Abstract
Abstract: Poly(methyl methacrylate) (PMMA) microfibers were used as a template for development of a capillary-like network in agarose hydrogel. Microfibers with diameter 10-20 μm, which is comparable to the diameter of native capillary vessels, were fabricated using a wet spinning technique. The microfibers were embedded in agarose gel and dissolved by immersing the gel in dichloromethane. The resultant microchannels in the gel had the same diameter as the microfibers, and allowed an aqueous solution to be perfused through the gel. The methodology is promising for fabricating a capillary-like network in tissue engineering scaffolds of various water-soluble polymers.
INTRODUCTION
Significant and lasting progress in the field of tissue engineering over the past few decades has led to the creation of implantable artificial tissues such as skin and cartilage [Citation1]. The next challenge in the field is to create thicker (three-dimensional, 3D) tissues and organs in vitro. However, in most tissue-engineering strategies, delivery of oxygen and nutrients to the cells in artificial tissues is based on diffusion from the surrounding environment [Citation2]. That mode of delivery provides an insufficient supply of oxygen and nutrients to the cells, which constrains researchers to design only thin tissues (e.g. skin) and those composed of cells with low oxygen demand (e.g. cartilage). A possible strategy for creating 3D tissues in vitro is development of a capillary network in the constructs to deliver sufficient oxygen and nutrients to whole cells [Citation3–5].
To date, several techniques for fabricating a vasculature-like network in vitro have been reported [Citation2–4, Citation6–12]. A technique introduced by Bellan et al. is the most promising for creating a biomimetic and dense capillary-like network into which an aqueous solution can be perfused [Citation13]. They created a capillary-like network by dissolving melt-spun sugar microfibers embedded in a water-insoluble polymer matrix (polycaprolactone). However, it is impossible for a capillary-like network to be fabricated in matrices of water-soluble polymers using this method, because the sugar microfibers rapidly dissolve in an aqueous environment.
The purpose of the present study was to develop a technique for fabricating a capillary-like network in matrices of water-soluble polymers. The materials of tissue engineering scaffolds influence cell adhesion, proliferation, and differentiation behavior [Citation14]. Water-soluble polymers include many natural proteins and polysaccharides (e.g. collagen, gelatin, hyaluronate, fibrin, alginate, agarose, and dextran). These polymers have been widely used as scaffold materials due to their inherent biological characteristics [Citation14], and recent advances in polymer science have led to further improvement of their mechanical and biological properties [Citation15, Citation16]. Use of such water-soluble polymers as the base material of support matrices for capillary-like networks would contribute to creation of biomimetic 3D artificial tissues.
To accomplish our objective, we selected poly(methyl methacrylate) (PMMA), a water-insoluble polymer, as the microfiber material. Unlike sugar microfibers, water-insoluble microfibers eliminate the problem of dissolution of microfibers in an aqueous environment. A wet spinning technique was employed to fabricate PMMA microfibers with comparable diameter to capillary blood vessels. Agarose, a model water-soluble polymer, was used as the support matrix material for a capillary-like network.
MATERIALS AND METHODS
Fabrication of PMMA Microfibers Using the Wet Spinning Technique
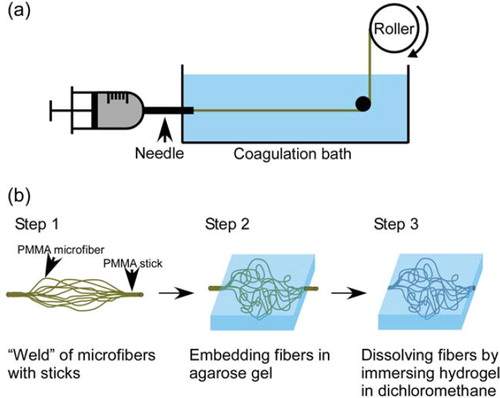
A wet spinning process is shown schematically in . A 45% (w/v) solution of PMMA (Mw: 75000 g mol−1, Scientific Polymer Products, Inc., Ontario, NY, USA) in dichloromethane was extruded at a constant rate (0.13 μl s−1) from a syringe equipped with a stainless-steel needle (270 μm inner diameter) into ethanol. The length of the coagulation bath and the air gap between the roller and the bath were kept constant at 90 and 10 cm, respectively. The PMMA microfibers thus formed were taken up by the roller. The mean cross-sectional diameters of the microfibers were determined using an optical microscope. The theoretical diameter of the microfibers was estimated from the equation
Figure 1. (a) Schematic depiction of the wet spinning process. (b) Procedure for creation of a capillary-like network in agarose gel.
