Abstract
Abstract: To explore the biocompatibility of acellular nerves of different mammalian species, for the acellular nerves derived from rats and rabbits, the morphology, immunocompatibility, and cytocompatibility with bone marrow stromal cells (BMSCs) were evaluated. The results indicated that the tridimensional architecture and main proteins of endoneurial tubes in both biomaterials were well retained. The nerve scaffolds did not show immunogenicity or cytotoxicity, but facilitated growth of BMSCs and secretion of neurotrophic factors in vitro. In conclusion, acellular nerves of different species possess favorable biocompatibility, and xenogenic acellular nerves combined with BMSCs have potential to replace allografts for peripheral nerve reconstruction.
INTRODUCTION
The treatment of peripheral nerve injuries with long gaps usually involves nerve grafting. For autologous and allogenic nerve transplantation, however, problems caused by the restricted source of donator and denervation of donor site are difficult to avoid [Citation1]. With the development of nerve tissue engineering, research has been conducted on the development of natural or artificial biomaterials. By integrating with seed cells, the constructed grafts can be utilized to effectively bridge and repair neurologic defects [Citation2–4].
Satisfactory nerve grafts should possess excellent biocompatibility. Accordingly, great interest has recently been triggered on the related systematic estimation via cytology, histology, as well as zoology [Citation5]. It has been proved that the internal structure and the compositions of the extracellular matrix (ECM) in nerve grafts play an important role in facilitating cell migration and axon elongation [Citation6,Citation7]. Sondell et al. [Citation8] fabricated acellular nerve allografts (ANA) by employing chemical detergents including Triton X-100 and sodium deoxycholate. With this method, the cells of peripheral nerves were removed and the basilar membrane of Schwann cells was preserved. As bioactive alternatives of nerves, acellular nerves consist of biogenic tissue proteins and possess the structural and biochemical features similar to those of naive nerves. After allogeneil graft, acellular nerves are capable of impelling cells to adhesion, migration, proliferation, and producing marked biological effects [Citation9–11], thus the microenvironment of neural regeneration can be optimized. In addition, the source of allogenic nerves is relatively extensive, and it is available to obtain the nerve segments of various sizes. In particular, the application of xenogenic nerves greatly increases the source of grafts; thus it has a promising prospect for the clinical treatment. To the authors’ knowledge, so far the investigation on the biocompatibility of acellular nerves of different mammalian species and the application of acellular nerve xenografts (ANX) for repairing neural injuries are very limited.
Schwann cells, as structural and functioning cells in peripheral nerves, play an important role in neural regeneration [Citation12]. However, because of the limited source of cell donor and cell population as well as the time-consuming amplification of cells in vitro, there are still some obstacles in the clinical application of Schwann cells. Eligible seed cells are suggested to exhibit characteristics such as easy accessibility, rapid proliferation, immunological compatibility, and enduring survival. BMSCs are attractive adult stem cells, since they can be drawn from bone marrow readily, and are characterized by multi-directional differentiation, fast multiplication, low immunogenicity, and secretion of neurotrophic factors. In nerve tissue engineering, numerous studies have successfully repaired central and peripheral neural injuries by using BMSCs as the seed cells [Citation13–16].
In this study, the acellular nerves of different mammalian species were prepared through chemically extracted protocol, and then the tissue-engineered transplants were constructed by the combination of acellular nerve scaffolds and BMSCs. For both acellular nerves the morphology was assayed, and the immunocompatibility as well as the cytocompatibility with BMSCs were evaluated.
MATERIALS AND METHODS
Animals
Sixteen healthy Wistar rats and ten Sprague-Dawley (SD) rats of either gender, weighing 200-250 g, and 6 New Zealand rabbits weighing 3 kg were provided by the Experimental Animal Center of China Medical University [Certification No. SCXK (Liao) 2008-0005]. All the experimental animals were housed under standard conditions, and the empirical procedures were approved by the Experimental Animal Administration and Ethics Committee of China Medical University. Animals were anesthetized i.p. with 100 g/l chloral hydrate (350 mg/kg weight) during the surgical proceedings.
Preparation of ANA and ANX
Under aseptic condition, bilateral sciatic nerves of anesthetized SD rats were exposed and the nerve segments were excised to prepare ANA. Meanwhile, the segments of ulnar nerves, which served as ANX, were harvested from rabbits. Upon harvest, the connective tissue of the nerves was removed, and then the nerves were immediately placed in sterilized distilled water and cut into 1-cm segments. Acellular nerves were prepared via chemically extracted processing, and all the subsequent steps were conducted based on the previously developed protocol [Citation8]. Briefly, nerve tissues were immersed in deionized distilled water (ddH2O) for 12 h, and then exposed to 4% Triton X-100 (Sigma, US) in ddH2O and digested for 12 h. After being rinsed in ddH2O for 3 h, the nerves were agitated in 3% sodium deoxycholate (Sigma, USA) in ddH2O for 12 h. The above-mentioned procedures were then repeated, and followed by a final wash in ddH2O overnight. All the treatments were carried out at room temperature. The prepared tissues were stored in 10 mM PBS buffer (pH 7.4) containing penicillin and gentamycin sulfate at 4 °C.
Morphologic Observation and Immunohistochemical Analysis of Acellular Nerves
The acellular nerves were fixed in 4% paraformaldehyde and embedded in paraffin. Cross-sections of the tissues (5 μm) were cut with a microtome and mounted on gelatin pre-coated slides. The sections were then stained with chromotropic acid 2R-brilliant green and images were acquired by using a light microscope (BX51, Olympus, Japan).
