Abstract
Abstract: Polymerized Porcine Hemoglobin (pPolyHb), a hemoglobin-based oxygen carrier (HBOC), was developed as a potential red blood substitute for clinical applications. Assessment of its effects on the immune system is an important component of the overall safety evaluation of HBOC. For this purpose, we assessed three inflammation indicators, including complement C3a, IL-6, and TNF-α in cultured cells and in a rat model when pPolyHb was incubated or administrated with the cells/animals. Our results suggested that the levels of these three indicators were not statistically changed upon pPolyHb stimulation, indicating that pPolyHb is not immunotoxic to cells and animals in this aspect.
INTRODUCTION
Hemoglobin-based oxygen carriers (HBOCs), with their capacity of delivering oxygen, could function as potential red blood cell substitutes or primary resuscitation solutions. Different from saline or colloid-based solutions, HBOCs carry and deliver oxygen to tissues in addition to restoring intravascular volume and pressure and, unlike RBCs, hemoglobin solutions do not need cross-matching and can be prepared virus- and bacteria-free [Citation1–3]. So far, the principle raw material for HBOCs’ manufacture comes from human and bovine hemoglobin (Hb) [Citation4,Citation5]. However, due to the limited supply of human Hb and the possible threat of human blood-transmitted diseases such as hepatitis and HIV and cross-species transmission of prions existing in bovine blood [Citation6], porcine Hb has been developed as a new source of HBOCs [Citation7].
The primary concern over HBOC-201 is the hypertensive effect hemoglobin solutions exhibit in animals, which is thought to be due in part to their affinity for nitric oxide [Citation8–10]. Low-MW Hb scavenges nitric oxide, thus preventing smooth muscle relaxation and elevating blood pressure. However, in our earlier work we demonstrated that pPolyHb maintained hemodynamic stability well and wouldn't cause hypertension in an exchange transfusion rat model in that 120–140% of estimated total blood volume was replaced by pPolyHb. Furthermore, pPolyHb increased tissue oxygen, reversed anaerobic metabolism, and provided for effective life-sustaining ability [Citation11].
Although HBOCs have been extensively studied on the various physiological properties, such as effectiveness and toxicity, the effects of pPolyHb on the host inflammatory response have not yet been further investigated. In an in vitro study by McFaul et al., the incubation of isolated human monocytes with purified Hb resulted in an increase of IL-8 and TNF-α at 4 hours and that quantities of these cytokines released were sufficient to induce significant PMN chemotaxis and PMN adherence to HUVECs [Citation1,Citation12]. Ortegon et al. observed that HBOC-201 had immune-activating potential, especially enhancement of neutrophilic β2 integrin (CD11b) expression at high concentration in vitro [Citation13]. However, in vivo in moderate and severe controlled hemorrhagic shock (HS) in swine, with the exception of mildly increased plasma levels of the anti-inflammatory Th-2 cytokine, IL-10, HBOC-201 had minimal effects on innate immune responses [Citation14–16]. As well, in trauma patients human polymerized hemoglobin (Polyheme) diminished immune activation in comparison with blood transfusions [Citation17]. Valeski et al. reported that bolus injection of αα -Hb did not activate a significant accumulation of complement C3 in the intestinal mucosa of the rats [Citation18]. In another study, in which complement activation by liposome-encapsulated Hb (LEH) was investigated, a positive result was obtained by Szebeni et al. In this case, bovine stroma-free tetrameric Hb from Biopure Corporation (Cambridge, MA) did not activate a complement, similar to the results in vivo with αα-Hb [Citation19].
The objective of this study is to evaluate the effects of pPolyHb on the host inflammatory response through assessment of the level of three inflammatory indicators, including complement C3a, IL-6, and TNF-α in rat models, especially immune effects in rats with more severe hemorrhagic shock, since the course of hemorrhagic shock (HS) exhibits a possible challenge to the immune system, which may contribute to multiorgan failure (MOF) or death [Citation20,Citation21]. Current pre-hospital resuscitation fluids and blood products present potential intrinsic immunomodulating properties [Citation22,Citation23]. It's necessary to investigate whether pPolyHb invokes innate immune response in such critical circumstances.
