Abstract
Abstract: Resuscitation of hemorrhagic shock requires volume replacement and restoration of oxygen metabolism. Artificial oxygen carriers that can both expand blood volume and deliver oxygen have been developed as resuscitation fluids. We employed hemoglobin vesicles (HbV), a cellular-type artificial oxygen carrier, in a Beagle dog hemorrhagic shock model to prove the efficacy of HbV. Hemorrhagic shock was introduced in splenectomized Beagle dogs by withdrawing 50% of circulating blood from the femoral artery. Shock was maintained for 60 minutes before isovolemic resuscitation with HbV dispersed in 5% albumin in saline (HbV), lactated Ringer's solution (LR), 5% human serum albumin in saline (HSA), or autologous shed blood (ASB). One animal in the LR group died 150 min after resuscitation. All other animals survived 4 h of the experiment. The mean arterial pressure remained significantly lower in the LR group than in the HbV group but did not differ significantly among the HbV, Alb, and ASB groups. Immediately after resuscitation, the HbV group showed a significantly higher mean pulmonary arterial pressure, which decreased within 10 minutes to the baseline level. The cardiac output was significantly higher in the Alb group than in the others, indicating compensation for low oxygen delivery per unit blood. The post-resuscitation hematocrit was 36% in the ASB group and decreased in the other groups (20–22%). Serum chemistry data from the HbV group were unremarkable. HbV contributed 32% of the post-resuscitation oxygen delivery. Collectively, HbV is comparable to ASB and HSA as a resuscitation fluid and is an effective oxygen carrier.
INTRODUCTION
Massive hemorrhage due to trauma requires large-volume fluid resuscitation as well as blood transfusion to maintain organ circulation and oxygen metabolism [Citation1–3]. Blood type determination and cross-match testing must be performed before blood transfusion. Blood cannot always be immediately accessed in the emergency setting, and it may take hours to reach the trauma center or another facility that can provide definitive therapy. Artificial oxygen carriers may be beneficial in such situations.
Hemoglobin Vesicles (HbV) have been developed by Tsuchida's group [Citation4–6], and we have previously reported their potential utility in small animals [Citation7–9]. Rodent models preclude evaluation of the right heart and pulmonary circulation. To evaluate these circulatory parameters and the response to HbV in larger animals, we developed a 50% hemorrhagic shock model in Beagle dogs and conducted a pilot study to clarify the safety, efficacy, and influence on right heart function of HbV as an artificial oxygen carrier.
MATERIALS AND METHODS
Animals
The study was conducted on 13 healthy young male Beagle dogs (6.84 ± 0.41 kg body weight, Sankyo Animal, Shinagawa, Japan) after approval by the Institutional Animal Care and Use Committee of Keio University and in compliance with the Guide for the Care of Laboratory Animals (National Institute of Health publication 86–23, revised 1985). The animals were maintained in cages with a 12-hour light-dark cycle, dog chow was provided twice daily, and water could be accessed freely. The animals were fasted overnight before the experiments but had free access to water up to 2 hour prior to anesthesia.
Animal Preparation and Instrumentation
Anesthesia was induced with ketamine (10 mg/kg intramuscularly) and atropine (0.02 mg/kg intramuscularly). After orotracheal intubation, animals were mechanically ventilated with an animal ventilator (Shinano SN 480–4, Tokyo, Japan) using a tidal volume (VT) of 20 mL/kg and respiratory rate of 15 breaths/min. Sevoflurane was administered via a vaporizer throughout the experiment. The concentration of sevoflurane (2.0–2.5%) was adjusted as necessary to maintain the animal at a stable plane of anesthesia. Prior to catheterization, a small pararectal laparotomy was made and splenectomy performed, a tissue oxygen tension (PtO2) needle electrode (POE-10N; Eiko Kagaku Co., Tokyo, Japan) was placed at the cortical area of the left kidney and connected to the tissue oxygen tension monitor (PO2 100; Intermedical. Co., Tokyo, Japan), and the peritoneal cavity was closed. In dogs, hemorrhage induces contracture of the spleen, which supplies additional blood from the spleen parenchyma to the systemic circulation. This mechanism alleviates the severity of hemorrhagic shock. To avoid this compensation, we surgically removed the spleen prior to the experiment. The resected spleen was placed in a tin basin and allowed to contract at room temperature for 30 minutes. The blood that was expressed from the spleen was collected and measured, and this volume was added to the experimental blood loss.
Electrocardiogram (EKG) electrodes were attached to the feet. A 5.5-F Thermo-dilution catheter (631Hf55; Edwards Lifescience, Irvine, CA, USA) was placed in the pulmonary artery via the right femoral vein. The left femoral artery was cannulated to monitor arterial pressure as well as for blood sampling. The pressure line was connected to transducers (5100TW; Edwards Lifescience, Irvine, CA, USA), and these transducers and the EKG line were connected to a polygraph system (LEG-1000, Nihon Kohden Co., Tokyo, Japan). The right femoral artery was cannulated with a 16G I.V. cathter (Angiocath; Becton Dickinson, Sandy, UT, USA) to control the bleeding. rSO2 (regional saturation of oxygen) was monitored using the rSO2 monitor INVOS 4100 (Somanetics Inc., Troy, MI) at the forehead (brain rSO2) and abdomen (rectus abdominis muscle rSO2).
