Abstract
Abstract: Injection of endothelial progenitor cells (EPCs) into arteries for cell therapy is a promising field in regenerative medicine. However, adhesion of EPCs during capillary passage is restricted, and non-adhering cells are lost into circulation. Here we demonstrate that it is possible to achieve a three- to sevenfold higher rate of EPC adhesion to endothelium and extracellular matrix molecules after short-term activation with phorbol myristate acetate (PMA). In addition, differentiation and toxicity analyses of PMA activated EPCs showed no impact on cell differentiation and negligible impact on cell survival.
INTRODUCTION
The formation of new blood vessels and capillaries is one of the key features of wound healing and repair of damaged tissues [Citation1,Citation2]. In the last decade, our understanding of new blood vessel and capillary formation has changed dramatically. For the process of postnatal vasculogenesis, a coordinated pattern of endothelial progenitor cell (EPC) recruitment, migration, and differentiation is crucial [Citation1,Citation2]. These cells contribute to blood vessel regeneration by incorporating themselves into newly generated vessels and, with their paracrine effects, participate in the subsequent chemoattraction of mature endothelial cells [Citation3,Citation4]. Intra-arterially injected EPCs have also been reported to reduce scar formation in heart tissue following cardiac infarction in mice, increase left ventricular output in coronary disease patients, improve pain-free walking time in patients suffering from intermittent claudication [Citation5], and accelerate wound closure and neovascularization in a mouse ear wound model [Citation6]. Therefore, cell therapy seems to be a promising field in regenerative medicine for patients with traumatic or ischemic tissue degeneration. Initial clinical cell therapy trials were performed using catheter administration of stem/progenitor cells to arteries supplying regions where the administered cells are supposed to have their therapeutic effect [Citation7,Citation8]. However, following intra-arterial injection, adhesion and extravasation of EPCs during capillary passage are limited to cells that have a definite level of activation (stickiness) and are thus able to make and maintain contact with local endothelium or, in cases of damaged vasculature, with the basement membrane or molecules of the extracellular matrix (ECM). Cell therapies performed to date use unstimulated stem/progenitor cells harvested from bone marrow or peripheral blood [Citation7,Citation8]. Since these cells have to be enriched from patient samples, their total number is limited, and cells used for therapy that pass through the capillary system are lost to circulation. Hence, it was our intention to evaluate in vitro whether endothelial progenitor cells can be stimulated before administration to maximize their adhesion to endothelium and molecules of the ECM. Data describing the regulation of adhesion receptor expression, such as of the selectin or integrin adhesion receptor family, which are most presumably responsible for EPC homing, adhesion and extravasation, are scarce or controversial [Citation9]. Chemical substrates inducing EPC adhesion to endothelium and/or ECM have not yet been reported. Therefore we used the unspecific protein kinase C (PKC) activator phorbol ester phorbol 12-myristate 13-acetate (PMA, TPA), which is known to boost the stickiness of a variety of immunological cells, to increase EPC activation/stickiness [Citation10,Citation11]. To date, PMA has mainly been analyzed for its ability to induce transformation of cell cultures into cell lines due to its tumor-promoting capability [Citation12]. The ability of “tumor promotion” is ascribed to a group of chemicals that enhance carcinogenesis in the presence of carcinogenic substances [Citation12]. However, PMA does not affect DNA and is therefore not muta- or carcinogenic by itself; it acts by mimicking the glycerol derivative and second messenger diacylglycerol (DAG) and temporarily activates DAG-dependent conventional protein kinase C (PKC alpha, beta I, beta II and gamma), transferring cells to a high and unspecific state of transient activation [Citation12]. In contrast to models of carcinogenesis, which require either a few days of PMA or carcinogen exposure or repetitive treatment, cell adhesion properties are rapidly altered within minutes after PMA contact without de novo protein synthesis [Citation13].
We here present the analysis of experiments investigating EPC adhesion to endothelium and the ECM molecules fibronectin, collagen and laminin after short-term activation of EPCs using different PMA concentrations. To ensure that short-term PMA activation does not induce cell death, we evaluated necrosis and apoptosis rates 3 and 24 hours after incubation using flow cytometry and microscopy. Finally, in the interest of seeing whether PMA-activated EPCs maintain their endothelial differentiation status, we performed RT-PCR experiments, measuring expression of the endothelial differentiation markers von-Willebrand factor (vWF) and DiLDL uptake (1,1′-dioctadecyl-3,3,3′, 3′-tetramethylindo-carbocyanine-labeled acetylated low density lipoprotein), which is considered to be a prototypic endothelial attribute [Citation8,Citation14].