MATERIALS
Reagents
6% Hetastarch 200-0.5 in sodium chloride solution (Fresenius Kabi), Pentobarbital Sodium (Sigma), Hepalean 1000U.S.P. units/ml (Organon), Sodium Chloride (Sigma), Potassium Chloride (Sigma), Calcium Chloride (Sigma), Sodium Phosphate Monobasic (Sigma), Disodium Hydrogen Phosphate (Sigma), Phosphate Buffered Saline (PBS) (Sigma), Sodium Hydroxide (Sigma), Tris Base (Sigma), Phenolred (Sigma), Bovine Serum Albumin (BSA) (Sigma), Fetal Bovine Serum (FBS) (Gibco), Dulbecco's Modification of Eagle's Medium (DMEM) (Gibco), Lipopolysaccharide (LPS) (Sigma), Rat/Mouse IL-6 Elisa Kit, Rat/Mouse TNF-α Elisa Kit, Rat Complement C3a Elisa kit (R&D System).
Animals
Male Sprague–Dawley rats (Xian Jiaotong University, China) weighing 240 ± 20 g were used in the study. The experiments described in this study were performed in adherence to National Institutes of Health guidelines on the use of experimental animals. Approval of the Animal Care Committee of Northwest University was obtained prior to initiating the experiments.
Test Solutions
pPolyHb (10.5 ± 0.5 g/dl polymerized porcine hemoglobin, methemoglobin <5%, endotoxin <1.0 EU/mL, osmolality 300–330 mOsm, pH 7.4 ± 0.05, average molecule of pPolyHb 600 ± 50 kD, 64 kD tetramer <2%) was formulated in buffer consisting of Na+ 135–155 mmol/L, K+ 3.0−5.0 mmol/L, Ca2+ 1−3 mmol/L, Cl− 140−160 mmol/L and stored at 4°C under nitrogen gas until use.
METHODS
In Vitro Study
Complement C3a Measurement
Blood was drawn from Sprague–Dawley rats. Plasma was isolated by centrifuging at 5500g for 20 min, and kept frozen at −80°C in small aliquots until it was analyzed. 400 ul of plasma was mixed with 100 ul of saline (negative control), LPS (positive control), and pPolyHb, respectively. The reaction mixtures were incubated at 37°C for 1 hour with shaking. Reaction was stopped by adding 1.6 ml saline into 400 ul of the reaction mixture. Complement C3a levels were detected using a Rat complement C3a Elisa kit (R&D System) according to the manufacturer's protocol. The detailed method is as described [Citation24].
IL-6 and TNF-α Measurement
Macrophage Raw 264.7 cells were purchased from China Center for Type Culture Collection (CCTCC), and the cells were harvested with permission from the Institutional Review Board (IRB) for the Protection of Human Subjects. Cells were grown in DMEM with 20% FBS and maintained in 100-mm dishes at 37°C in a humidified atmosphere of 95% air and 5% CO2. Cells were treated with different concentrations of PBS, BSA (negative control), LPS (positive control), and pPolyHb, respectively, for 12–18 h. Supernatant of cell culture was pipetted, then centrifuged at 2800 g for 20 min, and the supernatant fluids were transferred to a clean centrifuge tube and kept frozen at –20°C until they were analyzed for cytokines by ELISA. IL-6 and TNF-α levels were detected using Mouse IL-6 ELISA kits and Mouse TNF-α ELISA kits (R&D Systems) according to manufacturer's protocol. Assays were performed in triplicate.
In Vivo Study
Complement C3a, IL-6, and TNF-α Measurement in Healthy Rats
Twelve Sprague–Dawley rats were randomized into two groups: pPolyHb and Hetastarch (HES, control). Before the immunization, a blood sample was drawn from each rat. Serum was isolated and stored in small aliquots as pre-immune control serum. The equal amount of pPolyHb (3 g/dl) and HES were injected into rats intravenously. Serum was collected 12 h after injection and stored in small aliquots at −80°C until they were analyzed for cytokines.
