Abstract
Abstract: A low-cost and sensitive amperometric biosensor was developed for the determination of α-amylase activity. The biosensor was constructed by immobilizing glucose oxidase–gelatin via glutaraldehyde on the Au electrode surface. Measurements were carried out chronoamperometrically at -0.7 V. Several parameters such as glucose oxidase activity, gelatin amount, and glutaraldehyde percentage for cross-linking were optimized. Optimum pH, optimum temperature, repeatability, and storage stabilities of the biosensor were identified. Under the optimum experimental conditions, a linear calibration curve was obtained for α-amylase between 0.819 and 13.110 U/ml. Sample analyses were carried out by detecting α-amylase activities in baker's yeast samples.
INTRODUCTION
Biosensors, one of the newer areas in analytical chemistry, are simple analysis methods with high reproducibility, sensitivity, short response time, and inexpensive fabrication. For 40 years, the deposition of biological macromolecules onto transducer surfaces has constituted a common task in the development of biosensors. A number of established immobilization procedures have been investigated, ranging from physio-adsorption to covalent attachment [Citation1–4]. α-amylases (EC. 3.2.1.1) catalyze the hydrolysis of starch, resulting in a significant release of maltotriose and maltose from amylose, or maltose, glucose and dextrin from amylopectin. α-amylases are extensively used in biotechnology and synthetic chemistry industries for starch degradation, oligosaccharide production, elimination of starch sizing from textiles, the liquefaction of starch, proper formation of dextrins in baking, and also used as a supplement of detergents [Citation5, Citation6]. Different methods have been described for α-amylase activity determination by using the Wohlgemuth method [Citation7], capillary isoelectric focusing [Citation8], ESR assay method [Citation9], flow injection analysis [Citation10–12], viscometric assay [Citation13, Citation14], DNS method [Citation15], sensitive agar diffusion method [Citation16], and polarographic method [Citation17]. Among the many methods developed for this purpose, amperometric biosensor is especially promising because of its low cost, simplicity, and high sensitivity [Citation18, Citation19]. Due to its high selectivity to glucose and high activity over a broad range of pH values, amperometric biosensors based on glucose oxidase (GOD) have been widely used in glucose biosensor construction [Citation20]. Sensitivity and stability of a glucose biosensor are the key features for its quantitative analysis applications [Citation21, Citation22].
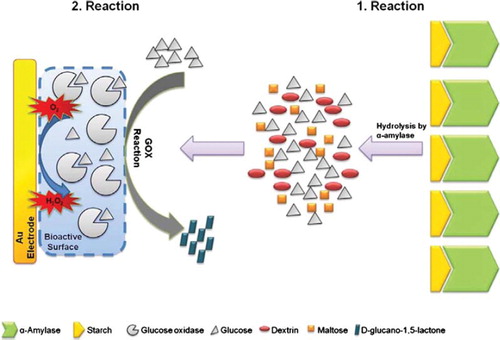
In this study, α-amylases digested starch into glucose units and glucose oxidase that was immobilized onto an Au electrode surface used these units as substrates afterwards. In the enzymatic reaction between glucose oxidase and glucose units, glucose oxidase uses dissolved oxygen existing in the reaction medium. Thus, the consumption of oxygen rate was proportional to the level of glucose produced from the hydrolysis of starch by α-amylase, and was directly related to the α-amylase activity.
EXPERIMENTAL
Chemicals
Glucose oxidase (50 U/mg, of Aspergillus niger origin), potassium dihydrogene phosphate (KH2PO4), citric acid (C6H8O7), acetic acid (CH3CO2H), sodium hydroxide (NaOH), sodium chloride (NaCl), bovine skin gelatin, sodium dodecyl sulfate (CH3(CH2)11OSO3Na), glycine and glutaraldehyde (25%) were purchased from Sigma (St. Louis, MO, USA). Starch (from potatoes) was purchased from Fluka (Switzerland) and α-amylase (65.55 U/mg, of Bacillus suptilis origin) was purchased from NOVO INDUSTRI AS (Copenhagen, Denmark). All reagents used were of analytical grades. All solutions were prepared with double distilled water just before their use.