METHODS
Isolation, Identification, and Quantification of EPCs
EPCs were isolated as previously described [Citation14–16]. Briefly, peripheral blood mononuclear cells (PBMCs) were isolated from buffy coat (German Red Cross, Frankfurt, Germany) by density gradient centrifugation (20 min, 600 g) with Ficoll (1.077 g/ml, Biochrom, Berlin, Germany). PBMCs were washed twice with cold PBS without calcium and magnesium (PBSw/o) (10 min, 350 g), and 8*106 cells were cultivated on fibronectin-coated (10 μg/ml, Sigma, Deisenhofen, Germany) 12-well culture dishes in 800 μl of Endothelial Basal Medium (EBM, Cambrex, Verviers, Belgium) supplemented with endothelial growth medium SingleQuods at 37°C, 5% CO2. After 72 h, nonadherent cells were removed by washing twice with PBS+/+, and the remaining cells were incubated in EBM for a further 48 hours.
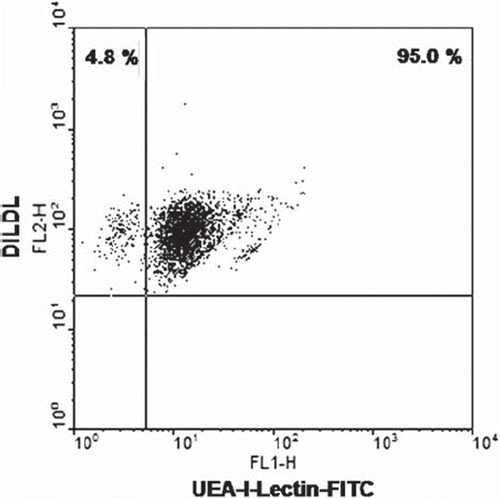
EPCs were identified using the method previously described [Citation14–16]. Cells were incubated for 1 h with 2.4 μg/ml 1,1′-dioctadecyl-3,3,3′,3′-tetramethylindo-carbocyanine-labeled, acetylated low-density lipoprotein (DiLDL, Cell-Systems, St. Katharinen, Germany) in EBM supplemented with 20% FCS. Cells were fixed with 2% paraformaldehyde for 10 min and after washing with PBS+/+FITC-labelled Ulex europaeus agglutinin-1 [10 μg/ml] (lectin, Sigma) were incubated for 1 h. Cells presenting double-positive fluorescence were considered to be EPC [Citation14–16] (.).
Figure 1. Characterization of EPC cell cultures. Peripheral blood mononuclear cells (PBMCs) were incubated in EBM for 5 days, stained with DiLDLD and FITC-lectin, and analyzed using flow cytometry. Double-positive cells are considered to be EPCs. Cell cultures with an EPC fraction of at least 90% were used for all experiments (representative dot plot is shown).
Isolation and Cell Culture of Human Umbilical Vein Endothelial Cells (HUVECs)
Endothelial cells (HUVECs) were isolated from human umbilical veins and harvested by enzymatic treatment with chymotrypsin. HUVECs were grown in Medium 199 (Biozol, Munich, Germany), 10% fetal calf serum (Invitrogen, Karlsruhe, Germany), 10% pooled human serum (German Red Cross), 20 μg/ml endothelial cell growth factor (Boehringer, Mannheim, Germany), 0.1% heparin (Roche, Basel, Switzerland), 100 ng/ml gentamicin (Invitrogen), and 2% 1 mol/L HEPES buffer (Seromed, Berlin, Germany). To check for the purity of HUVEC cultures, cells were stained with fluorescein isothiocyanate (FITC)-labeled monoclonal antibody against factor VIII-associated antigen (von Willebrand factor; clone F8/86; Dako, Hamburg, Germany) and analyzed microscopically, or by FACscan (BD Pharmingen; FL-1H (log) channel histogram analysis; 10.000 cells/scan). Cell cultures with a purity >95% were serially passaged. Subcultures from passages 2–3 were selected for experimental use.
EPC Activation by PMA
EPCs were harvested by trypsinization for 10 minutes. Cells were pooled and washed with PBS−/− and adjusted in HUVEC medium (see above) to a total number of 5*105 cells/ml. Cell suspension was pipetted into 6 Falcon tubes (each 1 ml, BD Bioscience, Heidelberg, Hamburg). Thereafter PMA (stock solution 10−4 M in 100% DMSO, Sigma) in different concentrations (0, 10−9, 10−8, 10−7, 10−6 mol/l) and the PMA solvent DMSO (Sigma) were put into tubes, and cells were incubated at 37°C, 5% CO2 for 15 minutes. Cells were then washed twice with PBS−/− and resuspended in 1 ml of HUVEC medium (see above).
EPC Adhesion Experiments on HUVECs
HUVECs were cultured to confluent monolayers on 6-well plates. Before experiments HUVECs were washed once with PBS+/+. Thereafter activated EPCs (see “EPC activation by PMA”) were added to the top of the HUVEC monolayers, and co-cultures were incubated at 37°C, 5% CO2 for 1 hour. Nonadherent EPCs were removed by washing HUVEC monolayers three times with PBS+/+. Thereafter cells were fixed with 2% glutaraldehyde for 10 minutes. The number of EPCs on endothelium was determined by counting five fields of view (×200) using an inverse fluorescence microscope (Olympus, Hamburg, Germany).