Complement C3a, IL-6, and TNF-α levels were detected using a Rat complement C3a Elisa kit, Rat IL-6 ELISA kits, and Rat TNF-α ELISA kits (R&D Systems) according to the manufacturer's protocol. Assays were performed in triplicate.
Surgical Preparation
Male Sprague–Dawley rats (n = 12) were anesthetized with sodium pentobarbital (45 mg/kg, intraperitoneally). The left jugular vein was cannulated (PE 50 tubing) for drug administration. The left femoral artery was cannulated (PE 50 tubing) and connected to a MP150 Data Acquisition System (BIOPAC, USA) for recording the blood pressure, ECG, and heart rate. The right femoral artery was cannulated to induce controlled hemorrhage. The animals were allowed to stabilize for 60 min before starting the experiment. Blood gas analysis was performed on an ABL 800 FLEX (Radiometer, Copenhagen, Denmark).
Hemorrhagic Shock (HS) Model and Complement C3a, IL-6, and TNF-α Measurement
Male Sprague–Dawley rats were heparinized before HS through the venous catheter at 60 units/100 g body weight. The test solution was warmed to the body temperature of 37°C. Rats (n = 12) were subjected to volume-controlled hemorrhage. 55 ± 5% estimated blood volume (EBV) was withdrawn via the right femoral artery in two steps at a rate of 0.5 ml/min. Rats were hemorrhaged over 90 min, after which they were resuscitated with pPolyHb or HES equal volume to the lost blood through the left jugular venous catheter at a rate of 0.5 mL/min.
Mean arterial blood pressure (MAP), systolic blood pressure (SP), diastolic blood pressure (DP), heart rate (HR), and respiration rate were monitored every 5 min throughout the experiment. Blood samples were withdrawn before HS (base), at the end of shock, at the end of resuscitation, 45 min, 3 h, and 6 h after resuscitation. Plasma was collected by centrifuging at 5500 g for 5 min and stored in small aliquots at −80°C until they were analyzed for cytokines. Complement C3a, IL-6, and TNF-α levels were detected according to the manufacturer's protocol. Assays were performed in triplicate.
Statistical Analysis
Data was represented as means ± SD for replicate experiments. The differences between treatment groups were assessed by one-way ANOVA followed by unpaired Student's t-test. Statistical significance was defined as p < 0.05 to reject a null hypothesis. All statistical calculations were performed with JMP version 3.2 for the Macintosh (SAS Institute, Cary, NC).
RESULTS
In Vitro Study
Assessment of Complement Cascades in an In Vitro System
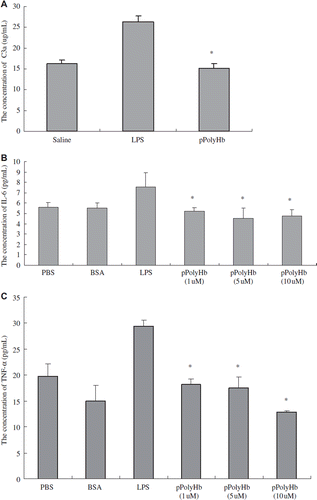
Various observed toxicities have arisen during the preclinical and clinical development of the current generation of hemoglobin-based products, of which pro-inflammatory activity may result in disseminated intravascular coagulation. For hemoglobin-based oxygen carriers, it is hypothesized that the adverse effects of HBOCs caused are in part the result of activation of complement and procoagulant cascades or any number of triggered enzyme or cellular systems [Citation25]. Complement component 3 (C3) plays a central role in the activation of complement system. Its activation is required for both classical and alternative complement activation pathways. Given the consideration of the safety described above, we conducted a test for complement and procoagulant cascades by an in vitro assay to examine the level of C3a in a cell culture system. pPolyHb was incubated with the rat plasma for 4 hrs, and the concentration of C3a was determined by Elisa. As shown in , there were no significant differences for C3a levels between the plasma incubated with pPolyHb and the saline control, whereas the amount of C3a in the plasma stimulated by LPS (positive control) is much higher than that by pPolyHb stimulation, suggesting that pPolyHb didn't induce complement C3a activation.