Apparatus
In this study, all electrochemical experiments were carried out in a 20 ml thermostated glass cell. Cyclic voltammetric and chronoamperometric measurements were performed with an Ivium Compactstat potentiostat. A three-electrode electrochemical cell set-up was used in the experiments: a gold electrode (Metrohm, Switzerland) as the working electrode, an Ag/AgCl electrode (3 M KCl saturated with AgCl as an internal solution, Metrohm, Switzerland) was used as the reference electrode, and a platinum electrode (Metrohm, Switzerland) was used as the counter electrode. A water bath was used for the preparation of bioactive material (Nüve, BM 302, UK). All the measurements were carried out at constant temperature by using a thermostat (Haake JF, Germany). A magnetic stirrer (IKA-Combimag, RCO) and a pH meter with electrode (WTW, pH538, Germany) were used to prepare buffer solutions.
Preparation of the Biosensor
For the construction of the bioactive layer, the Au electrodes were polished on a piece of velvet with alumina slurry, washed thoroughly with distilled water, and then sonicated for 5 min in ethanol and distilled water, respectively. Five milligrams of glucose oxidase and five milligrams of gelatin were dissolved in 100 μL pH 7,5 50 mM phosphate buffer at 40°C temperature. 65 μL of the solution was dispersed on the Au electrode surface and allowed to dry for 30 minutes at 4°C. For cross-linking, electrode surface was immersed into glutaraldehyde solution (5% v/v) for 5 minutes. Then the electrode was rinsed with distilled water to eliminate the excess glutaraldehyde.
Measurement Procedure
The biosensor based on glucose oxidase was replaced into the reaction cell containing 20 ml pH 6.5 50 mM phosphate buffer including 5 mg/ml starch and the magnetic stirrer was fixed at a constant speed. The measurements were carried out at steady-state conditions. After obtaining the first steady-state signal approximately in five minutes, α-amylase was injected into the reaction cell. In the experiments, different volumes of α-amylase standard solution between 50 to 1000 μl were injected. Sensor responses were determined as the differences between the first and second steady-state chrono-amperometric signal (ΔI) that was obtained after the addition of α-amylase. Each measurement was completed in 900 seconds. In the reaction, α-amylase hydrolyzes the starch molecules which were present in the phosphate buffer (50 mM, pH 6.5). When α-amylase hydrolyzes starch molecules, dissolved oxygen concentration in the intermediate surface decreases by the reactsion of glucose and oxygen that was catalyzed by glucose oxidase immobilized on the Au electrode surface (). During our measurements, oxygen reduction was monitored at −700 mV versus Ag/AgCl reference electrode, chronoamperometrically. Each measurement has been conducted in triplicate sets.
RESULTS AND DISCUSSION
Optimization of the Bioactive Surface of the Biosensor
Optimization studies include development of the biosensor and detailed investigations of components of bioactive layer, namely the glucose oxidase activity, gelatin amount, and glutaraldehyde percentage.
Optimization of Glucose Oxidase Amount. In this part of the study, the effect of glucose oxidase activity on the biosensor response was investigated. Measurements were accomplished by using five different biosensors containing 1.25 mg, 2.5 mg, 5.0 mg, 7.5 mg, and 10.0 milligrams of glucose oxidase while gelatin amount and glutaraldehyde percentage were kept constant as 2.5 mg and 5%, respectively. Standard curves were obtained for α-amylase activities of each biosensor. The results revealed that enzyme activity affected the biosensor response. When we used 1.25 milligrams of glucose oxidase, low and incoherent signals were obtained from the biosensor. Therefore, the effect of 1.25 mg of glucose oxidase amount was disregarded. Experiments showed that optimum biosensor response was obtained with 2.5 milligrams of glucose oxidase. Glucose oxidase amounts, linear ranges and R2 values of the different biosensors are given in .