For further ultrastructural analysis, the acellular nerves were immersed for 24 h in 2.5% glutaraldehyde in 0.1 M PBS buffer (pH 7.4), washed in the same buffer followed by 0.1 M cacodylate buffer (pH 7.4), post-fixed for 2 h in 4% osmium tetroxide containing 0.8% potassium ferrocyanide and 5 nM calcium chloride in 0.1 M cacodylate buffer (pH 7.4), dehydrated in a graded series of ethanol solutions, dried at a critical point drier (Hitachi, Japan), and then gold-coated. The specimens were subsequently observed using the scanning electron microscope (SEM, JSM-T300, Japan) with an accelerating voltage of 5 kV.
According to the Streptavidin-Biotin Complex method, paraffin sections were incubated in a solution of 3% H2O2 for 10 min at room temperature, then washed three times in PBS. The sections were blocked with 5% BSA for 30 min at 37 °C in a humidified chamber followed by an overnight incubation in the primary antibody at 4 °C. The working concentration of rabbit anti-laminin polyclonal antibody or mouse anti-S100 monoclonal antibody (Sigma, USA) were 1:200 and 1:400, respectively. Phosphate buffered solution was used as the negative control with the replacement of the primary antibody. The slides were washed three times in PBS, and then incubated in biotin labeled goat anti-rabbit or mouse IgG (Zhongshan, China) for 30 min at 37 °C. After being washed in PBS and stained with 3, 3’-diaminobenzidine, the sections were observed under a light microscope.
Subcutaneous Implantation Assay
Under anesthesia, ANA and ANX were placed in the subcutaneous pockets on the back of Wistar rats. Each animal received two grafts, which were explanted simultaneously at different time points, and whereafter the rats were sacrificed. At the seventh and fourteenth postoperative day, the grafts together with the surrounding tissues were taken out and fixed by 4% paraformaldehyde, and then embedded in paraffin. Cross-sections of tissues (5 μm) were cut with a microtome and captured on glass slides. The tissue from each nerve segment was evaluated via H&E and immunohistochemical staining. Rabbit anti-CD3 polyclonal antibodies (Boster, China) were diluted up to 1:100 as the primary antibody, then the sections were incubated in FITC labeled goat anti-rabbit secondary antibody (Zhongshan, China) for 30 min at 37 °C. After washing the sections three times in PBS, images were acquired from a CCD camera connected with a fluorescence microscope (TS100, Nikon, Japan). Phosphate buffered solution was used as the negative control with the replacement of the primary antibody.
Culture and Differentiation Characterization of BMSCs
Adult SD rats were sacrificed by cervical dislocation, and BMSCs were isolated from bilateral femurs. Briefly, by using a 5 ml syringe filled with culture medium, bone marrow was flushed out of the femur via several flushes through the bone. After centrifugation for 10 min at 1000 rpm at 4 °C, the supernatant was discarded, and the cells were then resuspended in Dulbecco's modified Eagle's medium (DMEM) containing 10% fetal bovine serum (FBS, Gibco, USA) and 1% penicillin/streptomycin. The cultures were incubated at 37 °C with 5% CO2 under fully humidified condition, and the non-adherent cells were removed after 48 h. Thereafter the fresh culture medium was replaced every three days. When the cells reached 80-85% confluence, the cultures were treated with Trypsin-EDTA solution (0.25% trypsin, 0.02% EDTA, Sigma, USA), and then harvested and diluted to 1:2 per passage for the further expansion.
To demonstrate the differentiation potentials of mesodermal stem cells, BMSCs at passage 4 were seeded in six-well plates at a density of 1 × 105 cells/ml and cultured in complete medium up to 80% confluence. The medium was then replaced with osteogenic medium (DMEM supplemented with 10% FBS, 0.1 μM dexamethason, 10 mM β-glycerol phosphate and 50 μM ascorbate) or adipogenic medium (DMEM supplemented with 10% FBS, 1 μM dexamethason, 5 μg/ml insulin, 0.5 mM isobutylmethylxanthine and 60 μM indomethacin), and cultured for 3 weeks. For the differentiation cultures, calcium deposits and the production of intracellular lipid droplets were detected via alizarin red and Oil Red O staining, respectively.
Indirect Cytotoxicity Evaluation
A MTT test was performed to indirectly assess the toxicity of acellular nerves to BMSCs. According to the standard formulated by ISO10993, 0.6 g samples were placed in 6 ml complete medium and incubated at 37 °C for 72 h to prepare the leaching liquor of the nerve grafts. Meanwhile, 1 × 105/ml single BMSC suspension was added into 96-well plates and allowed to adhere and grow within 24 h. Afterwards the cells were cultured with the leaching liquor for 1, 3, 5 and 7 days, respectively, and then the activity of BMSCs was tested. Briefly, the culture medium in each well of the plates was replaced with 180 μl DMEM and 20 μl MTT (5 mg/ml). After 4 h incubation at 37 °C, the supernatant was discarded and the pellet was dissolved by adding 150 μl DMSO. Following 10 min shaking, the absorbance was detected by a spectrophotometer (ElX-800, Bio-Tek, USA) at 490 nm. BMSCs were cultured in a common complete medium as the negative control. Cell relative growth rate (RGR) was calculated as follows: RGR = (absorbance value of experimental group − absorbance value of blank control group)/(absorbance value of negative control group − absorbance value of blank control group). Cytotoxicity of ANA and ANX was classified in accordance with the standard of United States Pharmacopeia (USP).