Figure 1. Concentrations of the inflammatory mediators treated with different agents in vitro. A: Comparison of complement C3a level treated with different agents: saline (negative control), LPS (positive control), and pPolyHb. Plasma from Sprague–Dawley rats was mixed with saline (negative control), LPS (positive control), and pPolyHb, respectively. One h later, complement C3a levels were detected using Rat complement C3a Elisa kit (R&D System) according to manufacturer's protocol. *P < 0.05 in comparison to LPS group; B: Comparison of IL-6 level treated with different agents: PBS, BSA (negative control), LPS (positive control), and pPolyHb. Raw264.7 cells were treated with different concentrations of PBS, BSA (negative control), LPS (positive control) and pPolyHb, respectively, for 12–18 h. Supernatant was separated. IL-6 and TNF-α levels were detected using Mouse IL-6 ELISA kits and Mouse TNF-α ELISA kits (R&D Systems) according to manufacturer's protocol. Assays were performed in triplicate. *P < 0.05 in comparison to LPS group; C: Comparison of TNF-α level treated with different agents: PBS, BSA (negative control), LPS (positive control), and pPolyHb. *P < 0.05 in comparison to LPS group.
Assessment of Pro-inflammatory Activity of pPolyHb
Pro-inflammatory activity is an important safety consideration of HBOC. Assessment of the cytokine release such as IL-6 and TNF-α therefore is a very important component for drug safety evaluation. In our design, Raw264.7 cells were cultured and incubated with different amount of pPolyHb or LPS for 16 hours; IL-6 and TNF-a concentration were then measured from the cell culture medium. As shown in , the cells released significantly higher amounts of IL-6 and TNF-α in the presence of LPS (7.5 pg/mL and 29.4 pg/mL, respectively), demonstrating that the cells respond well to exogenous stimulation, whereas the concentrations of IL-6 and TNF-α were kept in a low level in the presence of pPolyHb (6 and 18 pg/mL, respectively), a concentration similar to a nonspecific protein or medium, as evidenced by their release in the presence of BSA and PBS, demonstrating that pPolyHb did not induce pro-inflammatory activation.
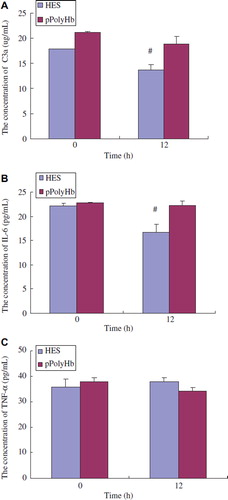
Figure 2. Changes of the levels of inflammatory mediators in healthy rats immunized by different agents. Sprague–Dawley rats (n = 12) were randomized into two groups: pPolyHb and Hetastarch (HES, control). The equal amount of pPolyHb (3 g/dl) and HES were injected into rats intravenously. Serum was collected before the immunization (base) and 12 h after injection. Complement C3a, IL-6, and TNF-α levels were detected using Rat Complement C3a Elisa kit, Rat IL-6 ELISA kits, and Rat TNF-α ELISA kits (R&D Systems) according to manufacturer's protocol. Assays were performed in triplicate. *P < 0.05 in comparison to baseline. A: Changes of complement C3a levels in healthy rats immunized by pPolyHb and HES at different periods; B: Changes of IL-6 levels in healthy rats immunized by pPolyHb and HES at different periods; C: Changes of TNF-α levels in healthy rats immunized by pPolyHb and HES at different periods.