Table 1. The effects of glucose oxidase amount, gelatin amount, and glutaraldehyde percentage on the biosensor response
Optimization of Gelatin Amount. In order to reveal optimum immobilization conditions for the biosensor, the effect of gelatin amount in the bioactive layer was investigated. Three different biosensors were prepared containing 2.5, 5.0 and 7.5 milligrams of gelatin. 2.5 and 5.0 milligrams of gelatin amounts led to higher biosensor responses than the response obtained from 7.5 mg of gelatin; however, the increase in the signals did not have a correlation with the increased amount of gelatin. Moreover, low amounts of gelatin caused an unstable bioactive surface. On the other hand, both the best gelatinization of the bioactive layer and the maximum biosensor response were obtained using 7.5 mg of gelatin. Thus, 7.5 mg of gelatin was chosen as the optimum gelatin amount. The relation between gelatin amount and biosensor response is shown at .
Effect of Glutaraldehyde Percentage on the Biosensor Response. The effect of the cross-linking agent, glutaraldehyde, on the biosensor response was investigated. For this purpose, the amounts of glucose oxidase and gelatin were kept constant as 2.5 and 7.5 mg, respectively, and each of the newly prepared biosensors was treated with 1.25, 2.5, and 5.0 % glutaraldehyde for 5 minutes. The relationship between biosensor response and glutaraldehyde percentage is indicated in . From the table, it can be determined that the most suitable and best linear range was obtained with the biosensor, which contained 5.0% glutaraldehyde. Lower percentages of glutaraldehyde led to less cross-linking, so they can permit enzyme or substrate to escape from the bioactive layer. Using higher percentages than 5.0% of glutaraldehyde results in the formation of tighter bioactive layer, thus diffusion of glucose monomers from reaction medium to bioactive layer gets more difficult. As a consequence, when the results are compared, the best detection limit and linear range were obtained when 5.0% glutaraldehyde was used in the biosensor construction.
Optimization of Working Conditions
pH Effect on Biosensor Response. pH of medium has an important role in biosensor studies for obtaining the best biosensor response. In order to determine the effect of pH value on the biosensor response, different buffer systems were investigated. Phosphate buffers containing 10 mg/ml starch were used in the experiments and all of the buffers had a concentration of 50 mM. From the experiments, the optimum pH value was found to be 6.5 (). The response at pH 6.5 was set as 100%. The oxygen consumption rates were 97.98% and 95.51% at pH 6.4 and 6.6, respectively. Below and above pH 6.5, decreases were observed in the biosensor response. Therefore, optimum pH was accepted as 6.5. shows optimum pH graph.
Figure 1. The effect of pH on the biosensor response. [Working conditions: phosphate buffers, 50 mM and pH 6.0, 6.2, 6.4, 6.5, 6.6, 6.8, 7.0. T = 35°C. 5 mg/ml starch solution and 6.555 U/ml standard solution of α-amylase was used. The amount of glucose oxidase and gelatin was kept constant at 5 mg and glutaraldehyde percentage was %2.5.]
![Figure 1. The effect of pH on the biosensor response. [Working conditions: phosphate buffers, 50 mM and pH 6.0, 6.2, 6.4, 6.5, 6.6, 6.8, 7.0. T = 35°C. 5 mg/ml starch solution and 6.555 U/ml standard solution of α-amylase was used. The amount of glucose oxidase and gelatin was kept constant at 5 mg and glutaraldehyde percentage was %2.5.]](/cms/asset/a860d42d-cc15-47c6-a09a-2404c9dbcc01/ianb19_a_597757_f0002_b.gif)
Effect of the Temperature on the Biosensor Response. To determine the influence of temperature on the response of the biosensor, experiments were carried out between 25 and 50°C under the best working conditions obtained from the optimization studies. Results are given in . The results showed that the best biosensor response was obtained at 35°C. Below and above this temperature, decreases in the biosensor responses were recorded.
Figure 2. The effect of temperature on the biosensor response. [Working conditions: Phosphate buffer; pH 6.5, 50 mM; 5 mg/ml starch solution and 6.555 U/ml standard solution of α-amylase was used. Amount of glucose oxidase, amount of gelatin and glutaraldehyde percentage was kept constant as 2.5 mg, 7.5 mg, and 5%, respectively.]
