Abstract
Abstract: Objective: To investigate the biocompatibility of diamond-like carbon (DLC) coated nickel-titanium shape memory alloy (NiTi SMA) in vitro and in vivo. Methods: The in vitro study was carried out by co-culturing the DLC coated and uncoated NiTi SMA with bone marrow mesenchymal stem cells (MSCs), respectively, and the in vivo study was carried out by fixing the rabbits’ femoral fracture model by DLC coated and uncoated NiTi SMA embracing fixator for 4 weeks, respectively. The concentration of the cells, alkaline phosphatase (AKP), and nickel ion in culture media were detected, respectively, at the first to fifth day after co-culturing. The inorganic substance, osteocalcin, alkaline phosphatase (ALP), and tumor necrosis factor (TNF) in callus surrounding fracture and the Ni+ in muscles surrounding fracture site, liver and brain were detected 4 weeks postoperatively. Results: The in vitro study showed that the proliferation of MSCs and the expression of AKP in the DLC-coated group were higher than the uncoated group (P < 0.05), while the uncoated group released more Ni2+ into the culture media than that in the coated group (P < 0.05). The in vivo study revealed that the inorganic substance and AKP, osteocalcin, and TNF expression were significantly higher in the DLC coated NiTi SMA embracing fixator than that in the uncoated group (P < 0.05). Ni2+ in liver, brain, and muscles surrounding the fracture were significantly lower in the DLC coated groups than that in the uncoated group (P < 0.05). Conclusion: Nickel-titanium shape memory alloy coated by diamond-like carbon appears to have better biocompatibility in vitro and in vivo compared to the uncoated one.
INTRODUCTION
Because of hyper-elastic and memory effect, Nickel-Titanium (NiTi) shape memory alloy (SMA) has become a commonly used biological material in medicine, especially in orthopedics. In spite of some good successes and excellent research of NiTi-SMA in orthopedics, there are still serious limitations to the clinical applications of NiTi alloy today. Meanwhile, damage of these materials on the body is unavoidable. Due to the presence of high amounts of Ni2+, the cytotoxicity of such alloys is under scrutiny [Citation1]. The potential leakage of elements and ions could be toxic to cells, tissues, and organs. Therefore, it is important to appraise these materials. Surface modification can improve the surface condition of alloy, enhancing its ability of anti-causticity [Citation2]. In this study, cytotoxicity of surface modified and untreated NiTi-SMA was observed in vitro and its histocompatibility was evaluated in rabbits fracture model in vivo to provide the theoretical basis for further application of these materials.
Materials and Methods
Materials
In an in vitro study, thirty pieces of DLC coated NiTi SMA (6 mm×7 mm×0.5 mm) and uncoated NiTi SMA of equal size and equal amount were prepared by Material Collage of Lanzou University. In the in vivo study, diamond-like carbon coated and uncoated nickel-titanium alloy embracing fixator (type 4H8-40) were provided by Lanzhou Ximai Co., Ltd., China. Intramedullary nails (type ZQY-01) used in this study were purchased from Tianjin Jinxingda Industries Co., Ltd., China.
Animals
Thirty Chinchilla rabbits of ordinary grade, aged 4–6 months and weighed 2.5–3.5 kg, were provided by Lanzhou Institute of Biological Products, China (License No. 12-004). The experimental animals were sacrificed according to Regulations for the Administration of Affairs Concerning Experimental Animals published by the Ministry of Science and Technology in 1988 [Citation3]. The animal experimental protocols were approved by the scientific research institute of Lanzhou University.
Main Reagents and Instruments
ICP optical emission spectrometer (type IRIS ER/S, TJA Company, USA). Image analyzer (type JX-2000A, Chengdu Measuring & Testing Equipment Co., Ltd. China). Graphite furnace atomic absorption spectrometry (BD9AA7001, Beijing East-West Electronic Company, China). Image analysator (type JX-2000A, Chengdou, China), Alkaline phosphatase (ALP), osteocalcin, tumor necrosis factor (TNF) first antibodies and SABC immunohistochemical kit (Wuhan Boster Bioengineering Co. Ltd., China).