Preparation of Resuscitation Fluid
The Hb vesicles were manufactured according to the method developed by Waseda's group and provided by Oxygenic (Oxygenic Co., Ltd., Japan), and 25% human recombinant albumin solution was provided by Nipro (Nipro Co., Osaka, Japan) [Citation4–6]. For the HbV group, Hb vesicles were re-suspended in a 5% albumin solution. Hb concentration of the supplied HbV suspension fluid was adjusted to 10 g/dL in saline. The HbV suspension fluid was then mixed with 25% albumin solution at a ratio of 8.6 to 1.4 to be used as a colloidal resuscitation fluid containing 5% albumin. This mixture was filtered through a 0.45-μm filter unit (DISMIC® 25cs; Toyo Roshi Kaisha, Ltd., Japan) to dissociate any large aggregates. The final Hb vesicle solution contained 8.6 g/dL of hemoglobin.
For the ASB group, the exsanguinated blood was drawn into a 50-mL syringe containing 7 mL of citrate-phosphate-dextrose (CPD) solution (Karmi C; Kawasumi, Tokyo, Japan). This blood was preserved at room temperature until use.
For the Alb group, 5% albumin solution was prepared by diluting 25% albumin solution with saline. Lactated Ringer's solution (Lactec; Otsuka Pharmaceutical Co., Tokushima, Japan) was used for the LR group.
Study Protocol
After establishment of stable anesthesia, animals were assigned randomly to four experimental groups, the Autologous shed blood (ASB) (n = 4), HbV (n = 3), Albumin (HSA) (n = 3), and Lactated Ringer's solution (LR) (n = 3) groups. The mean arterial pressure (MAP), pulmonary arterial pressure (PAP), central venous oxygen saturation (SvO2), EKG, heart rate (HR), and regional saturation of oxygen (rSO2) of the brain and muscle and tissue oxygen tension (PtO2) of the renal cortex were continuously monitored throughout the experiment. Pulmonary capillary wedge pressure (PCWP), central venous pressure (CVP), arterial blood gas analysis (BGA), mixed venous BGA, and cardiac output were measured and complete blood count and serum chemistry panels obtained every 30 minutes after resuscitation for the first 2 hours and every 60 minutes thereafter until 4 hours post-resuscitation.
Fifty percent of the estimated circulating blood volume was withdrawn from the right femoral artery catheter at a rate of 20 mL/min. The estimated blood volume was calculated from the following formula: Estimated blood volume (mL) = 86 × body weight (kg).
Shock was maintained for 60 minutes after exsanguination. After the shock period, fluid resuscitation with the designated resuscitation fluid (either autologous shed blood (ASB), HbV suspension fluid (HbV), Albumin (5% albumin in saline), or Lactated Ringer's solution) was performed. A volume of resuscitation fluid equal to that of the withdrawn blood was administered via the right femoral vein at a rate of 20 mL·kg 1·min 1. After resuscitation, no additional intravenous fluid was allowed except for the cold 5% glucose required to measure cardiac output.
The arterial and pulmonary arterial pressures and the PtO2 of the renal cortex were monitored continuously and cardiac output and arterial blood gases measured every 30 minutes for the first 2 hours after resuscitation and every 60 minutes thereafter until 4 hours after resuscitation.
We took serum samples before exsanguination and after measuring other parameters 240 min post- resuscitation. Blood samples were ultracentrifuged to collect serum as ordinary centrifugation could not separate HbV. Standard laboratory testing was performed for TP, Alb, A/G ratio, AST, ALT, LDH, ALP, GGTP, LAP, ChE, Tbil, Dbil, Cr, BUN, UA, Amylase, Lipase, CPK, Tcho, Free Cholesterol, beta Lipase, HDL-C, TG, Total lipid, FFA, Phospholipid, K, Cl, Ca, IP, Mg, Fe, and Cu.
Statistical Analysis
Because of the small sample size, the Tukey Kramer test was used to evaluate the differences between groups. P < 0.05 was considered statistically significant. Student's t-test was used for comparisons within the same group.
RESULTS
Survival
We observed one death in the LR group 150 min after resuscitation. All other animals survived the study.
Heart Rate
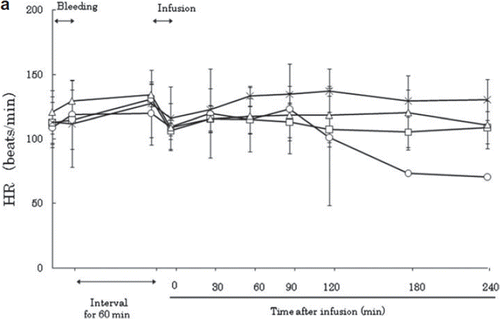
After exsanguination, the HR increased slightly in all groups and remained stably high until the beginning of resuscitation (). After resuscitation, the HR first decreased slightly to its initial level, then increased again in all groups at 30 minutes post-resuscitation. In the LR group, HR decreased after 120 minutes, and one animal subsequently died before the end of the experiment. The severity of shock in this model was thought to be such that isovolemic LR resuscitation could not compensate for the hemorrhagic shock. EKG showed no changes in the ST elevation or heart rhythm throughout the experiment.