In Vivo Study
Assessment of Complement Cascades and Pro-inflammatory Activity in Healthy Rat Model
Given that there is no immunotoxicity in an in vitro system, we continued our assessment in an animal model, since our product will eventually be tested in clinical practice. 3 g/dl pPolyHb and the same amount of HES (a general treatment in clinical settings) were administrated via IV route to healthy rats. 12 h after the administration, plasma was collected at different time interval and C3a, IL-6, and TNF-α were measured. As shown in , , and , the concentrations of C3a, IL -6 and TNF-α were 18.8 μg/mL, 22.3 pg/mL and 34.5 pg/mL, respectively, which is near to the level of 21.2 μg/mL, 22.8 pg/mL, and 37.8 pg/mL at baseline, suggesting that pPolyHb administration would not activate inflammatory response in healthy rats. A similar trend was observed in the HES group.
Assessment of Complement Cascades and Pro-inflammatory Activity in Hemorrhagic Shock Rat Model
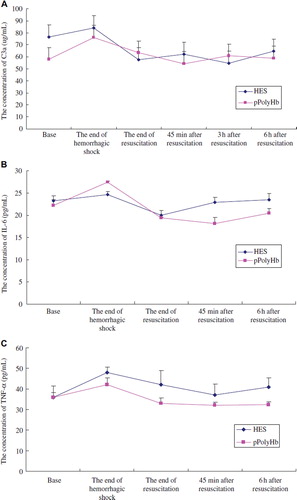
We then conducted a similar experiment in a severe hemorrhagic shock (HS) model. HS was induced by withdrawing 55 ± 5% estimated blood volume (EBV) via the right femoral artery over 90min, and mean arterial pressure was maintained at 30–40 mmHg. Ninety minutes later, rats were resuscitated with pPolyHb or HES equal volume to the lost blood. Plasma was collected at the following several time points: before HS (base), the end of shock, the end of resuscitation, 45 min, 3 h, and 6 h after resuscitation. As shown in , , and , the three indicators elevated in the period of HS and climbed to the highest at the end of HS. The concentrations of C3a, IL-6, and TNF-α came to 76 μg/mL, 27.2 pg/mL, and 41.1 pg/mL at the end of HS, compared to 57.8 μg/mL, 22.4 pg/mL, and 36.7 pg/mL before HS. Following the infusion of pPolyHb or HES, the three indicators decreased and backed to the baseline, indicating that administration of pPolyHb would improve the inflammation caused by HS.
Figure 3. Changes of the levels of inflammatory mediators in hemorrhagic shock rats resuscitated with different fluids. Sprague–Dawley rats (n = 12) undergo volume-controlled hemorrhage. 90 min later, they were resuscitated with pPolyHb or HES equal volume to the lost blood. Blood samples were withdrawn before HS (base), at the end of shock, at the end of resuscitation, 45 min, 3 h, and 6 h after resuscitation. Plasma was collected and complement C3a, IL-6, and TNF-α levels were detected according to manufacturer's protocol. Assays were performed in triplicate. A: Changes of complement C3a levels in hemorrhagic shock rats resuscitated with pPolyHb and HES; B: Changes of IL-6 levels in hemorrhagic shock rats resuscitated with pPolyHb and HES; C: Changes of TNF-α levels in hemorrhagic shock rats resuscitated with pPolyHb and HES.
DISCUSSION
pPolyHb has been studied for years in our group; it shows a good potential for tissue oxygenation and survival benefit in rat models [Citation11], but pPolyHb’ effects on host immune responses are not well described.
In this study, we assessed three inflammation indicators, including complement C3a, IL-6, and TNF-α in cultured cells and in healthy rats when pPolyHb was incubated or administrated with the cells/animals. The results showed that these parameters were not significantly changed compared with the control. We also investigated pPolyHb's immune effects in a controlled hemorrhagic shock and resuscitation model. C3a, IL-6, and TNF-α climbed to the highest at the end of shock, which may attribute to the infiltration and activation of a large amount of leukocytes and tissue cells to release inflammatory mediators and initiate inflammatory response in the tissues. However, pPolyHb resuscitation could reverse inflammation and restore the level of three inflammatory mediators to normal level, which means the infusion of pPolyHb would not induce inflammatory response. To the contrary, it may help to attenuate the existing inflammatory symptom. Similar results were also seen in a swine model of HBOC-201, which showed that HBOC-201 had no significant adverse or beneficial effects on immune function in a swine model of moderately severe HS and severe controlled HS [14-16]. Our in vitro results are consistent with in vivo results in that there is no inflammatory reaction caused by addition or infusion of pPolyHb. These works add to the emerging body of evidence, again suggesting that pPolyHb is a safe resuscitative fluid on immune function. These data are important because improved outcome in animal models of HS (our unpublished data) of pPolyHb will prompt us to propose a pre-hospital clinical trial in HS, compared with HES.