![Figure 2. The effect of temperature on the biosensor response. [Working conditions: Phosphate buffer; pH 6.5, 50 mM; 5 mg/ml starch solution and 6.555 U/ml standard solution of α-amylase was used. Amount of glucose oxidase, amount of gelatin and glutaraldehyde percentage was kept constant as 2.5 mg, 7.5 mg, and 5%, respectively.]](/cms/asset/1f867c71-f6a7-4653-999a-74cd2ff1a61c/ianb19_a_597757_f0003_b.gif)
Characterization Studies of the Biosensor
Linear Range of the Biosensor. Regarding the α-amylase activity, the linear standard curve between 0.819 – 13.110 U/ml was obtained under the optimum experimental conditions. The linear graph was defined by the equation of y = 58.515x – 20.217 and R2 = 0.9952, where y represents ΔI (nA) and x represents α-amylase activity (U/ml). The results acquired for the determination of detection limits for α-amylase activity are given in .
Figure 3. Linear range of the biosensor. [Working conditions: Phosphate buffer; pH 6.5, 50 mM; T = 35°C; 5 mg/ml starch solution and α-amylase activity between 0.819-13.110 U/ml. Amount of glucose oxidase, amount of gelatin and glutaraldehyde percentage was kept constant as 2.5 mg, 7.5 mg, and 5%, respectively.]
![Figure 3. Linear range of the biosensor. [Working conditions: Phosphate buffer; pH 6.5, 50 mM; T = 35°C; 5 mg/ml starch solution and α-amylase activity between 0.819-13.110 U/ml. Amount of glucose oxidase, amount of gelatin and glutaraldehyde percentage was kept constant as 2.5 mg, 7.5 mg, and 5%, respectively.]](/cms/asset/1f6081a5-86ef-4695-917d-184cc54b85a2/ianb19_a_597757_f0004_b.gif)
The detection limit of the biosensor was obtained as 1.695 3 10−3 U/ml. At concentrations higher than 13.110 U/ml, the standard curve showed a deviation from linearity.
Repeatability. Repeatability is another important parameter for the evaluation of the biosensor. For the determination of the repeatability of the biosensor, repetitive injections with 6.555 U/ml α-amylase activity standards were made. According to the results of 10 replicates of trials, the average value (x), the standard deviation (S.D.) and variation coefficient (C.V.) were calculated as 6.558 U/ml, ± 0.250 U/ml and 3.812%, respectively.
Storage Stability. The aim of storage stability experiments was to collect evidence about the performance of the biosensor based on glucose oxidase for α-amylase activity, which could be affected by the environmental factors such as temperature and humidity. Experiments were carried out periodically every 7 days for a 15-day storage period by detecting the decreases in the biosensor response. When not in use, the biosensor was stored in a flask containing phosphate buffer (pH 6.5, 50 mM) at 4°C. During the storage period, the biosensor preserved about 23% of its initial activity.
Sample Analysis. To determine α-amylase activity in baker's yeast samples, the developed glucose oxidase immobilized amperometric biosensor was used. For this purpose, 2.5 grams of baker's yeast samples were dissolved in double distilled water. For the liberation of α-amylase enzymes from yeast cells, the mixtures were treated with ultrasonicator. Experiments were carried out by applying the prepared sample to the reaction cell containing phosphate buffer (pH 6.5, 50 mM) that includes starch molecules (5 mg/ml concentration) instead of α-amylase enzyme solution. α-amylase activity was calculated and the results were compared with those obtained with a reference method that is called the DNS method [Citation16]. The comparison of the results is shown in . The given data was the average of three replicates of trials ± S.D. and high compatibility between the results of the biosensor and the reference method was observed.
Table 2. Results for determinations of α-amylase activity in baker's yeast samples
CONCLUSION
In this study, we presented a novel α-amylase activity determination method by constructing an amperometric glucose oxidase based biosensor. Certain amounts of glucose oxidase and gelatin mixture were immobilized on Au electrode and glutaraldehyde was used as a cross-linking agent. The response time of the biosensor was 15 minutes. This study can be an alternative method among the other α-amylase activity determination methods. The improved biosensor does not need any expensive equipment or material. Measurement method is rapid, simple, reliable, sensitive, and not time-consuming.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
REFERENCES
- Tuner, A.P.F., Karube, I., Wilson, G.S. (1987). Biosensors: Fundamentals and Applications. New York: Oxford University Press.
