell Tissue Res 332: 469–478.
- Sanz-Ruiz, R., Fernández Santos, M.E., Domínguez Muñoa, M., Martín, I.L., Parma, R., Sánchez Fernández, P.L., Fernández-Avilés, F. (2008). Adipose tissue-derived stem cells: The friendly side of a classic cardiovascular foe. J. of Cardiovasc. Trans. Res. 1: 55–63.
- Zuk, P.A., Zhu, M., Mizuno, H., Huang, J., Futrell, J.W., Katz, A.J., Benhaim, P., Lorenz, H.P., Hedrick, M.H. (2001). Multilineage cells from human adipose tissue: Implications for cell-based therapies. Tissue Eng. 7(2): 211–28.
- Jiang, Y., Vaessen, B., Lenvik, T., Blackstad, M., Reyes, M., Verfaillie, C.M. (2002). Multipotent progenitor cells can be isolated from postnatal murine bone marrow, muscle, and brain. Exp Hematol 30(8): 896–904.
- Bieback, K., Kern, S., Klüter, H., Eichler, H. (2004). Critical parameters for the isolation of mesenchymal stem cells from umbilical cord blood. Stem Cells 22(4): 625–634.
- Kuznetsov, S.A., Mankani, M.H., Gronthos, S., Satomura, K., Bianco, P., Robey, P.G. (2001). Circulating skeletal stem cells. J Cell Biol. 153(5): 1133–40.
- Kern, S., Eichler, H., Stoeve, J., Klüter, H., Bieback, K. (2006). Comparative analysis of mesenchymal stem cells from bone marrow, umbilical cord blood, or adipose tissue. Stem Cells 24(5): 1294–301. Epub 2006 Jan 12.
- Koga, H., Muneta, T., Nagase, T., Nimura, A., Ju, Y.J., Mochizuki, T., Sekiya, I. (2008). Comparison of mesenchymal tissues-derived stem cells for in vivo chondrogenesis: suitable conditions for cell therapy of cartilage defects in rabbit. Cell Tissue Res 333: 207–215.
- The Ministry of Science and Technology of the People's Republic of China. (1988). Regulations for the Administration of Affairs Concerning Experimental Animals, 1988-10-31, Beijing, China.
- O'Driscoll, S.W., Recklies, A.D., Poole, A.R. (1994). Chondrogenesis in periosteal explants: An organ culture model for in vitro study. J Bone Joint Surg Am 76(7): 1042–51.
- Kang, S.W., Bada, L.P., Kang, C.S., Lee, J.S., Kim, C.H., Park, J.H., Kim, B.S. (2008). Articular cartilage regeneration with microfracture and hyaluronic acid. Biotechnol Lett 30: 435–439.
- Ferkel, R.D., Zanotti, R.M., Komenda, G.A., Sgaglione, N.A., Cheng, M.S., Applegate, G.R., Dopirak, R.M. (2008). Arthroscopic treatment of chronic osteochondral lesions of the talus: long-term results. Am J Sports Med. 36(9): 1750–62.
- Cook, J.L., Hudson, C.C., Kuroki, K. (2008). Autogenous osteochondral grafting for treatment of stifle osteochondrosis in dogs. Vet Surg. 37(4): 311–21.
- Hipp, J., Atala, A. (2008). Sources of stem cells for regenerative medicine. Stem Cell Rev 4: 3–11.
- Dominici, M., Le Blanc, K., Mueller, I., Slaper-Cortenbach, I., Marini, F., Krause, D., Deans, R., Keating, A., Prockop, D.J., Horwitz, E. (2006). Minimal criteria for defining multipotent mesenchymal stromal cells: The International Society for Cellular Therapy position statement. Cytotherapy 8: 315–317.