Construction of BMSCs-laden Nerve Grafts In Vitro
BMSCs at passage 4 were utilized for allogenic seeding. Single cell suspension was prepared at a concentration of 2 × 107 cells/ml, and the acellular nerves were pre-incubated in complete medium at 37 °C for 3 h. A total volume of 30 μl cell suspension was injected using a microinjector under a dissecting microscope, resulting in a loading of 6 × 105 cells per graft. To perform the injection, the microinjector was inserted through the full length of nerve segments, and the cells were injected in equal volumes at four evenly spaced points as the injector was withdrawn. The nerve grafts implanted with BMSCs were then immersed in DMEM supplemented with 10% FBS and incubated at 37 °C with 5% CO2 under fully humidified condition.
After 5 days culture, the BMSCs-laden nerve scaffolds were prepared as the specimens for ultrastructural study with SEM. Additionally, the expression of neurotrophic factors secreted by BMSCs in the nerve grafts was detected. The tissues were routinely embedded in paraffin and cut into 5 μm cross-sections for immunostaining. The working concentration of either rabbit anti-nerve growth factor (NGF) or brain-derived neurotrophic factor (BDNF) polyclonal antibody was 1:100, and the concentration of FITC labeled goat anti-rabbit secondary antibody was 1:100. Then, the sections were observed under a fluorescence microscope.
The gene expression of NGF and BDNF was also determined by applying the quantitative real-time PCR. Primers were designed and synthesized by TaKaRa Bio Inc. (China), and the oligonucleotide sequences of primers are detailed in . The specimens were loaded into 1 ml Trizol reagent (Invitrogen, US) and then homogenated. Total RNA was extracted according to the Trizol extraction protocol. The RNA concentration was determined via the measurement of absorbance at 260 and 280 nm using a UV-spectrometer (UV-2800AH, UNICO, US). Purified RNA was diluted up to 500 ng/μl and 3 μl RNA was utilized to synthesize cDNA with PrimeScript® RT reagent kit (TaKaRa, China). Briefly, the reaction mix was composed of 1.5 μl 50 μM Oligo dT Primer, 6 μl 5 × PrimeScript® Buffer, 1.5 μl PrimeScript® RT Enzyme Mix Ⅰ, 1.5 μl 100 μM Random 6 mers, 3 μl extracted total RNA and 16.5 μl RNase free ddH2O. The mixture was incubated at 37 °C for 45 min, then incubated at 85 °C for 5 sec for reaction termination. After that, an incubation at 4 °C for 7 min was performed. Real-time PCR was performed with SYBR® Premix Ex Taq™ kit (TaKaRa, China) following the manufacturer's instruction. The reactions were carried out in a 20 μl reaction volume containing 2 μl 1:10 diluted original cDNA, 10 μl 2 × SYBR® Premix Ex Taq™, 0.4 μl forward and reverse primers (10 μM), 0.4 μl 50 × ROX Reference Dye Ⅱ and 6.8 μl ddH2O. Using qPCR system (Prism 7000, ABI, USA), cycling parameters were set as follows: initial denaturation at 95 °C for 30 sec, followed by 40 cycles at 95 °C for 5 sec and 60 °C for 30 sec, and final melting curve analysis for distinguishing the main PCR products from primer-dimers. Each real-time PCR reaction was conducted in triplicate to evaluate the data reproducibility. The cycle number where the amplification curve crossed the threshold line was denoted as critical threshold (CT) and the gene expression was calculated by the comparative CT (2-ΔΔCT) method. β-actin was used as the control housekeeping gene for the normalization of the amount of RNA added to the reverse transcription reactions.
Table 1. Oligonucleotide sequences and product sizes of primers.
Statistical Analysis
To determine the significant difference among groups, the data were analyzed with SPSS 13.0 software using analysis of variance followed by the Student's t-test for the data of normal distribution, otherwise the Kruskal-Wallis H test was used. Results are expressed as mean ± SD, and p values < 0.05 were considered statistically significant.
RESULTS
Structure and Components of Acellular Nerves
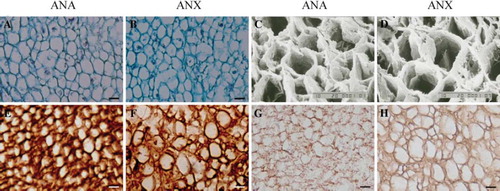
Following decellularization, histomorphological observation of ANA and ANX was performed. For both acellular nerves, the figures of chromotropic acid 2R-brilliant green staining indicated that the endoneurial tubes were hollow and the structural integrity of the basal lamina tubes was well retained. There was little red residual debris of myelin sheath in the remaining vessels of basilar membrane (). Ultrastructural analysis via SEM further demonstrated that cells, axons, and myelin sheath were absent in both of the extracted nerves. The orderly arranged basal lamina tubes remained, presented as the mesh-like structure in transections (). Immunostaining images confirmed that laminin, which was one of the main components in ECM, has been largely preserved, while S100-positive Schwann cells almost disappeared in both ANA and ANX ().
Figure 1. The structure and components of acellular nerves. In the cross-sections of ANA and ANX, chromotropic acid 2R-brilliant green staining showed that the endoneurial tubes were apparently hollow and the structural integrity of the basal lamina tubes was well retained. There was little red residual debris of myelin sheath in the remaining vessels of basilar membrane (A, B). SEM further revealed that the cells, axons, and myelin sheath were absent, and the orderly arranged basal lamina tubes were remained, as presented as the mesh-like structure in the transections (C, D). Immunostaining confi rmed that laminin was largely preserved (E, F), while S100-positive Schwann cells were completely eliminated in both biomaterials (G, H). Scale bar = 10 μm.