theoretical diameter = 2(V/aπ)0.5
where V is extrusion rate of PMMA solution, and a is roller speed (take-up rate of microfibers) [Citation17].
Development of Capillary-like Network in Agarose Gel
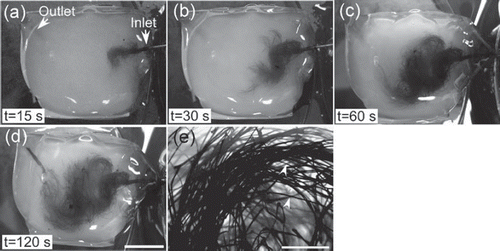
The procedure for creation of capillary-like network in agarose gel is shown schematically in (b). PMMA microfibers were collected from the roller and cut into pieces 15 cm long. To form fluidic inlet and outlet macrochannels, we prepared PMMA sticks 1 mm in diameter by injecting the PMMA solution in dichloromethane into a silicone tube, and evaporating the solvent. The 1-cm-long sticks were sprayed with a small amount of dichloromethane and welded to the ends of individual PMMA microfibers. The PMMA structure was immersed in 9 ml of 2% (w/v) agarose aqueous solution at 45°C (SeaPlaque® Agarose, Cambrex Bio Science Rockland, Inc., Rockland, ME, USA), and the solution cooled to 4°C to effect gelation. The agarose gel was immersed in ethanol-water solution and the concentration of ethanol in the solution was gradually increased from 70 to 100% (v/v) over 60 h. The resultant hydrogel was then immersed in dichloromethane for 72 h to dissolve the PMMA structure, leaving a capillary-like network inside the agarose matrix. Subsequently, the matrix was immersed in ethanol, and the ethanol concentration was gradually decreased from 100 to 70% (v/v) over 36 h. Finally, the matrix was immersed in distilled water for 12 h. The water was exchanged every 3 h to remove ethanol from the gel. The experiment was performed in duplicate.
RESULTS AND DISCUSSION
In our methodology for creating a capillary-like network in matrices of water-soluble polymers, the first aim was fabrication of water-insoluble microfibers equivalent to the diameter of native capillary vessels (5—20 μm in diameter) [Citation18]. Decreased diameter of fibers results in loss of their mechanical strength. Consequently, it was feared that such fine microfibers would be fractured during the process described in (b). To eliminate that potential obstacle we used PMMA as the microfiber material because PMMA has high stiffness. The wet spinning technique that was used to fabricate the fine PMMA microfibers had been employed previously to make microfibers of water-insoluble synthetic polymers [Citation19, Citation20]. However, to our best knowledge, there are no reports concerning fabrication of PMMA microfibers using the technique. Consequently, we investigated the effect of the take-up rate of microfibers on their diameter.
The take-up rate was varied from 2.3 to 31.0 cm s−1. At 2.3 cm s−1 the diameter of the PMMA microfibers was 56 ± 2 μm (), and the diameter decreased to 14 ± 4 μm with increase in the take-up rate to 31.0 cm s−1 (). These results show that fine PMMA microfibers with diameter comparable to the diameter of native capillary vessels can be obtained by wet spinning. At take-up rates greater than 31.0 cm s−1 the PMMA microfibers frequently broke and a continuous fiber could not be obtained.