Immune safety evaluation of investigational new drugs routinely includes five aspects. They are immunosuppression, immunogenicity, hypersensitivity, autoimmunity, and adverse immunostimulation. This study only completes a small part of the immune safety investigation, and additional studies on other aspects of immune safety evaluation will be conducted to address our concern about the safety of pPolyHb in human clinical use.
CONCLUSION
We examined the inflammatory properties of Porcine Polymerized Hemoglobin (pPolyHb). Both in vitro and in vivo studies demonstrated that pPolyHb didn't result in significant inflammatory response. In addition, it could alleviate the existing inflammatory symptom caused by HS. These results provide evidence for future administration of pPolyHb in the clinical trial.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper. We acknowledge with thanks the Grant from National Natural Science Foundation of China (Program Number: 81102367), the “Science and Technology Research and Development Project” (2011K12-03-10) from Shaanxi Province, and the grant from Xi'an Science and Technology Bureau (Grants No.CXY1131), as well as the “Special Research Foundation” grant (Grants No.06JK171) from the Education Department of Shaanxi Province.
REFERENCES
- McFaul, S.J., Bowman, R.D., Villa, V.M. (2000). Hemoglobin stimulates the release of proinflammatory cytokines from leukocytes in whole blood. J. Lab. Clin. Med. 135:263–269.
- Nucci, M.L., Abuchowski, A. (1998). The search for blood substitutes. Scientific America 2:72–77.
- Cohn, S.M. (1997). Is blood obsolete? J Trauma 42: 730–732.
- Goodnough, L.H., Brecher, M.E., Kanter, M.H., Aubuchon, J.P. (1999). Transfusion medicine. First of two parts-blood transfusion. N Engl J Med. 340:438–447.
- Stowell, C.P., Levin, J., Spiess, B.D., Winslow, R.M. (2001). Progress in the development of RBC substitutes. Transfusion 41:287–299.
- Winslow, R.M. (2000). Alphaalpha-crosslinked hemoglobin: Was failure predicted by preclinical testing? Vox Sang. 79:1–20.
- Zhu, X.L., Chu, W., Wang, T., Wang, F., Fan, D., Dan, N., Chen, C. (2007). Variations in dominant antigen determinants of glutaraldehyde polymerized human, bovine and porcine hemoglobin. Artificial Cells, Blood Substitutes and Biotechnology 35:518–532.
- Hess, J.R., Macdonald, V.W., Brinkley, W.W. (1993). Systemic and pulmonary hypertension after resuscitation with cell-free hemoglobin. J Appl Physiol 74:1769–1778.
- Kim, H.W., Greenburg, A.G. (1997). Ferrous hemoglobin scavenging of endothelium derived nitric oxide is a principal mechanism for hemoglobin mediated vasoactivities in isolated rat thoracic aorta. Artif Cells Blood Substit Immobil Biotechnol. 25:121–133.
- Loscalzo, J. (1997). Nitric oxide binding and the adverse effects of cell-free hemoglobins: What makes us different from earthworms. J Lab Clin Med. 129:580–583.
- Zhu, H.L., Dang, X.D., Yan, K.P., Dai, P.G., Ma, J., Li, Y., Chang, T.M.S. Chen, C. (2011). Pharmacodynamic study of polymerized porcine hemoglobin (pPolyHb) in a rat model of exchange transfusion. Artificial Cells, Blood Substitutes and Biotechnology 39:119–126.