Immunological Reaction of Subcutaneous Implant ation
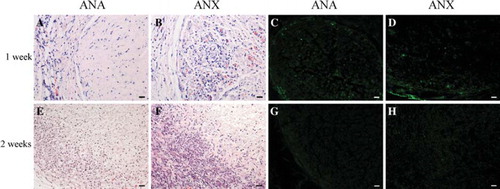
At the time points of 1 and 2 weeks post-implantation, the wounds of either ANA group or ANX group did not exhibit swelling, effusion, and inflammatory reaction. While the implants and the surrounding tissues were harvested en bloc for H&E staining and immunohistochemical analysis, it was observed that there was minimal adherence and the morphology of the implanted nerves was intact without edema. After 1 week, for both groups, there was a large amount of fibroblasts, neutrophils, and neogenetic blood capillaries surrounding the implants, whereas only very few lymphocytes were observed. The epineurium was partially absorbed and the formative granulation tissue appeared maturating (). A minority of CD3-positive T lymphocytes appeared in the grafts and host tissues (), while CD4-positive T lymphocytes were not detected (data not shown). After 2 weeks, the epineurium of both implants was absorbed evidently, and the endoneurium and collagen fibers remained. A large number of fibroblasts and vascular endothelial cells invaded to grow at the interface between the implants and host tissues, with continuous blood vessels being formed. Meanwhile, neutrophils decreased on a large scale (). CD3 expression of the infiltrated T lymphocytes was reduced significantly (Figure 2G, H), and CD4-positive T lymphocytes were still absent. In vivo, the extent of tissue response to the implanted ANX appeared similar to that of ANA at each time point.
Figure 2. Immunological reaction elicited by ANA or ANX following subcutaneous implantation. H&E staining at 1 and 2 weeks post-implantation (A, B, E, and F). Immunostaining showed that a minority of CD3-positive T lymphocytes infiltrated in the grafts and host tissues after 1 week (C, D), and CD3 expression of the infiltrated T lymphocytes were reduced markedly after 2 weeks (G, H). Scale bar = 20 μm.
Culture and Differentiation Characteristics of BMSCs
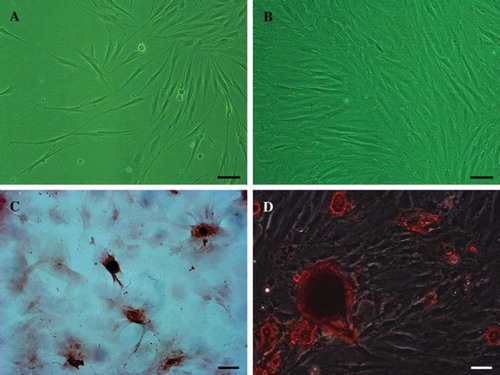
As the cell suspension was seeded in culture flasks, many rounded cells adhered to the bottom of flasks after 24 h. BMSCs were typically isolated from hematopoietic cells and other cells by their adherence to the plastic. Following the subsequent medium changes, the non-adherent cells were removed and most of the adherent cells displayed fusiform shapes. These cells began to proliferate at the third day and gradually grew to form small colonies. By 7-10 days in culture, the number of cellular colonies of various sizes obviously increased; most cells in the colonies grew and exhibited a spindle-shaped morphology (). Shortly, a morphologically homogeneous population of fibroblast-like adherent cells with the colonies expanded and reached 80-85% confluence (). Following more than twice subcultures, BMSCs proliferated abundantly, and tended to be sufficient and vigorous for subsequent cell seeding. After 3 weeks of induction in vitro by using osteogenic and adipogenic medium, respectively, red calcium deposits appeared in the cytoplasm via alizarin red staining (), and cells presented lipid-laden adipocyte phenotype via Oil Red O staining (). Thus, BMSCs exhibited osteogenic and adipogenic potentials, indicative of the differentiation features of mesodermal stem cells.
Figure 3. Morphological characteristics and mesodermal differentiation of the cultured BMSCs. The primary BMSCs exhibited spindle-shaped morphology at day 7 and morphological homogenicity when cells reached 85% confluence at day 12 (A, B, phase contrast). After differentiation in vitro for 3 weeks, calcium deposits in the cytoplasm were detected via alizarin red staining © and intracellular lipid globules were visualized via Oil Red O staining (D). Scale bar = 20 μm (A, B), 50 μm (C, D).
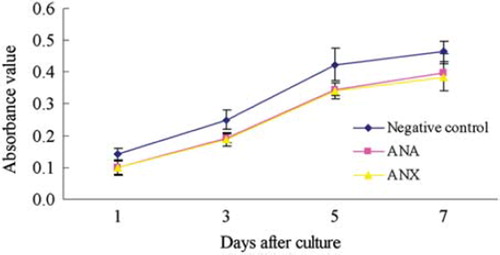
Assessment of Indirect Cytotoxicity
The activity of BMSCs cultured with acellular nerve extracts after 1, 3, 5, and 7 days was assayed by MTT test, respectively. At each time point, there was no significant difference in the mean absorbance values between the two biomaterial groups, and the absorbance values increased with increasing incubation time (). The cytotoxicity of ANX was comparable to that of ANA. In addition, the RGR of ANA was 70%, 77%, 81%, and 86% after 1, 3, 5, and 7 days, respectively. The RGR of ANX was 69%, 76%, 80%, and 83%, respectively. Aggregate analysis revealed that the grade of cytotoxicity for both acellular nerves was assessed as one. Both ANA and ANX processed by chemical extraction exhibited qualified responses and showed no toxicity to BMSCs.