- Kauffmann, J. M., Guilbault, G. G. (1992). Bioanalytical Applications of Enzymes, Vol. 36. New York: Wiley.
- Eggins, B. R. (1996). Biosensors: An Introduction. New York: Wiley.
- Gass, A. E. G. (1990). Biosensors: A Practical Approach. New York: Oxford University Press.
- Terashima, M., Katoh, S. (1996). Modification of alpha-amylase functions by protein engineering. Ann Ny Acad Sci, 799: 65–69.
- Shaw, J. F., Lin, F. P., Chen, S. C., Chen, H. C. (1995). Purification and properties of an extracellular α-amylase from Thermus sp. Bot Bull Acad Sin, 36: 195–200.
- Wohlgemuth, J. (1908). Über eine neue methode zur quantitativen bestimmung des diastatischen ferments. Biochem Z, 9: 1–9.
- Watanabe, T., Yamamoto, A., Nagai, S., Terabe, S. (1998). A novel method for measurement of > -amylase activity in rice koji by capillary isoelectric focusing. J Ferment Bioeng, 85: 451–453.
- Marcazzan, M., Vianello, F., Scarpa, M., Rigo, A. (1999). An ESR assay for α-amylase activity toward succinylated starch, amylose and amylopectin. J Biochem Bioph Meth, 38: 191–1202.
- Schindler, R., Lendl, B., Kellner, R. (1998). Simultaneous determination of α-amylase and amyloglucosidase activities using flow injection analysis with fourier transform infrared spectroscopic detection and partial least-squares data treatment. Anal Chim Acta, 366: 35–43.
- Zajoncová, L., Jilek, M., Beranová, V., Peč, P. (2004). A biosensor for the determination of amylase activity. Biosens Bioelectron, 20: 240–245.
- Van Staden, J.F., Mulaudzi, L. V. (2000). Flow injection spectrophotometric assay of α-amylase activity. Anal Chim Acta, 421: 19–25.
- González, C. F., Fariña, J. I., Figueroa, L. I. C. (2002). A critical assessment of a viscometric assay for measuring Saccharomycopsis fibuligera α-amylase activity on gelatinised cassava starch. Enzyme Microb Tech, 30: 169–175.
- Singh, S., Gupta, A. K., Gupta, S. K., Kaur, N. (2010). Effect of sowing time on protein quality and starch pasting characteristics in wheat (Triticumaestivum L.) genotypes grown under irrigated and rain-fed conditions. Food Chem, 122: 559–565.
- Miller, G. L. (1959). Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal Chem, 31: 426–428.
- Farias, D. F., Carvalho, A. F. U., Oliveira, C. C., Sousa, N. M., Rocha-Bezerra, L. C. B., Ferreira, P. M. P., Lima, G. P. G., Hissa, D. C. (2010). Alternative method for quantification of alfa-amylase activity. Braz J Biol, 70: 405–407.
- Strand, G., Renneberg, R., Scheller, F. (1985). Polarographic determination of α-amylase activity. J Electroanal Chem Interfac, 194: 123–130.
- Qian, J. M., Suo, A. L., Yao, Y., Jin, Z. H. (2004). Polyelectrolyte-stabilized glucose biosensor based on wood ceramics as electrode. Clin Biochem, 37: 155–161.
- Luong, J. H. T., Male, K. B., Glennon, J. D. (2008). Biosensor technology: Technology push versus market pull. Biotech Adv, 26: 492–500.
- White, B. J., Harmon, H. J. (2002). Novel optical solid-state glucose sensor using immobilized glucose oxidase. Biochem Bophys Res Commun, 296: 1096–1071.
- Gavalas, V. G., Chaniotakis, N.A. (2000). Polyelectrolyte stabilized oxidase based biosensors: Effect of diethylaminoethyl-dextran on the stabilization of glucose and lactate oxidases into porous conductive carbon. Anal Chim Acta, 404: 67–73.
- Delvalux, M., Demoustier-Champagne, S. (2003). Immobilisation of glucose oxidase within metallic nanotubes arrays for application to enzyme biosensors. Biosens Bioelectron, 18: 943–951.