- McFaul, S.J., Bowman, R.D., Villa, V.M., Gutierrez-Ibanez M.J., Johnson M., Smith D. (1994). Hemoglobin stimulates mononuclear leukocytes to release interleukin-8 and tumor necrosis factor-α. Blood 84:3175–81.
- Ortegon, D.P., Dixon, P.S., Crow, K.K., Mueller, D.L., Kerby, J.D. (2003). The effect of the bovine hemoglobin oxygen therapeutic HBOC-201 on human neutrophil activation in vitro. J. Trauma 55:755–760.
- Dong, F., Hall, C.H., Golech, S.A., Philbin, N.B., Rice, J.P., Gurney, J., Arnaud, F.G., Hammett, M., Ma, X., Flournoy, W.S., Hong, J., Kaplan, L., Pearce, L.B., McGwin, G., Ahlers, S., McCarron, R., Freilich, D. (2006). Immune effects of resuscitation with HBOC-201, a hemoglobin-based oxygen carrier, in swine with moderately severe hemorrhagic shock from controlled hemorrhage. Shock 25:50–55.
- Hall, C.H., Malkevich, N., Handrigan, M., Vander Molen, C., Arnaud, F.G., Hong, J., Dong, F., Rice, J.P., Philbin, N.B., Ahlers, S., McCarron, R., Freilich, D., McGwin, G., Flournoy, W.S., Pearce, L.B. (2007). Innate immune responses in swine resuscitated from severe traumatic hemorrhagic shock with hemoglobin-based oxygen carrier-201. Artificial Cells, Blood Substitutes and Biotechnology 35:259–274.
- Vander Molen, C., Malkevich, N., Philbin, N., Rice, J., Collier, S., Hall, C., Ahlers, S., McCarron, R., Freilich, D., McGwin, G., Bruce P. L. (2007). Immune effects of decreasing low-molecular weight hemoglobin components of hemoglobin-based oxygen carriers (HBOC) in a swine model of severe controlled hemorrhagic shock. Artificial Cells, Blood Substitutes, and Biotechnology 35: 507–517.
- Johnson, J.L., Moore, E.E., Gonzalez, R.J., Fedel, N., Partrick, D.A., Silliman, C.C. (2003). Alteration of the postinjury hyperinflammatory response by means of resuscitation with a red cell substitute. J. Trauma 54:133–139.
- Valeski, J.E., Gritzuk, M.M., Baldwin, A.L. (2004). Role of complement in diaspirin crosslinked hemoglobin-induced intestinal mucosal injury. Fed. Am. Soc. Exper. Biol. J. 18:195–197.
- Szebeni, J., Wassef, N.M., Hartman, K.R. . (1997). Complement activation in vitro by the red cell substitute, liposome-encapsulated hemoglobin: Mechanism of activation and inhibition by soluble complement receptor type 1. Transfusion 37:150–159.
- Giannoudis, P.V. (2003). Current concepts of the inflammatory response. Injury 34:397–404.
- Mannick, J.A., Rodrick, M.L., Lederer, J.A. (2001). The immunologic response to injury. J. Am. Coll. Surg. 193: 237–244.
- Biffl, W.L., Moore, E.E., Offner, P.J., Ciesla, D.J., Gonzalez, R.J., Silliman, C.C. (2001). Plasma from aged stored red blood cells delays neutrophil apoptosis and primes for cytotoxicity: Abrogation by poststorage washing but not prestorage leukoreduction. J. Trauma 50:426–431.
- Rhee, P., Wang, D., Ruff, P., Austin, B., DeBraux, S., Wolcott, K., Burris, D., Ling, G., Sun, L. (2000). Human neutrophil activation and increased adhesion by various resuscitation fluids. Critical Care Medicine 28:74–78.
- Chang, T.M.S. (1997). Blood Substitutes: Principles, Methods, Products and Clinical Trials, Vol. 1, Karger, Basel (full text available for free online viewing at http://www.artcell.mcgill.ca).
- Winslow, R.M. (2006). Blood Substitute, Elsevier Inc., London.