Construction of BMSCs-laden Nerve Grafts
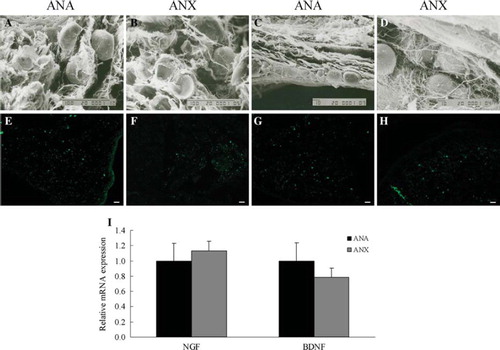
The BMSCs-laden ANA and ANX were allowed to be cultured in vitro for 5 days; thereafter the morphology and attachment of the cells growing in the nerve scaffolds were appraised. Scanning electron micrographs of the cross and longitudinal sections showed that BMSCs were uniformly distributed in the lumens of endoneurial tubes, adhering and growing in oval shapes, and the filiform fibers and spherical particles appeared on the cell surfaces. BMSCs exhibited the semblable performance in both grafts (). Immunostaining confirmed the stable expression of neurotrophic factors including NGF and BDNF, which were secreted by the implanted BMSCs in the constructed nerve grafts (). In addition, the expression of mRNA detected by real-time PCR was consistent with that of the proteins (). It was manifested that in vitro either ANA or ANX resembled each other in the levels of the neurotrophic factors secreted by BMSCs.
Figure 5. SEM observation of the cross- and longitudinal sections of BMSCs-laden nerve scaffolds after 5 days culture in vitro (A-D). NGF (E, F) and BDNF (G, H) expression in the constructed nerve grafts by immunostaining. The mRNA expression of the neurotrophic factors determined by real-time PCR (I). β-actin was used as the control housekeeping gene and the mRNA expression was calculated by the 2-ΔΔCT method (mean ± SD, n = 4). The relative mRNA levels were represented as the ratios by comparing the expression of each group with that of the ANA group (designated as 1). Scale bar = 100 μm (A, B), 10 μm (C, D), 20 μm (E–H).
DISCUSSION
In clinical repair of the peripheral nerve lesion, the most essential issue is to search the nerve grafts with favorable bionic property and biocompatibility, so as to provide a suitable microenvironment for nerve regeneration. Currently, a majority of research has focused on the construction of tissue-engineered nerve grafts. These biomaterials organically comprise bridging scaffolds, seed cells, and growth factors [Citation5,Citation17–19]. Previous studies showed that acellular nerve grafts possessed a natural tubular frame and components of ECM, and the allografts could repair peripheral nerve defects to some extent [Citation20–22]. Heterogenetic nerve transplants seem to have a more sufficient source. However, the report on this is very limited [Citation23,Citation24]. In nerve tissue engineering, BMSCs are applied as seed cells with attractive features of easy accessibility, rapid amplification in vitro, low immunogenicity as well as successful integration within the host tissue [Citation25]. Numerous research has proved the therapeutic potential of the cultured BMSCs in the repair of nerve injuries [Citation11,Citation26]. Hence, our interest is directed to evaluate the biocompatibility of acellular nerves of different mammalian species involving the cytocompatibility with BMSCs.
The acellular nerves are mainly constituted by basal lamina of Schwann cells, i.e. ECM, which presents a complicated grid-like structure constructed by the macromolecules such as secreted proteins and polyoses and is the basic framework for the adhesion and metabolism of cells [Citation27]. We applied moderate chemical detergents of the same concentration to prepare acellular peripheral nerves with the same diameter. The processed nerves were derived from rats and rabbits, respectively. To achieve optimal balance between the preservation of vessels of basilar membrane and the removal of cells, the morphological detection of the acellular nerves were performed. The ultramicrostructural analysis showed that the acellular nerves derived from both species presented a biomimetic three-dimensional architecture maintaining natural physical characteristics of basal lamina tubes. Compared with the acellular nerves of rats, the epineurium of the rabbits’ nerves was thicker, the amount of fasciculus was greater, and the average inner diameter of vessels of basilar membrane was larger. The motor nerve graft has been proved to allow more regenerating nerve fibers to pass through, due to its vessels of basilar membrane with larger diameter [Citation28,Citation29]. Thus, if the acelluar nerve transplants derived from particular species, possessing thicker epineurium and larger vessels of basilar membrane, are utilized to bridge peripheral nerve gaps, the conjugation of suture would be steadier, the axial diffusion of soluble substances in endoneurial tubes can be facilitated, the pressure on nerve fibers caused by mild swelling of the scaffold may be relieved, and the extension of nerve fibers is improved accordingly [Citation2].