EPC Adhesion to Extracellular Matrix
Collagen (Biochrom) was resuspended to a concentration of 0.4 mg/ml, laminin (BD Biosciences) and fibronectin (Sigma) to a concentration of 0.1 mg/ml in PBS+/+. Six-well tissue culture plates were coated with 500μl of diluted matrices per well and stored at 4°C overnight. Then plates were washed three times with PBS−/− and blocked with 1% BSA in PBS−/− for 1 hour at room temperature. Thereafter activated EPCs (see “EPC activation by PMA”) were placed on the top of extracellular matrix plates and incubated at 37°C, 5% CO2 for 1 hour. Nonadherent EPCs were removed by washing extracellular matrix plates three times with PBS+/+, and cells were then fixed with 2% glutaraldehyde (Sigma) for 10 minutes. The number of EPCs on the extracellular matrix plates was determined by counting five randomized fields of view (×200) using an inverse fluorescence microscope (Olympus).
Detection of Apoptosis and Necrosis of EPCs after Short-term PMA Activation
EPCs were cultured as described above. Thereafter culture plates were washed with PBS+/+, and cells were incubated with PMA (0, 10−9, 10−8, 10−7, 10−6 mol/l) and the PMA solvent DMSO (with the same volume as used for highest PMA concentration) for 15 minutes. Cells were then washed three times with PBS+/+ and incubated for 3 or 24 hours in EBM. After 3 or 24 hours, cells were washed once with PBS+/+, and the EPC number was determined by counting five randomized fields of view (×200). Thereafter cells were detached, and apoptosis and necrosis of the EPCs were determined by propidium iodide (PI) and annexin-V staining using the TACSAnnexin-V-FITC assay (R&D Systems, Wiesbaden, Germany) following the manufacturer's instructions. For measurement a three-channel FACScan (BD Pharmingen) was used.
Gene Expression of vWF and DiLDL Uptake in EPCs after Short-term Activation with PMA
EPCs were cultured and stimulated with PMA as described above. Cells were then washed three times with PBS+/+ and further incubated in EBM. Three days after PMA stimulation, RNA for RT-PCR was isolated using RNeasy (Qiagen, Hilden, Germany). First-strand cDNA-synthesis was performed with each 1 μg RNA sample using Superscript RT (Invitrogen) according to manufacturer's instructions. The vWF and GAPDH primer were synthesized by MWG-Biotech (Ebersberg, Germany) [Citation17]. The PCR products were analyzed on an ethidium bromide-stained 2% agarose gel. For DiLDL uptake analysis EPCs were incubated with DiLDL (Cell-Systems) for 1 hour, three days after PMA stimulation, as described in the “Isolation, identification and quantification” of EPCs section and measured on a FACScan.
Statistics
Statistical evaluation was conducted using the Wilcoxon-Mann-Whitney U-test. Results are presented as mean+/− SEM or SD. A P value of less than 0.05 indicates statistical significance; all significance tests were performed and should be interpreted in a two-sided manner.
RESULTS
Characterization and Quantification of EPCs
Peripheral blood mononuclear cells (PBMCs) were cultured in Endothelial Basal Medium (EBM) for 5 days, thereafter double-stained for DiLDL and FITC-lectin, and then analyzed by flow cytometry (.). Double-positive cells in the upper right quadrant were considered to be endothelial progenitor cells (EPCs) [Citation14–16,Citation18]. EPC cultures with a purity of at least 90% were used for all subsequent experiments.
EPC Adhesion on HUVECs after Short-term PMA Activation
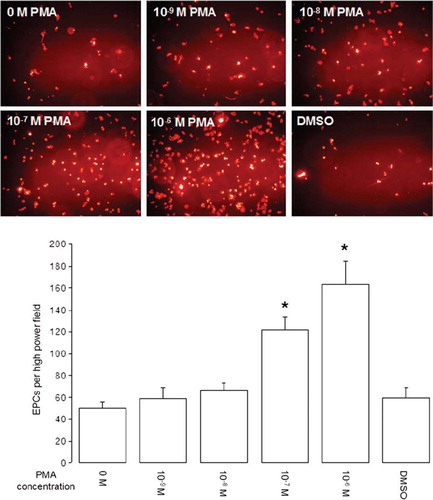
EPCs were activated by different concentrations of PMA, and adhesion experiments on endothelial monolayers were carried out. To distinguish between endothelial cells and EPCs, EPCs were stained for DiLDL prior to experiments. Photos in show representative examples of EPCs per field of view on endothelial monolayers with a clear correlation between increasing cell numbers and the concentration of PMA used for cell activation. A significant increase in EPC number compared with the control was observed for PMA concentrations of 10−7 to 10−6 mol/l with maximum cell number when using 10−6 mol/l PMA (, graph).
Figure 2. EPC adhesion on HUVECs after short-term PMA activation. EPCs were co-cultured on HUVECs for 1 hour, and nonadhered cells were removed by washing co-cultures 3 times with PBS+/+. Photos show representative fields of view (×200 magnification) of DiLDL-positive cells. Graph shows adhesion of EPCs on HUVECs after short-term PMA activation with different concentrations (n = 6). Stars indicate significant difference vs. control; for p < 0.05 groups were considered to be significantly different.