Figure 2. Dependence of the diameter of PMMA microfibers on take-up rate. The data (actual diameter) represent mean values (n > 100) and the bars show standard deviations.
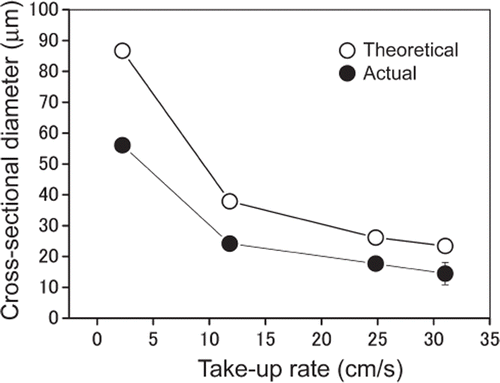
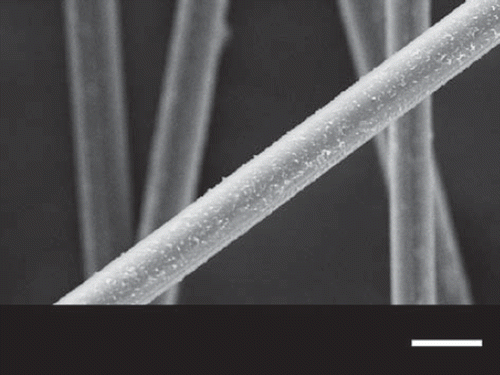
Figure 3. Scanning electron microscopic image of PMMA microfibers prepared with take-up rate 31.0 cm s−1. Scale bar: 20 μm.
The theoretical diameters at each take-up rate were 87 μm (at 2.3 cm s−1), 38 μm (at 11.8 cm s−1), 26 μm (at 24.8 cm s−1), and 23 μm (at 31.0 cm s−1). The shrinkage ratio (actual diameter/theoretical diameter) was almost constant (0.60—0.69), which is useful for prediction of the diameter of PMMA microfibers when the microfibers are fabricated at take-up rates different from those in this study. The shrinkage is due to elution of dichloromethane and coagulation of PMMA.
We confirmed (using optical microscopy) that there were no fragments of microfibers in the agarose gel after immersion in dichloromethane. To verify that an aqueous solution could be perfused in the microchannel network, India ink was injected into the agarose gel through the inlet macrochannel at flow rate 0.17 μl s−1. The ink flowed in the individual microchannels and out of the outlet macrochannel (). Examination using an optical microscope showed that there was no significant difference between the diameters of the PMMA microfibers (14 ± 4 μm) and the resultant microchannels (15 ± 5 μm). These results indicate that our methodology is promising for creating capillary-like networks in matrices of water-soluble polymers.
CONCLUSIONS
PMMA microfibers with diameters comparable to those of native capillary vessels were fabricated using wet spinning. Dissolution of the microfibers embedded in agarose gel resulted in the creation of a capillary-like network in the gel. From these results we conclude that our methodology is effective for creating capillary-like networks in tissue engineering scaffolds of water-soluble polymers.
Declaration of interest: This work was supported by a Grant-in-Aid for Young Scientists (B), 23760752, 2011, from the Ministry of Education, Culture, Sports, Science and Technology (MEXT) of Japan.
REFERENCES
- Shi, J., Votruba, A.R., Farokhzad, O.C., Langer, R. (2010). Nanotechnology in drug delivery and tissue engineering: From discovery to applications. Nano Lett. 10: 3223–30.
- Ko, I.K., Iwata, H. (2001). An approach to constructing three-dimensional tissue. Ann N Y Acad Sci. 944: 443–455.
- Takei, T., Sakai, S., Ono, T., Ijima, H., Kawakami, K. (2006). Fabrication of endothelialized tube in collagen gel as starting point for self-developing capillary-like network to construct three-dimensional organs in vitro. Biotechnol Bioeng. 95: 1–7.