Laminin is the main protein in ECM and capable of identifying and binding the corresponding receptors located in the growth cone. It provides an adherent medium for regenerating axons, guides, and sustains the advance of neurites [Citation4,Citation30]. Additionally, laminin is favorable to the differentiation, migration, and basal lamina deposition of Schwann cells as well as myelin sheath formation in vitro [Citation20,Citation31]. Immunostaining showed that after acellularization, laminin in the acellular nerves of the both species was well retained. In peripheral nerves the expression product of major histocompatibility complex (MHC) mainly exists on the surface of the Schwann cell membrane and the myelin sheath, and constitutes the membrane antigens of Schwann cells. These primarily contribute to the immunologic rejection post transplant [Citation32,Citation33]. When the antigenic cells and myelin sheath are removed, the allogenic acellular nerves obviate the immunoreaction mediated by cells after being implanted in vivo [Citation9,Citation21]. In this study, the residual Schwann cell debris was labeled with S100, the marker existing in the cytoplasm and the cell membrane of Schwann cells. By means of immunostaining and chromotropic acid 2R-brilliant green staining, it was confirmed that there was no apparent S100-positive expression or red-stained fragment of myelin sheath in the acquired acellular nerves. This implied the complete removal of cells and myelin sheath in the both mammalian peripheral nerves following decellularization.
Gulati [Citation32] proved that the macromolecular substances in ECM exhibited no immunogenicity between allogeneic individuals via immunological assay. Even between the different species, the evolution of compositions of ECM is generally conservative and the components could be immunologically tolerated by heterogenic recipients [Citation34]. Due to the complete removal of cells and myelin sheath, the immunogenicity of acellular nerves is minimized. Thus the cell and humor-mediated immunological rejections are largely avoided after the allogeneil graft [Citation21,Citation35]. The immunological rejection elicited by the transplant is principally related to the infiltration of helper T lymphocytes and cytotoxic T lymphocytes in both the implant and the surrounding tissue [Citation36]. Following subcutaneous implantation in rats, we found that the acute inflammatory reaction induced by acellular nerves of either species decreased gradually. At 1 week post-implantation, only a small amount of CD3-positive T lymphocytes infiltrated around the grafts. After 2 weeks, the cells decreased significantly. CD4-positive T lymphocytes did not appear at any scheduled time point (data not shown), and the differences in CD3-positive expression between the two groups were not distinct. Based on the previous research [Citation24], our result further illustrated that neither of the acellular nerves elicited cell-mediated immunoreaction, and the nerve scaffolds could be immunologically tolerated by heterogenic recipients.
As the seed cells, BMSCs could be conveniently drawn from animals, amplified persistently and proliferate rapidly in vitro. In addition, the mesenchymal stem cells only show a low immunogenicity [Citation25,Citation37]. BMSCs are characterized as the potentiality of multi-directional differentiation, they could be induced in vitro and then differentiate into osteoblasts, chondrocytes, adipocytes, neurons, neurogliocytes, etc. With the aim of evaluating the cytotoxicity of biomaterials, BMSCs were cultured with the leaching liquor of either acellular nerve. It was found that RGR increased gradually with increasing incubation time, the grade of cytotoxicity for both acellular nerves was assessed as one, and at each time point of incubation there was no significant difference in the activity of BMSCs between the two groups. Furthermore, there was no adverse residual of the chemical detergents in the nerve scaffolds after chemical extraction, and the prepared grafts of both species did not show cytotoxicity.
The components of ECM in nerves mainly comprise laminin, fibronectin, sulfate-proteoglycan, mucoprotein-C, collogen IV, collogen V, etc. [Citation38]. In addition, our study demonstrated that the acellular nerves prepared through chemical extraction retained the naive structure of vessels of basilar membrane, and the collagen fibers of the vessel wall were arranged orderly. These features make the acellular nerves conducive to cell adhesion, migration, mitotic division, and proliferation [Citation39,Citation40]. It was further confirmed that after 5 days culture in vitro, BMSCs were uniformly distributed in the lumens of endoneurial tubes, adhered to the vessel wall and grew, presenting oval shapes. The filiform filaments and spherical particles secreted by cells were also observed on the surfaces of BMSCs. Both acelluar nerve scaffolds revealed nearly the same contribution to the cell survival and adhesion.
It has been reported that BMSCs, which were cultivated alone [Citation41–43] or co-cultured with neurons in vitro [Citation14,Citation44], synthesized and secreted various neurotrophic factors such as NGF, BDNF, ciliary neurotrophic factor (CNTF), glial cell line-derived neurotrophic factor (GDNF), etc. In the present study, both acellular nerves seeded with BMSCs were cultured for 5 days in vitro. Between the ANX group and the ANA group, the differences in the levels of NGF and BDNF secreted by BMSCs were insignificant at the levels of the gene and the protein. It was demonstrated that BMSCs contributed equivalently in the acellular nerve scaffolds derived from different species.
CONCLUSIONS
The acellular nerve grafts of different mammalian species that are prepared via chemically extracted protocol show favorable biocompatibility. The combination of xenogenic acellular nerves and BMSCs has potential to provide an appropriate neurotrophic microenvironment for the axon elongation and myelinization, indicating that this study can be referred to as an experimental basis for the clinical selection of acellular nerve grafts with broad sources. An in vivo study utilizing ANX integrated with BMSCs as the substitutes of allogenic nerves for repairing peripheral nerve injuries is ongoing in our laboratory.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
REFERENCES
- Terzis, J.K., Sun, D.D., and Thanos, P.K. (1997). Historical and basic science review: past, present, and future of nerve repair. J Reconstr Microsurg, 13(3):215–225.
- Ciardelli, G., and Chiono, V. (2006). Materials for peripheral nerve regeneration. Macromol Biosci, 6(1):13–26.