EPC Adhesion to Extracellular Matrix Molecules after Short-term PMA Activation
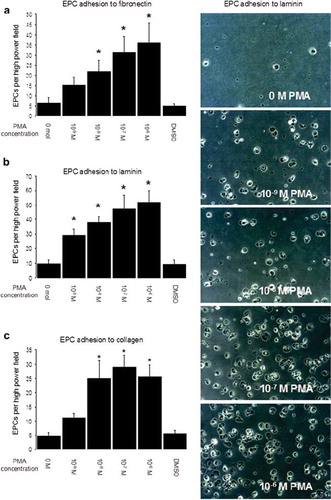
EPCs were activated by different concentrations of PMA, and adhesion experiments on extracellular matrix (fibronectin, collagen, laminin) were carried out. Photos in show representative examples of EPCs per field of view on laminin-coated culture plates with a clear correlation between increasing cell numbers and the concentration of PMA used for cell activation. For fibronectin-coated culture plates, a significant increase in EPC number vs. control was observed for PMA concentrations of 10−8 to 10−6 mol/l with maximum cell number when using 10−6 mol/l PMA (). Adhesion to laminin-coated dishes was significantly increased even when using the low PMA concentration of 10−9 mol/l (). For collagen-coated culture plates, significant increases in EPC number vs. control were observed for PMA concentrations of 10−8 to 10−6 mol/l with maximum cell number when using 10−7 mole/l PMA ().
Figure 3. EPC adhesion on extracellular matrix after short-term PMA activation. EPCs were incubated on fibronectin, collagen or laminin for 1 hour, and nonadhered cells were removed by washing plates 3 times with PBS+/+. Photos show representative fields of view (×200 magnitude) of EPC adhering to laminin. Graph a shows EPC adhesion to fibronectin, Graph b shows EPC adhesion to laminin, and Graph c shows EPC adhesion to collagen (all experiments n = 6). Stars indicate significant difference vs. control; for p < 0.05 groups were considered to be significantly different.
Apoptosis and Necrosis of EPCs after Short-term PMA Activation
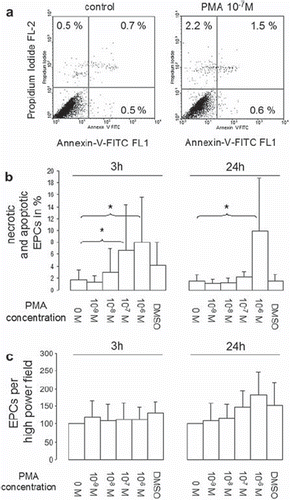
We measured apoptosis and necrosis of EPCs after PMA activation to evaluate the toxicity of short-term PMA treatment. shows representative dot plots of PMA-treated (right) and control (left) EPCs after staining with propidium iodide (channel 2) and annexin-V-FITC (channel 1). The percent results of total cells in the upper left (necrosis), upper right (late apoptosis) and lower right (early apoptosis) quadrant were summed up to calculate the total percentage of dying and dead cells in a population. Results are presented in . A significantly increased dead cell fraction compared to control was identified three hours after EPC treatment with 10−7 and 10−6 mol/l PMA. Twenty-four hours after incubation of EPCs with PMA a significantly increased dead cell fraction was seen only for cells treated with 10−6 mol/l PMA. Since the cytometric analyses presented give information about the percentage of dead cells in the whole cell population but lack information on increasing or decreasing cell numbers, we counted EPCs per high power field 3 and 24 hours after treatment with PMA (). At these time points, no significant differences in cell numbers compared to control were seen for any of the PMA concentrations used ().
Figure 4. Apoptosis and necrosis of EPCs after short-term PMA activation. EPCs were activated with different PMA con centrations for 15 minutes, after which cells were washed 3 times with PBS+/+ and incubated for a further 3 or 24 hours in EBM. Apoptosis and necrosis was determined by propidium iodide (PI) and annexin-V-FITC staining. Necrotic (PI-positive cells), late apoptotic (double-stained cells) and early apoptotic (annexin-V-FITC-positive cells) cells were determined by flow cytometry (representative dot plots after 3 hours are shown, fig. 4a). To determine the dead cell fraction, necrotic, early, and late apoptotic cell fractions were summed up. Figure 4b shows dead cell fractions 3 and 24 hour after short-term PMA stimulation. Figure 4c shows EPCs per high power field before collection for flowcytometric necrosis/apoptosis analysis (all experiments n = 6). Stars indicate significant differences between groups; for p < 0.05 groups were considered to be sig nificantly different.