In Vitro Study
Culture of bone marrow mesenchymal stem cells (MSCs). MSCs were derived from Chinchilla rabbits aged 4∼6 months and weighted 2.5∼3.5 kg in vitro. In the state of asepsis, 3∼4 ml bone marrow was abstracted from the inferior extremity of the femoral by a syringe contacted with myeloid puncture needle. Isochoric Perciol separating medium was mixed into the marrow and centrifugated by 3000 r/min for 20 minutes, drawing flocc layer and adding D-Hank's solution to 10 ml, centrifugated by 1500 r/min for another 10 min, abandoning clear supernatant, adding in DMEM medium 10 ml containing bovine serum 2.5 ml. Then these cells were incubated under condition of 5%CO2 and 37°C for 4 days, abandoning the cells which were not yet adhered, and continuing cultivation. When these cells overgrew into the monolayer, they were digested by 0.25% trypsin and passaged. The media were exchanged every 3 days. The third passage of cells was used for this study. Immunohistochemically staining was performed using a CD44 immunohistochemical kit. The result show that MSCs are actively expressed.
Mixed culture of MSCs with coated and uncoated NiTi SMA. The concentration of the 3rd passage MSCs was adjusted to 10×108 L−1. The suspension was added into 12-well culture plates. Two kinds of NiTi alloys (30 pieces, respectively) were immersed into each well of the culture plate, while the control group (no alloy added) was established simultaneously. All the alloy-suspension mixture was co-cultured in 5% CO2 at 37°C. During the observation, the culture media were not changed.
Growth of cells, expression of AKP, and concentration of nickel ion (Ni+). On the days of 1∼5 after culturing, the cells were counted by blood cell counting plate and growth curves were drawn. Using disodium phenyl phosphate as substrate, the expression of alkaline phosphatase (AKP) was detected with 6 out of 30 samples on each day by collecting the culture solution. The absorption value (A) at 520 nm wavelength was converted into King Armstrong's unit (U/L). The concentration of Ni+ in the corresponding culture medium was determined by graphite furnace atom absorption spectrophotosmetry.
In Vivo Study
Thirty rabbits were randomly divided into 3 groups (n =10), which were diamond-like carbon coated nickel-titanium alloy embracing fixator group, uncoated nickel-titanium alloy embracing fixator group, and intramedullary needle fixator group. Following anesthesia by injection of 1% sodium pentobarbital (25 mg/kg), transverse fracture models in the middle part of the femur were made through a lateral femoral incision and fixed with diamond-like carbon coated nickel-titanium alloy embracing fixator, uncoated nickel-titanium alloy embracing fixator, and intramedullary needle fixator, respectively.
Quantitative analysis of inorganic substance and semiquantitative analysis of ALP, osteocalcin, and TNF in callus surrounding the fracture. Four weeks postoperatively, the calcination method was used to analyze the inorganic substance around the fracture. The difference of weight before and after calcination was considered as the weight of inorganic substance. Another part of callus around the fracture was collected and fixed with 40 g/L paraformaldehyde, and then embedded with paraffin and made the microtome section. These sections were inmmunohistochemically stained for semiquantitative analysis of ALP, osteocalcin, and TNF by image analysator. Specifically, each slice takes a 10 field of vision under 20 time of objective lens; after gradation adjustment, the average absorbance of positive cells was determined.
Determination of Ni+ in the liver, brain, and the muscles surrounding fracture. Four weeks postoperatively, the same amount of liver tissue, brain tissue, and the muscle surrounding fracture was collected and the same volume of distilled water was added in the tube. Then the tissues were grinded into homogenate for detection of nickel ion in each of the sample units by graphite furnace atomic absorption spectrometry.
Statistical Analysis
Experimental data were expressed by ![]() ± s. We input the data into SPSS statistical package version 13.0 (SPSS Inc., Chicago, IL, USA). Analysis of variance (one-way-ANOVA) and student-newman-keuls (SNK-q) test were used in this study.
± s. We input the data into SPSS statistical package version 13.0 (SPSS Inc., Chicago, IL, USA). Analysis of variance (one-way-ANOVA) and student-newman-keuls (SNK-q) test were used in this study.