- Crouzier, T., McClendon, T., Tosun, Z., and McFetridge, P.S. (2009). Inverted human umbilical arteries with tunable wall thicknesses for nerve regeneration. J Biomed Mater Res A, 89(3):818–828.
- Verdú, E., Labrador, R.O., Rodríguez, F.J., Ceballos, D., Forés, J., and Navarro, X. (2002). Alignment of collagen and laminin-containing gels improve nerve regeneration within silicone tubes. Restor Neurol Neurosci, 20(5):169–179.
- Stang, F., Keilhoff, G., and Fansa, H. (2009). Biocompatibility of different nerve tubes. Materials, 2(4):1480–1507.
- Oudega, M., Gautier, S.E., Chapon, P., Fragoso, M., Bates, M.L., Parel, J.M., and Bunge, M.B. (2001). Axonal regeneration into Schwann cell grafts within resorbable poly(alpha-hydroxyacid) guidance channels in the adult rat spinal cord. Biomaterials, 22(10):1125–1136.
- Previtali, S.C., Nodari, A., Taveggia, C., Pardini, C., Dina, G., Villa, A., Wrabetz, L., Quattrini, A., and Feltri, M.L. (2003). Expression of laminin receptors in Schwann cell differentiation: Evidence for distinct roles. J Neurosci, 23(13):5520–5530.
- Sondell, M., Lundborg, G., and Kanje, M. (1998). Regeneration of the rat sciatic nerve into allografts made acellular through chemical extraction. Brain Res, 795(1–2): 44–54.
- Hudson, T.W., Liu, S.Y., and Schmidt, C.E. (2004). Engineering an improved acellular nerve graft via optimized chemical processing. Tissue Eng, 10(9-10):1346–1358.
- Kim, B.S., Yoo, J.J., and Atala, A. (2004). Peripheral nerve regeneration using acellular nerve grafts. J Biomed Mater Res A, 68(2):201–209.
- Wang, D., Liu, X.L., Zhu, J.K., Jiang, L., Hu, J., Zhang, Y., Yang, L.M., Wang, H.G., and Yi, J.H. (2008). Bridging small-gap peripheral nerve defects using acellular nerve allograft implanted with autologous bone marrow stromal cells in primates. Brain Res, 1188:44–53.
- Mosahebi, A., Fuller, P., Wiberg, M., and Terenghi, G. (2002). Effect of allogeneic Schwann cell transplantation on peripheral nerve regeneration. Exp Neurol, 173(2): 213–223.
- Cuevas, P., Carceller, F., Garcia-Gómez, I., Yan, M., and Dujovny, M. (2004). Bone marrow stromal cell implantation for peripheral nerve repair. Neurol Res, 26(2):230–232.
- Hokari, M., Kuroda, S., Shichinohe, H., Yano, S., Hida, K., and Iwasaki, Y. (2008). Bone marrow stromal cells protect and repair damaged neurons through multiple mechanisms. Neurosci Res, 86(5):1024–1035.
- Polisetti, N., Chaitanya, V.G., Babu, P.P., and Vemuganti, G.K. (2010). Isolation, characterization and differentiation potential of rat bone marrow stromal cells. Neurol India, 58(2):201–208.
- Zhang, P.X., Xu, H.L., Zhang, D.Y., Fu, Z.H.G., Zhang, H.B., and Jiang, B.G. (2006). The biocompatibility research of functional Schwann cells induced from bone mesenchymal cells with chitosan conduit membrane. Artif Cell Blood Sub, 34:91–99.
- Bozkurt, A., Deumens, R., Beckmann, C., Olde Damink, L., Schügner, F., Heschel, I., Sellhaus, B., Weis, J., Jahnen-Dechent, W., Brook, G.A., and Pallua, N. (2009). In vitro cell alignment obtained with a Schwann cell enriched microstructured nerve guide with longitudinal guidance channels. Biomaterials, 30(2):169–179.
- Cheung, H.Y., Lau, K.T., Lu, T.P., and Hui, D. (2007). A critical review on polymer-based bio-engineered materials for scaffold development. Compos B: Eng, 38(3):291–300.
- Ribeiro-Resende, V.T., Koenig, B., Nichterwitz, S., Oberhoffner, S., and Schlosshauer, B. (2009). Strategies for inducing the formation of bands of Büngner in peripheral nerve regeneration. Biomaterials, 30(29):5251–5259.
- Hu, J., Zhu, Q.T., Liu, X.L., Xu, Y.B., and Zhu, J.K. (2007). Repair of extended peripheral nerve lesions in rhesus monkeys using acellular allogenic nerve grafts implanted with autologous mesenchymal stem cells. Exp Neurol, 204(2):658–666.
- Hudson, T.W., Zawko, S., Deister, C., Lundy, S., Hu, C.Y., Lee, K., and Schmidt, C.E. (2004). Optimized acellular nerve graft is immunologically tolerated and supports regeneration. Tissue Eng, 10(11-12):1641–1651.
- Sun, X.H., Che, Y.Q., Tong, X.J., Zhang, L.X., Feng, Y., Xu, A.H., Tong, L., Jia, H., and Zhang, X. (2009). Improving nerve regeneration of acellular nerve allografts seeded with SCs bridging the sciatic nerve defects of rat. Cell Mol Neurobiol, 29(3):347–353.
- Accioli De Vaconcellos, Z.A., Duchossoy, Y., Kassar-Duchossoy, L., and Mira, J.C. (1999). Experimental median nerve repair by fresh or frozen nerve autografts and xenografts. Ann Chir Main Memb Super, 18(1): 74–84.