EPC Differentiation after Short-term PMA Activation
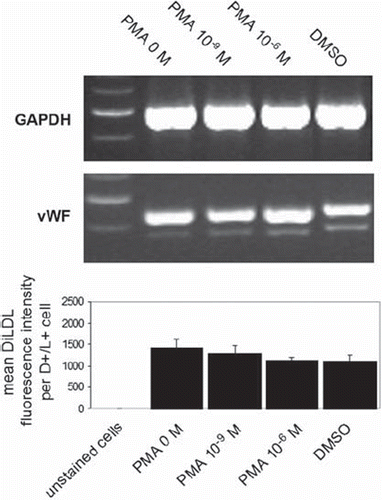
To analyze whether short-term PMA activation alters the cell differentiation of EPCs, we measured gene expression of the endothelial marker vWF and fluoroscopic DiLDL uptake 3 days after PMA exposure. shows representative lanes for GAPDH and vWF expression (n = 3), and the graph shows mean DiLDL fluorescence intensity in EPCs from 4 experiments. As depicted, significant differences in vWF expression or DiLDL uptake were not registered in EPCs after short-term PMA activation.
Figure 5. EPC differentiation after short-term PMA activation. EPCs were activated with different PMA concentrations for 15 minutes, after which cells were washed 3 times with PBS+/+ and incubated for a further 3 days in EBM. Gene expression of vWF was then measured using RT-PCR, and DiLDL uptake was determined using flow cytometry. Figure 5 shows representative lanes for GAPDH and vWF expression (n = 3), and the graph shows mean DiLDL fluorescence intensity in EPCs from 4 experiments.
DISCUSSION
The promising field of cell therapy by catheter-assisted injection of adult stem and/or progenitor cells into arteries is a newly emerging option for patients with chronic or nonhealing wounds or ischemic tissue assault such as myocardial infarction. For cell therapy to be effective, enough of the cells administered must be recruited to the target tissue. Using in vivo imaging techniques—iron oxide-labeled stem/progenitor cells for magnetic resonance imaging (MRI), radioisotope-labeled cells for single-photon emission computed tomography (SPECT) or tracers such as F18-FDG for positron emission tomography (PET)–the extent of stem and progenitor cell homing and engraftment was shown to be rather low in experimental and clinical studies [Citation19,Citation20]. The amount of radioactivity after intravenous injection of radiolabeled endothelial progenitor cells or purified CD34+ or CD133+ stem cells into rats after myocardial infarction was approximately 2% four days after infusion, indicating that only a minor percentage of cells homed to the heart. The majority of cells were detected in the spleen or liver [Citation21]. However, administration of bone marrow mononuclear cells (BMCs) and CD133+ cells using catheter-assisted arterial injection increased cell delivery to 7-11% and was even higher for purified CD34+ cells with a radioactivity of 14% of total administered cells in humans [Citation22–25]. These findings suggest that pharmacological manipulation of progenitor/stem cell engraftment and homing should be the next step in cell therapeutical trials to improve the transplanted cell fraction to designated tissues. Cell adhesion and extravasation from circulation is a process that takes place in local capillaries and has been analyzed extensively for cells of the immune system and for tumors to understand malignant cell dissemination. Mechanisms by which stem and progenitor cells adhere to endothelial layers of the capillary system or ECM molecules—in cases of endothelial monolayer disruption—are mainly unknown or controversial [Citation26]. However, initial findings in this field of research support the idea that the homing of EPCs to sites of neovascularisation shares at least some features with the homing of leukocytes to sites of inflammation [Citation26]. Taking this into account, there appear to be two possible options for improving cell migration to injured tissue. One is the activation of local endothelium using inflammatory cytokines to improve cell adhesion molecule expression (e.g. ICAM-1 or VCAM-1, both reported to be involved in CD34+ and CD133+ hematopoietic stem cell traffic [Citation27–29]. However, endothelium adjacent to a wound has a definitive high CAM (cell adhesion molecule) expression level due to exposure to inflammatory cytokines such as IL-1 and TNF-alpha. Thus, pharmacological enhancement of endothelial adhesiveness would be difficult to achieve [Citation30]. The second option is to improve cell delivery by activating homing receptors of stem/progenitor cells before administration. Recently published data suggest that, according to the recruitment of inflammatory cells, the known coordinate sequence of multistep adhesive and signalling events including selectin-mediated rolling and integrin-mediated firm adhesion to endothelial monolayers and finally invasion of the extracellular matrix are in parts alike [Citation26]. Unfortunately, selective pharmacological modulation of potentially involved adhesion receptors such as L-selectin or the integrins VLA-4 or LFA-1 is not possible at present because the mechanisms for receptor regulation and activity in progenitor/stem cells are still unclear. On this account we analyzed whether adhesion of EPCs can be enhanced by the well-known and intensively analyzed agent for unspecific induction of leukocyte stickiness, the protein kinase C (PKC) activator phorbol ester phorbol 12-myristate 13-acetate (PMA) [Citation31,Citation32]. Phorbol esters induce aggregation of monocytes and granulocytes as well as adhesion of T lymphocytes to either B cells or monocytes and generally of leukocytes to vascular endothelial cells [Citation33]. This rapidly induced and antigen-independent intercellular adhesion requires cellular metabolism, an intact cytoskeleton, and extracellular divalent cations; it is mediated by exocytosis of preformed cell adhesion molecules and not de novo protein synthesis [Citation33]. In our experiments presented here, short-term incubation of EPCs (15 minutes) with high-dose PMA (10−7–10−6 mole/l) was found to result in three to four times higher cell adhesion to endothelial monolayers compared with unstimulated EPCs. These findings correlate with observations made for short-term PMA activation of different leukocytes [Citation31,Citation32]. Furthermore, PMA was observed to enhance adherence of inflammatory cells to diverse ECM molecules [Citation34,Citation35]. As described in [Citation36,Citation37], severe tissue damage due to ischemia/radiation/trauma triggers local inflammation with partial disruption of the endothelial monolayer and exposure of the subjacent basement membrane. To take this into account we also analyzed PMA-mediated EPC adhesion to proteins of the extracellular matrix, namely the basement membrane components fibronectin, laminin and collagen. As demonstrated in , significantly increased EPC adhesion was seen for all tested matrix proteins, and adhesion to laminin-coated culture dishes showed significantly increased adhesion even at the low PMA concentration of 10−9 mol/l.