RESULTS
In Vitro Study
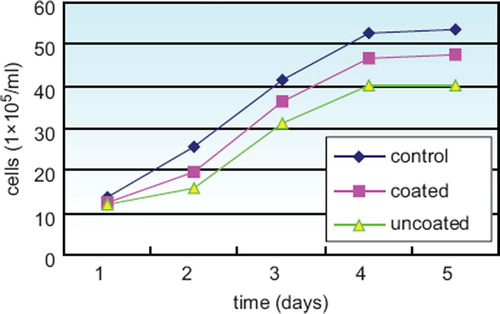
Growth of cells. The concentration of the cells was tested from the first to fifth day after culture. The results show that the coated group has a higher concentration on the third, fourth, and fifth day compared to the uncoated group (P < 0.05) (). The growth curves are as below ().
Table 1. The cell density at each time point (1 × 105/ml, ![]() ± s, n = 6)
± s, n = 6)
Expression of AKP in culture medium. In this study, the expression of AKP in the coated group on the third, fourth, and fifth day is significantly higher than that in the uncoated group (P < 0.05) ().
Table 2. The concentration of alkaline phosphatase (AKP) in cells culture media at each time point (King Armstrong's Unit/L, ![]() ± s, n = 6).
± s, n = 6).
Concentration of nickel ion (Ni+) in culture media. The concentration of Ni2+ in the culture medium of the coated group is lower than that in the uncoated group (P < 0.05) on the third, fourth, and fifth day ().
Table 3. The concentration of Ni2+ in culture media at each time point (μg/ml, ![]() ± s, n = 6).
± s, n = 6).
In Vivo Study
The in vivo study shows that the content of inorganic substance and the expression of ostcalcin, AKP, and TNF in coated and nickel-titanium alloy embracing fixator group was higher than that in the uncoated nickel-titanium alloy embracing fixator group ().
Table 4. Quantitative analysis of inorganic substance and semiquantitative analysis of ALP, osteocalcin, and TNF in callus surrounding the fracture (![]() ± s, g/g, A, n = 10).
± s, g/g, A, n = 10).
Ni+ in liver, brain, and muscles around the fracture was lower in DLC coated nickel-titanium alloy embracing fixator group and intramedullary needle fixator group than that in uncoated nickel-titanium alloy embracing fixator group, which shows that nickel ion released by uncoated nickel titanium is higher than the coated one. The ion consists not only of the tissue around the fracture, but also in farther organs such as the liver and brain ().
Table 5. Ni+ in liver, brain, and muscles around the fracture (![]() ± s, n = 10 (μg/g).
± s, n = 10 (μg/g).
DISCUSSION
Shape memory alloys (SMAs) are metallic materials with great potential to enhance medical engineering structures and have revolutionized the field of metallic biomaterials. Applications in the biomedical area are multiple and these materials improve significantly the quality of the diagnostics, treatments, and surgeries [Citation4,Citation5]. In spite of some good successes and excellent research of nickel-titanium shape memory alloy (NiTi-SMA) in reconstructive surgery, there are still serious limitations to the clinical applications of NiTi-SMA today.
The main disadvantages of the medical alloy are that it has no bioactivity and is difficult to attach to the body when embedded [Citation6]. Ni-Ti superelastic alloy is susceptible to environmental embrittlement in a corrosive atmosphere [Citation7]. It can release metal ion and even release debris into the surrounding tissues, which could be toxic to cells, tissues, and organs and lead to osteoporosis, even failure of internal fixation. There are endless interactions between body and metals, e.g. 1% NaCl is enough to have a corrosive effect on the medical alloy, which can influence the stabilization of the alloy.