- Wagner, W., Wein, F., Seckinger, A., Frankhauser, M., Wirkner, U., Krause, U., Blake, J., Schwager, C., Eckstein, V., Ansorge, W., Ho, A.D. (2005). Comparative characteristics of mesenchymal stem cells from human bone marrow, adipose tissue, and umbilical cord blood. Experimental Hematology 33: 1402–1416.
- Coleman, R.M., Case, N.D., Guldberg, R.E. (2007). Hydrogel effects on bone marrow stromal cell response to chondrogenic growth factors. Biomaterials 28(12): 2077–2086.
- Betre, H., Ong, S.R., Guilak, F., Chilkoti, A., Fermor, B., Setton, L.A. (2006). Chondrocytic differentiation of human adipose-derived adult stem cells in elastin-like polypeptide. Biomaterials 27(1): 91–99.
- Nawata, M., Wakitani, S., Nakaya, H., Tanigami, A., Seki, T., Nakamura, Y., Saito, N., Sano, K., Hidaka, E., Takaoka, K. (2005). Use of bone morphogenetic protein 2 and diffusion chambers to engineer cartilage tissue for the repair of defects in articular cartilage. Arthritis Rheum. 52(1): 155–163.
- Yoshimura, H., Muneta, T., Nimura, A., Yokoyama, A., Koga, H., Sekiya, S. (2007). Comparison of rat mesenchymal stem cells derivedfrom bone marrow, synovium, periosteum, adipose tissue, and muscle. Cell Tissue Res 327: 449–462.
- Sakaguchi, Y., Sekiya, I., Yagishita, K., Muneta, T. (2005). Comparison of human stem cells derived from various mesenchymal tissues: superiority of synovium as a cell source. Arthritis Rheum. 52: 2521–2529.
- Ju, Y.J., Muneta, T., Yoshimura, H., Koga, H., Sekiya, I. (2008). Synovial mesenchymal stem cells accelerate early remodeling of tendon-bone healing. Cell Tissue Res. 332: 469–478.
- Chen, J., Wang, C., Lü, S., Wu, J., Guo, X., Duan, C., Dong, L., Song, Y., Zhang, J., Jing, D., Wu, L., Ding, J., Li, D. (2005). In vivo chondrogenesis of adult bone-marrow-derived autologous mesenchymal stem cells. Cell Tissue Res. 319(3): 429–38.
- Grigolo, B., Lisignoli, G., Piacentini, A., Fiorini, M., Gobbi, P., Mazzotti, G., Duca, M., Pavesio, A., Facchini, A. (2002). Evidence for redifferentiation of human chondrocytes grown on a hyaluronanbased biomaterial (HYAFF-11): molecular, immunohistochemical and ultrastructural analysis. Biomaterials 23: 1187–1195.
- Rudert, M., Wilms, U., Hoberg, M., Wirth, C.J. (2005). Cell-based treatment of osteochondral defects in the rabbit knee with natural and synthetic matrices: cellular seeding determines the outcome. Arch Orthop Trauma Surg. 125: 598–608.
- Løken, S., Jakobsen, R.B., Arøen, A., Heir, S., Shahdadfar, A., Brinchmann, J.E., Engebretsen, L., Reinholt, F.P. (2008). Bone marrow mesenchymal stem cells in a hyaluronan scaffold for treatment of an osteochondral defect in a rabbit model. Knee Surg Sports Traumatol Arthrosc. 16: 896–903.
- Song, H.X., Li, F.B., Shen, H.L., Liao, W.M., Liu, M., Wang, M., Cao, J.L. (2006). Repairing articular cartilage defects with tissue-engineering cartilage in rabbits. Chin J Traumatol. 9(5): 266–71.
- Kuroki, H., Nakagawa, Y., Mori, K., Kobayashi, M., Okamoto, Y., Yasura, K., Nishitani, K., Nakamura, T. (2007). Sequential changes in implanted cartilage after autologous osteochondral transplantation: postoperative acoustic properties up to 1 year in an in vivo rabbit model. Arthroscopy 23: 647–654.