Besides its effects on cell adhesion after short-term incubation or on tumor promotion in the presence of carcinogens, PMA promotes, or in some cases prevents, apoptosis in cell cultures or alters cell differentiation to a higher as well as to a lower differentiation status [Citation38–40]. Both cell death and loss of differentiation are undesirable for cells designated for cell therapy. To ensure that PMA-activated EPCs are not forced towards apoptosis or become intoxicated and die due to necrosis, we counted EPCs 3 and 24 hours after PMA stimulation. No reduction in total cell numbers was observed, and the total cell count was even increasing in the 24h samples after high-dose PMA stimulation (), presumably due to better cell adherence during the washing procedures. Flowcytometric analyses of cell necrosis (propidium iodide) and apoptosis (annexin-FITC) revealed a significant induction of cell death for high-dose PMA 3 hours after exposure, and the dying cell fraction was still elevated in the 24-hour samples. Since the induction of cell death ranges from approximately 2% (no PMA) to 10% (10−6 mol/l PMA), we think that in light of the extent to which PMA induces cell adherence (three- to sevenfold) this is a negligible effect on EPC survival.
The second problem, loss of differentiation in EPCs, has the potential to make transplanted cells useless, as their differentiation to endothelial cells and the function as paracrine effector cells could be altered or abrogated completely. Expression of vWF and DiLDL uptake are described as prototypic for EPC differentiation [Citation4,Citation14,Citation18]. The RT-PCR we used shows no impact on the gene expression of vWF, and flow cytometric analyses of DiLDL uptake were not altered in EPCs after short-term PMA incubation, meaning that we did not demonstrate a negative impact on differentiation.
Finally, the most engaging question is whether PMA stimulation of EPCs can be seen as a prospective option for improved cell delivery in patients receiving cell therapy. PMA is a tumor-promoting agent, which means that it potentiates carcinogenic chemicals and tumor-inducing viruses in their tumor-evoking character in vitro and in vivo [Citation12]. This PMA attribute can be observed after long-term (couple of days) or repetitive PMA exposure in culture and in animal trials. Short-term ex vivo cell incubation with PMA cannot guarantee the safety of this agent. Tumor induction is either a nonstochastic or a stochastic, multistep and therefore unpredictable process. Even when in vitro augmented stem/progenitor cells desired for cell transplantation are cultured absolutely free of chemical carcinogens, it is impossible to rule out short-term activation due to cellular contamination with oncogenic viruses or mutagenic effects due to normal background UV light exposure and consequent tumor promotion by PMA. Hence, the experiments presented here should be regarded as a demonstration of pharmacological feasibility and should lay the groundwork for further research in directed stem and progenitor cell delivery. Moreover, we present a potent tool for further in vivo animal experiments to investigate pharmacological manipulation of EPC traffic and to answer the question whether a higher cell delivery to damaged organs/tissues ultimately results in a measurable benefit in tissue regeneration.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
REFERENCES
- Falanga, V. (2005). Wound healing and its impairment in the diabetic foot. Lancet, 366(9498): 1736–43.
- Reed, M.J., Edelberg, J.M. (2004). Impaired angiogenesis in the aged. Sci Aging Knowledge Environ, 7:pe7.
- Rumpold, H., Wolf, D., Koeck, R., Gunsilius, E. (2004). Endothelial progenitor cells: A source for therapeutic vasculogenesis?, J Cell Mol Med, 8(4): 509–18.
- Asahara, T., Kawamoto, A. (2004). Endothelial progenitor cells for postnatal vasculogenesis. Am J Physiol Cell Physiol, 287(3): C572–9.
- Vale, P.R., Isner, J.M., Rosenfield, K. (2001). Therapeutic angiogenesis in critical limb and myocardial ischemia. J Interv Cardiol, 14(5): 511–528.
- Sander, A.L., Jakob, H., Henrich, D., Powerski, M., Witt, H., Dimmeler, S., Barker, J., Marzi, I., Frank, J. (2009) Systemic transplantation of progenitor cells accelerates wound epithelialization and neovascularization in the hairless mouse ear wound model. J Surg Res [Epub ahead of print].