Although Ni2+ is one of the essential microelements for cells, it can influence the cell proliferation at a high concentration [Citation8]. In order to decrease the release of Ni2+, surface modifying of NiTi memory alloy is very important. The corrosion and abrasion effect will greatly decrease and the duration of the alloy will greatly increase because of the protective membrane at the surface of the materials. Various oxidation treatments have also been applied to nearly equiatomic NiTi alloys so as to form a Ni-free protective oxide on the surface. Studies have shown that the oxide significantly decreases Ni+ release into exterior medium compared with untreated surfaces [Citation9–11]. A study concerning artificial joint shows that the coated artificial joint has no overt toxicity to collagenoblast and macrophage, and the growth and phaenotype are kept normal [Citation12]. These indicate that the coated material has favorable biocompatibility. Ni+ release causes allergenic, toxic, and carcinogenic reactions. The factors affecting corrosion are manifold and may be grouped into two classes: (1) the medium surrounding the metal, characterized by its pH, temperature and chemical composition (presence of Cl−, F2+, O2−, et al.); and (2) the metal's surface state (roughness, due to product processing or wear use; geometry factors such as sharp-angled, edged faces). What makes the application of NiTi controversial is not the intermetallic compound itself but the corrosion products; in particular, the amount of leached Ni2+ in organic fluids. Other research [Citation13] indicates that the release of Ni+ is significantly reduced compared with the untreated NiTi. The sample with surface NiTi exhibits the highest amount of bone formation whereas stainless steel fares the worst.
Owing to its superior mechanical properties with corrosion resistance, biocompatibility, and hemocompatibility, DLC has emerged as a promising material for biomedical applications [Citation14–16]. Experiments indicate that DLC coatings are biocompatible in vitro and in vivo. In this study, the DLC was used for treatment of the surface of NiTi shape alloy. The DLC is a metastable non-crystal material. It has characteristics of chemical stabilization, anti-corrosion, and anti-fraying [Citation17]. DLC can induce the new bone formation, increase the affinity of body and material, and avoid the immunological rejection. Meanwhile, surface activity exists on the surface of the material that can avoid the direct contact between body and material. Therefore, corrosion resistance is increased and the dissolution of the ion is inhibited. Another study [Citation18] shows that tantalum is an excellent candidate for NiTi alloys as a coating to improve its anti-corrosion property and radiopacity. It is suggested that DLC has no effect on metabolism and proliferation of cells [Citation19]. Histological anatomy shows that the tissue around the material has no adverse effect. Owing to its superior tribological and mechanical properties with corrosion resistance, biocompatibility, and hemocompatibility, diamond-like carbon has emerged as a promising material for biomedical applications. Diamond-like carbon films with various atomic bond structures and compositions are finding places in orthopedic, cardiovascular, and dental applications. Cells grew onto diamond-like carbon coating without any cytotoxity and inflammation. Diamond-like carbon coatings in orthopedic applications reduced wear, corrosion, and debris formation. Diamond-like carbon coating also reduced thrombogenicity by minimizing the platelet adhesion and activation [Citation20,Citation21]. S. Kobayashi et al. [Citation22] also found that Ni+ level in the solutions had been reduced one-sixth by DLC films when compared with non-coated wire. This indicates that DLC films have the protective effect of the diffusion and the non-cytotoxicity in a corrosive environment.
Mesenchymal stem cells (MSCs) are an attractive cell source for regenerative medicine because they can be harvested in a minimally invasive manner, and they are easily isolated and expanded, with multipotentiality including chondrogenesis [Citation23–25]. Bone marrow mesenchymal cells transplantation may be an effective approach to promote the healing of fractures. Research on biocompatibility of SMZ with MSCs will enhance the better understanding of cell-based therapy in orthopedics.
We observed the biocompatibility in vitro of DLC coated SMA with MSCs of Chinchilla rabbit. The concentration of AKP in culture media and the Ni2+ released in culture media were detected to compare the differences between the coated and uncoated SMA. On the first and second days, the amount of cells and the concentration of AKP in culture media had no obvious difference between the coated and uncoated groups. However, on the 3rd, 4th and 5th days, the amount of cells and the concentration of AKP in culture media of the coated group were greater than the uncoated group and the Ni2+ was lower than the uncoated group. The increase of Ni2+ is a main reason that affects the proliferation of cells, which indicates that uncoated NiTi alloy is prone to abrase and release Ni2+; accordingly there is no benefit to surrounding tissues. DLC coated NiTi alloy releases less Ni2+ than the uncoated one, indicating the importance of surface reshaping. DLC coating has a perfect biocompatibility in several investigations [Citation26–30]. We also analyzed in vivo the biocompatibility of coated and uncoated NiTi alloys. It is shown that the surface modified NiTi shape memory alloys are superior to the uncoated alloy and those of conventional stainless steel such as intramedullary nail. This new surface treatment could be of great interest for biomedical applications, as it can minimize sensitization and allergies and improve biocompatibility and corrosion resistance of NiTi shape memory alloys [Citation31].