- Takei, T., Sakai, S., Yokonuma, T., Ijima, H., Kawakami, K. (2007). Fabrication of artificial endothelialized tubes with predetermined three-dimensional configuration from flexible cell-enclosing alginate fibers. Biotechnol Prog. 23: 182–186.
- Ochoa, E.R., Vacanti, J.P. (2002). An overview of the pathology and approaches to tissue engineering. Ann N Y Acad Sci. 979: 10–26.
- Neumann, T., Nicholson, B.S., Sanders, J.E. (2003). Tissue engineering of perfused microvessels. Microvasc Res. 66: 59–67.
- Bettinger, C.J., Cyr, K.M., Matsumoto, A., Langer, R., Borenstein, J.T., Kaplan, D.L. (2007). Silk fibroin microfluidic devices. Adv Mater. 19: 2847–2850.
- Choi, N.W., Cabodi, M., Held, B., Gleghorn, J.P., Bonassar, L.J., Stroock, A.D. (2007). Microfluidic scaffolds for tissue engineering. Nat Mater. 6: 908–915.
- Bettinger, C.J. (2009). Synthesis and microfabrication of biomaterials for soft-tissue engineering. Pure Appl Chem. 81: 2183–2201.
- Cabodi, M., Choi, N.W., Gleghorn, J.P., Lee, C.S.D., Bonassar, L.J., Stroock, A.D. (2005). A microfluidic biomaterial. >J Am Chem Soc. 127: 13788–13789.
- Bettinger, C.J., Weinberg, E.J., Kulig, K.M., Vacanti, J.P., Wang, Y.D., Borenstein, J.T., Langer, R. (2006). Three-dimensional microfluidic tissue-engineering scaffolds using a flexible biodegradable polymer. Adv Mater. 18: 165–169.
- Huang, J.H., Kim, J., Agrawal, N., Sudarson, A.P., Maxim, J.E., Jayaraman, A., Ugaz, V.M. (2009). Rapid fabrication of bio-inspired 3D microfluidic vascular networks. Adv Mater. 21: 3567–3571.
- Bellan, L.M., Singh, S.P., Henderson, P.W., Porri, T.J., Craighead, H.G., Spector, J.A. (2009). Fabrication of an artificial 3-dimensional vascular network using sacrificial sugar structures. Soft Matter. 5: 1354–1357.
- Lee, K.Y., Mooney, D.J. (2001). Hydrogels for tissue engineering. Chem Rev. 101: 1869–1879.
- Lee, K.Y., Alsberg, E., Mooney, D.J. (2001). Degradable and injectable poly(aldehyde guluronate) hydrogels for bone tissue engineering. J Biomed Mater Res. 56: 228–233.
- Liu, Y., Chan-Park, M.B. (2009). Hydrogel based on interpenetrating polymer networks of dextran and gelatin for vascular tissue engineering. Biomaterials. 30: 196–207.
- Su, J., Zheng, Y.Z., Wu, H.K. (2009). Generation of alginate microfibers with a roller-assisted microfluidic system. Lab Chip. 9: 996–1001.
- Borenstein, J.T., Terai, H., King, K.R., Weinberg, E.J., Kaazempur-Mofrad, M.R., Vacanti, J.P. (2002). Microfabrication technology for vascularized tissue engineering. Biomed Microdevices. 4: 167–175.
- Gupta, B., Revagade, N., Anjum, N., Atthoff, B., Hilborn, J. (2006). Preparation of poly(lactic acid) fiber by dry-jet-wet spinning. II. Effect of process parameters on fiber properties. J Appl Polym Sci. 101: 3774–3780.
- Oh, S.C., Wang, Y.S., Yeo, Y.K. (1996). Modeling and simulation of the coagulation process of poly(acrylonitrile) wet-spinning. Ind Eng Chem Res. 35: 4796–4800.