- Erbs, S., Linke, A., Schächinger, V., Assmus, B., Thiele, H., Diederich, K.W., Hoffmann, C., Dimmeler, S., Tonn, T., Hambrecht, R., Zeiher, A.M., Schuler, G. (2007). Restoration of microvascular function in the infarct-related artery by intracoronary transplantation of bone marrow progenitor cells in patients with acute myocardial infarction: The Doppler Substudy of the Reinfusion of Enriched Progenitor Cells and Infarct Remodeling in Acute Myocardial Infarction (REPAIR-AMI) trial. Circulation, 116(4): 366–74.
- Schächinger, V., Erbs, S., Elsässer, A., Haberbosch, W., Hambrecht, R., Hölschermann, H., Yu, J., Corti, R., Mathey, D.G., Hamm, C.W., Süselbeck, T., Werner, N., Haase, J., Neuzner, J., Germing, A., Mark, B., Assmus, B., Tonn, T., Dimmeler, S., Zeiher, A.M. (2006). REPAIR-AMI investigators: Improved clinical outcome after intracoronary administration of bone-marrow-derived progenitor cells in acute myocardial infarction: Final 1-year results of the REPAIR-AMI trial. Eur Heart J. 27(23): 2775–83.
- Hristov, M., Zernecke, A., Liehn, E.A., Weber, C. (2007). Regulation of endothelial progenitor cell homing after arterial injury. Thromb Haemost, 98(2): 274–7.
- Bochner, B.S., Peachell, P.T., Brown, K.E., Schleimer, R.P. (1988). Adherence of human basophils to cultured umbilical vein endothelial cells. J Clin Invest, 81(5): 1355–64.
- Schleimer, R.P., Rutledge, B.K. (1986). Cultured human vascular endothelial cells acquire adhesiveness for neutrophils after stimulation with interleukin 1, endotoxin, and tumor-promoting phorbol diesters. J Immunol, 136(2): 649–54.
- Umezawa, K., Weinstein, I.B., Horowitz, A., Fujiki, H., Matsushima, T., Sugimura, T. (1981). Similarity of teleocidin B and phorbol ester tumour promoters in effects on membrane receptors. Nature, 290(5805):411–3.
- Enomoto, T., Yamasaki, H. (1985). Phorbol ester-mediated inhibition of intercellular communication in BALB/c 3T3 cells: Relationship to enhancement of cell transformation. Cancer Res. 45(6): 2681–8.
- Henrich, D., Hahn, P., Wahl, M., Wilhelm, K., Dernbach, E., Dimmeler, S., Marzi, I. (2004). Serum derived from multiple trauma patients promotes the differentiation of endothelial progenitor cells in vitro: possible role of transforming growth factor-beta1 and vascular endothelial growth factor 165. Shock, 21(1): 13–6.
- Vasa, M., Fichtlscherer, S., Adler, K., Aicher, A., Martin, H., Zeiher, A.M., Dimmeler, S. (2001). Increase in circulating endothelial progenitor cells by statin therapy in patients with stable coronary artery disease. Circulation, 103(24): 2885–90.
- Kalka, C., Masuda, H., Takahashi, T., Kalka-Moll, W.M., Silver, M., Kearney, M., Li, T., Isner, J.M., Asahara, T. (2000). Transplantation of ex vivo expanded endothelial progenitor cells for therapeutic neovascularisation. Proc Natl Acad Sci, 97(7): 3422–7.
- Fernandez Pujol, B., Lucibello, F.C., Gehling, U.M., Lindemann, K., Weidner, N., Zuzarte, M.L., Adamkiewicz, J., Elsässer, H.P., Müller, R., Havemann, K. (2000). Endothelial-like cells derived from human CD14 positive monocytes. Differentiation, 65(5): 287–300.
- Henrich, D., Seebach, C., Wilhelm, K., Marzi, I. (2007). High dosage of simvastatin reduces TNF-alpha-induced apoptosis of endothelial progenitor cells but fails to prevent apoptosis induced by IL-1beta in vitro. J Surg Res. 142(1): 13–9.
- Beeres, S.L., Bengel, F.M., Bartunek, J., Atsma, D.E., Hill, J.M., Vanderheyden, M., Penicka, M., Schalij, M.J., Wijns, W., Bax, J.J. (2007). Role of imaging in cardiac stem cell therapy. J Am Coll Cardiol, 49: 1137–48.
- Bengel, F.M., Schachinger, V., Dimmeler, S. (2005). Cell-based therapies and imaging in cardiology. Eur J Nucl Med Mol Imaging, 32 (Suppl 2): S404–16.
- Aicher, A., Brenner, W., Zuhayra, M., Badorff, C., Massoudi, S., Assmus, B., Eckey, T., Henze, E., Zeiher, A.M., Dimmeler, S. (2003). Assessment of the tissue distribution of transplanted human endothelial progenitor cells by radioactive labelling. Circulation, 107: 2134–9.