From the different publications analyzed, it can be concluded that a passivated surface of NiTi shape memory alloys is highly recommended in reconstructive applications. However, the human body is a very complex place; it is not surprising that there is still a long way to go before we can readily, routinely, and successfully use the material in the human body. The interactions between materials and the human environment, degradation in vivo, and the possible toxicity must be better understood before the general use of NiTi-SMA. Therefore, it is advisable to avoid all Ni+-containing materials for already Ni-sensitized people. New solutions must be found to solve the problems that arise. A surface and structure treatment would perhaps make NiTi-SMA more tempting for human implantation. In effect, the surface treatment opens up unsuspected possibilities. The biocompatibility of SMA must be studied in a rigorous, orderly way to validate the long-term effects of the implant and eliminate the apprehensions of potential users. The direction of studies of biocompatibility, biostability, and biofunctionality of SMA implants must follow from their specific functions.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
REFERENCES
- Es-Souni, M., Es-Souni, M., Fischer-Brandies, H. (2005). Assessing the biocompatibility of NiTi shape memory alloys used for medical applications. Anal Bioanal Chem. 381(3):557–67.
- Poon, R.W., Yeung, K.W., Liu, X.Y., . (2005). Carbon plasma immersion ion implantation of nickel-titanium shape memory alloys. Biomaterials. 26(15):2265–72.
- The Ministry of Science and Technology of the People's Republic of China. Regulations for the Administration of Affairs Concerning Experimental Animals, Beijing, 1988-10-31.
- Yahia, L., Manceur, A., Chaffraix, P. (2006). Bioperformance of shape memory alloy single crystals. Biomed Mater Eng. 16(2):101–118.
- Guillemot, F. (2005). Recent advances in the design of titanium alloys for orthopedic applications. Expert Rev Med Devices. 2(6):741–748.
- Wu, L.L., Liang, H.F., Cai, C.L., . (2005). Properties of diamond-like carbon films on Ti-Ni alloy. Biaomian Jishu. 34(2):30–31.
- Asaoka, K., Yokoyama, K., Nagumo, M. (2002). Hydrogen embrittlement of nickel-titanium alloy in biological environment. Metallurgical and Materials Transactions A. 33(3):495–501.
- Tracana, R.B., Sousa, J.P., Carvalho, G.S. (1994). Mouse inflammatory response to stainless steel corrosion products. Mater Sci Mater Med. 5(10):596–600.
- Michiardi, A., Aparicio, C., Planell, J.A., . (2006). New oxidation treatment of NiTi shape memory alloys to obtain Ni-free surfaces and to improve biocompatibility. J Biomed Mater Res B Appl Biomater. 77(2):249–256.
- Chu, C.L., Hu, T., Wu, S.L., . (2007). Surface structure and properties of biomedical NiTi shape memory alloy after Fenton's oxidation. Acta Biomater. 3(5):795–806.
- Chrzanowski, W., Abou Neel, E.A., Armitage, D.A., . (2008). Surface preparation of bioactive Ni-Ti alloy using alkali, thermal treatments and spark oxidation. J Mater Sci Mater Med. 19(4):1553–1557.
- Allen, M., Law, F., Rushton, N. (1994). The effects of diamond-like carbon coatings on macrophages, fibroblasts and osteoblast-like cells in vitro. Clin Mater. 17(1):1–10.
- Yeung, K.W., Poon, R.W., Chu, P.K., . (2007). Surface mechanical properties, corrosion resistance, and cytocompatibility of nitrogen plasma-implanted nickel-titanium alloys: a comparative study with commonly used medical grade materials. J Biomed Mater Res A. 82(2):403–414.