- Zhang, Y., Luo, H., Zhang, Z., Lu, Y., Huang, X., Yang, L., Xu, J., Yang, W., Fan, X., Du, B., Gao, P., Hu, G., and Jin, Y. (2010). A nerve graft constructed with xenogeneic acellular nerve matrix and autologous adipose-derived mesenchymal stem cells. Biomaterials, 31(20):5312–5324.
- Tohill, M., and Terenghi, G. (2004). Stem-cell plasticity and therapy for injuries of the peripheral nervous system. Biotechnol Appl Biochem, 40(Pt 1):17–24.
- Choi, B.H., Zhu, S.J., Kim, B.Y., Huh, J.Y., Lee, S.H., and Jung, J.H. (2005). Transplantation of cultured bone marrow stromal cells to improve peripheral nerve regeneration. Int J Oral Maxillofac Surg, 34(5):537–542.
- Anderson, P.N., Campbell, G., Zhang, Y., and Lieberman, A.R. (1998). Cellular and molecular correlates of the regeneration of adult mammalian CNS axons into peripheral nerve grafts. Prog Brain Res, 117:211–232.
- Lloyd, B.M., Luginbuhl, R.D., Brenner, M.J., Rocque, B.G., Tung, T.H., Myckatyn, T.M., Hunter, D.A., Mackinnon, S.E., and Borschel, G.H. (2007). Use of motor nerve material in peripheral nerve repair with conduits. Microsurgery, 27(2):138–145.
- Moradzadeh, A., Borschel, G.H., Luciano, J.P., Whitlock, E.L., Hayashi, A., Hunter, D.A., and Mackinnon, S.E. (2008). The impact of motor and sensory nerve architecture on nerve regeneration. Exp Neurol, 212(2):370–376.
- Rangappa, N., Romero, A., Nelson, K.D., Eberhart, R.C., and Smith, G.M. (2000). Laminin-coated poly(L-lactide) filaments induce robust neurite growth while providing directional orientation. J Biomed Mater Res, 51(4): 625–634.
- Obremski, V.J., Wood, P.M., and Bunge, M.B. (1993). Fibroblasts promotes Schwann cell basal lamina deposition and elongation in the absence of neurons in culture. Dev Biol, 160(1):119–134.
- Gulati, A.K. (1995). Immunological fate of Schwann cell-poulated acellular basal lamina nerve allografts. Transplantation, 59(11):1618–1622.
- Trumble, T.E., and Shon, F.G. (2000). The physiology of nerve transplantation. Hand Clin, 16(1):105–122.
- Exposito, J.Y., D'Alessio, M., Solursh, M., and Ramirez, F. (1992). Sea urchin collagen evolutionarily homologous to vertebrate pro-alpha 2(I) collagen. J Biol Chem, 267(22): 15559–15562.
- Rovak, J.M., Bishop, D.K., Boxer, L.K., Wood, S.C., Mungara, A.K., and Cederna, P.S. (2005). Peripheral nerve transplantation: the role of chemical acellularization in eliminating allograft antigenicity. J Reconstr Microsurg, 21(3):207–213.
- Annselin, A.D., and Pollard, J.D. (1990). Immunopathological factors in peripheral nerve allograft rejection: Quantification of lymphocyte invasion and major histocompatibility complex expression. J Neurol Sci, 96(1):75–88.
- Barry, F.P., and Murphy, J.M. (2004). Mesenchymal stem cells: Clinical applications and biological characterization. Int J Biochem Cell Biol, 36(4):568–584.
- Agius, E., and Cochard, P. (1998). Comparison of neurite outgrowth induced by intact and injured sciatic nerves: A confocal and functional analysis. J Neurosci, 18(1): 328–338.
- Badylak, S.F., Freytes, D.O., and Gilbert, T.W. (2009). Extracellular matrix as a biological scaffold material: Structure and function. Acta Biomater, 5(1):1–13.
- Chafik, D., Bear, D., Bui, P., Patel, A., Jones, N.F., Kim, B.T., Hung, C.T., and Gupta, R. (2003). Optimization of Schwann cell adhesion in response to shear stress in an in vitro model for peripheral nerve tissue engineering. Tissue Eng, 9(2):233–241.
- García, R., Aguiar, J., Alberti, E., de la Cuétara, K., and Pavón, N. (2004). Bone marrow stromal cells produce nerve growth factor and glial cell line-derived neurotrophic factors. Biochem Biophys Res Commun, 316(3):753–754.
- Gu, Y., Wang, J., Ding, F., Hu, N., Wang, Y., and Gu, X. (2010). Neurotrophic actions of bone marrow stromal cells on primary culture of dorsal root ganglion tissues and neurons. J Mol Neurosci, 40(3):332–341.
- Yamaguchi, S., Kuroda, S., Kobayashi, H., Shichinohe, H., Yano, S., Hida, K., Shinpo, K., Kikuchi, S., and Iwasaki, Y. (2006). The effects of neuronal induction on gene expression profile in bone marrow stromal cells (BMSC): A preliminary study using microarray analysis. Brain Res, 1087(1):15–27.
- Kamei, N., Tanaka, N., Oishi, Y., Ishikawa, M., Hamasaki, T., Nishida, K., Nakanishi, K., Sakai, N., and Ochi, M. (2007). Bone marrow stromal cells promoting corticospinal axon growth through the release of humoral factors in organotypic cocultures in neonatal rats. J Neurosurg Spine, 6(5):412–419.