- Kurpisz, M., Czepczynski, R., Grygielska, B., Majewski, M., Fiszer, D., Jerzykowska, O., Sowiński, J., Siminiak, T. (2006). Bone marrow stem cell imaging after intracoronary administration. Int J Cardiol, 121(2):194–5.
- Caveliers, V., De Keulenaer, G., Everaert, H., Van Riet, I., Van Camp, G., Verheye, S., Roland, J., Schoors, D., Franken, P.R., Schots, R. (2007). In vivo visualization of 111In labeled CD133+ peripheral blood stem cells after intracoronary administration in patients with chronic ischemic heart disease. Q J Nucl Med Mol Imaging, 51: 61–6.
- Goussetis, E., Manginas, A., Koutelou, M., Peristeri, I., Theodosaki, M., Kollaros, N., Leontiadis, E., Theodorakos, A., Paterakis, G., Karatasakis, G., Cokkinos, D.V., Graphakos, S. (2006). Intracoronary infusion of CD133+and CD133− CD34+selected autologous bone marrow progenitor cells in patients with chronic ischemic cardiomyopathy: cell isolation, adherence to the infarcted area, and body distribution. Stem Cells, 24: 2279–83.
- Hofmann, M., Wollert, K.C., Meyer, G.P., Menke, A., Arseniev, L., Hertenstein, B., Ganser, A., Knapp, W.H., Drexler, H. (2005). Monitoring of bone marrow cell homing into the infarcted human myocardium. Circulation, 111: 2198–202.
- Chavakis, E., Urbich, C., Dimmeler, S. (2008). Homing and engraftment of progenitor cells: A prerequisite for cell therapy. J Mol Cell Cardiol, 45(4): 514–22.
- Chute, J.P. (2006). Stem cell homing. Curr Opin Hematol, 13(6): 399–406.
- Jin, H., Aiyer, A., Su, J., Borgstrom, P., Borgstrom, P., Stupack, D., Friedlander, M., Varner, J. (2006). Homing mechanism for bone marrow-derived progenitor cell recruitment to the neovasculature. J Clin Invest, 116(3): 652–662.
- Powerski, M.J., Henrich, D., Sander, A., Wastl, D., Ludwig, K., Marzi, I. (2010). CD133+CD34+ stem cells are mobilized after musculoskeletal surgery and target endothelium activated by surgical wound fluid. Langenbecks Arch Surg,
- Etzioni, A. (1996). Adhesion molecules-their role in health and disease. Pediatr Res, 39(2): 191–8.
- Webster, R.O., Wysolmerski, R.B., Lagunoff, D. (1986). Enhancement of human polymorphonuclear leukocyte adherence to plastic and endothelium by phorbol myristate acetate. Comparison with human C5a. Am J Pathol, 125(2): 369–78.
- Damle, N.K., Doyle, L.V. (1990). Ability of human T lymphocytes to adhere to vascular endothelial cells and to augment endothelial permeability to macromolecules is linked to their state of post-thymic maturation. J Immunol, 144(4): 1233–40.
- Patarroyo, M., Prieto, J., Rincon, J., Timonen, T., Lundberg, C., Lindbom, L., Asjö, B., Gahmberg, C.G. (1990). Leukocyte-cell adhesion: A molecular process fundamental in leukocyte physiology. Immunol Rev, 114: 67–108.
- Bohnsack, J.F., Akiyama, S.K., Damsky, C.H., Knape, W.A., Zimmerman, G.A. (1990). Human neutrophil adherence to laminin in vitro. Evidence for a distinct neutrophil integrin receptor for laminin. J Exp Med, 171(4): 1221–37.
- Roth, P., Polin, R.A. (1992). Induction of monocytic cell adherence to matrix-bound fibronectin by phorbol ester. J Clin Lab Immunol, 37(2): 51–63.
- Gute, D.C., Ishida, T., Yarimizu, K., Korthuis, R.J. (1998). Inflammatory responses to ischemia and reperfusion in skeletal muscle. Mol Cell Biochem, 179(1–2): 169–87.
- Li, Y.Q., Chen, P., Jain, V., Reilly, R.M., Wong, C.S. (2004). Early radiation-induced endothelial cell loss and blood-spinal cord barrier breakdown in the rat spinal cord. Radiat Res, 161(2): 143–52.
- Lowe, M.E., Pacifici, M., Holtzer, H. (1978). Effects of phorbol-12-myristate-13-acetate on the phenotypic program of cultured chondroblasts and fibroblasts. Cancer Res, 38(8): 2350–6.
- Greenberger, J.S., Newburger, P.E., Sakakeeny, M. (1980). Phorbol myristate acetate stimulates macrophage differentiation and replication and alters granulopoiesis and leukemogenesis in long-term bone marrow cultures. Blood, 56(3): 368–79.
- Cotter, T.G., Lennon, S.V., Glynn, J.M., Green, D.R. (1992). Microfilament-disrupting agents prevent the formation of apoptotic bodies in tumor cells undergoing apoptosis. Cancer Res, 52(4): 997–1005.