- Roy, R.K., Lee, K.R. (2007). Biomedical applications of diamond-like carbon coatings: a review. J Biomed Mater Res B Appl Biomater. 83(1):72–84.
- Schaefer, O., Lohrmann, C., Winterer, J., . (2004). Endovascular treatment of superficial femoral artery occlusive disease with stents coated with diamond-like carbon. Clin Radiol. 59(12):1128–1131.
- Linder, S., Pinkowski, W., Aepfelbacher, M. (2002). Adhesion, cytoskeletal architecture and activation status of primary human macrophages on a diamond-like carbon coated surface. Biomaterials. 23(3):767–773.
- Nurdin, N., François, P., Mugnier, Y., . (2003). Haemocompatibility evaluation of DLC- and SiC-coated surfaces. Eur Cell Mater. 5:17–26.
- Heng, Y., Cai, W., Li, H.T., . (2006). Surface modification of NiTi alloy with tantalum to improve its biocompatibility and radiopacity. J Mater Sci. 41(15):4961–4964.
- Allen, M., Myer, B.L., Rushton, N. (2001). In vitro and in vivo investigations into the biocompatibility of diamond-like carbon (DLC) coatings for orthopedic applications. J Biomed Mater Res. 58(3):319–328.
- Roy, R.K., Lee, K.R. (2007). Biomedical applications of diamond-like carbon coatings: a review. J Biomed Mater Res B Appl Biomater. 83(1):72–84.
- Schaefer, O., Lohrmann, C., Winterer, J., . (2004). Endovascular treatment of superficial femoral artery occlusive disease with stents coated with diamond-like carbon. Clin Radiol. 59(12):1128–1131.
- Kobayashi, S., Ohgoe, Y., Ozeki, K., . (2007). Dissolution effect and cytotoxicity of diamond-like carbon coatings on orthodontic archwires. J Mater Sci Mater Med. 18(12):2263–2268.
- Hideyuki, Koga, Takeshi, Muneta, Tsuyoshi, Nagase, . (2008). Comparison of mesenchymal tissues-derived stem cells for in vivo chondrogenesis: suitable conditions for cell therapy of cartilage defects in rabbit. Cell Tissue Res. 333: 207–215.
- Kuroda, R., Ishida, K., Matsumoto, T., . (2007). Treatment of a full-thickness articular cartilage defect in the femoral condyle of an athlete with autologous bone-marrow stromal cells. Osteoarthritis Cartilage. 15(2):226–31.
- Csaki, C., Matis, U., Mobasheri, A., . (2007). Chondrogenesis, osteogenesis and adipogenesis of canine mesenchymal stem cells: a biochemical, morphological and ultrastructural study. Histochem Cell Biol. 128(6):507–20.
- Soininen, A., Tiainen, V.M., Konttinen, Y.T., . (2009). Bacterial adhesion to diamond-like carbon as compared to stainless steel. J Biomed Mater Res B Appl Biomater. Apr 7 [Epub ahead of print].
- Fedel, M., Motta, A., Maniglio, D., . (2008). Surface properties and blood compatibility of commercially available diamond-like carbon coatings for cardiovascular devices. J Biomed Mater Res B Appl Biomater. Dec 17 [Epub ahead of print].
- Hasebe, T., Yohena, S., Kamijo, A., . (2007). Fluorine doping into diamond-like carbon coatings inhibits protein adsorption and platelet activation. J Biomed Mater Res A. 83(4):1192–1199.
- Okpalugo, T.I., Ogwu, A.A., Okpalugo, A.C., . (2008). The human micro-vascular endothelial cells in vitro interaction with atomic-nitrogen-doped diamond-like carbon thin films. J Biomed Mater Res B Appl Biomater. 85(1):188–195.
- Laube, N., Kleinen, L., Bradenahl, J., . (2007). Diamond-like carbon coatings on ureteral stents–a new strategy for decreasing the formation of crystalline bacterial biofilms? J Urol. 177(5):1923–1927.
- Michiardi, A., Aparicio, C., Planell, J.A., . (2006). New oxidation treatment of NiTi shape memory alloys to obtain Ni-free surfaces and to improve biocompatibility. J Biomed Mater Res B Appl Biomater. 77(2):249–256.