Figure 1a. Effects of Lactated Ringer's solution (LR), human serum albumin (HSA), autologous shed blood (ASB), and hemoglobin vesicles (HbV) on heart rate (HR) in anesthetized dogs subjected to 50% exsanguination. There were no significant differences between the HbV group and the other groups. (○: LR group; □: HSA group, × : ASB group; △: HbV group).
Mean Arterial Pressure
After exsanguination, the mean arterial pressure (MAP) measurements of each group were 18 ± 7.0 mmHg in the HSA group, 23.7 ± 1.15 mmHg in the ASB group, 21 ± 3 mmHg in the HbV group, and 22.3 ± 8.5 mmHg in the LR group, indicating that hemorrhagic shock was uniformly established (). Immediately after resuscitation, the MAP was 62.7 ± 6.8 mmHg in the HSA group, 51 ± 19 mmHg in the ASB group, 78 ± 11.0 mmHg in the HbV group, and 52 ± 8.7 mmHg in the LR group. There was no significant difference in pre- or immediate post-resuscitation MAP among the groups.
Figure 1b. Effects of Lactated Ringer's solution (LR), human serum albumin (HSA), autologous shed blood (ASB), and hemoglobin vesicles (HbV) on MAP (mean arterial pressure) in anesthetized dogs subjected to 50% exsanguination. There were significant differences between the LR and HbV groups 30 minutes after resuscitation. (*) (○: LR group; : HSA group; × : ASB group; △: HbV group).
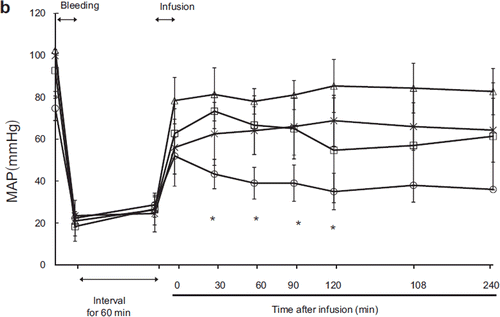
After resuscitation, MAP was low in the LR group and high in the HbV group. There were significant differences between the HbV and LR groups at 30 (p = 0.01), 60 (p = 0.02), 90 (p = 0.03), and 120 (p = 0.048) minutes after resuscitation. There were no significant differences between the HSA and ASB groups, the ASB and HbV groups, or the HSA and HbV groups at any timepoint.
Mean Pulmonary Arterial Pressure (MPAP)
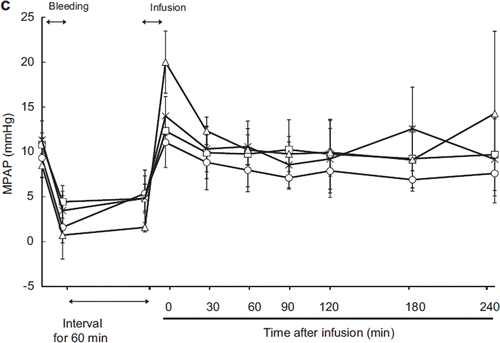
After exsanguination, MPAP dropped below 5 mmHg in all groups (). Immediately after resuscitation, MPAP recovery was observed in all groups. MPAP was 20.0 ± 3.5 mmHg in the HbV group, significantly higher than in the ASB, HSA, or LR group. This elevation quickly returned to the normal MPAP range, and 30 minutes post-resuscitation, there were no significant differences between HbV and the other groups.
Figure 1c. Effects of Lactated Ringer's solution (LR), human serum albumin (HSA), autologous shed blood (ASB), and hemoglobin vesicles (HbV) on mean pulmonary arterial pressure (MPAP) in anesthetized dogs subjected to 50% exsanguination. There were no significant differences between the HbV group and the other groups. (○: LR group; □: HSA group; × : ASB group; △: HbV group).
Pulmonary Capillary Wedge Pressure (PCWP)
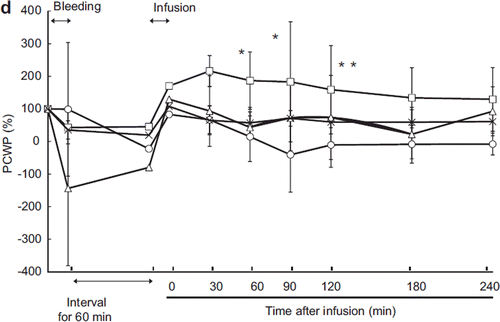
The change of PCWP was similar in all groups except the LR group (). Immediately after resuscitation, PCWP recovered to the baseline levels and remained stable in the ASB, LR, and HbV groups. However, the PCWP increased proportionally more in the HSA group than in the other groups and remained high throughout the experiment. This indicated that the HSA group required a higher end-diastolic left-ventricular pressure in order to maintain cardiac output.
Figure 1d. Effects of Lactated Ringer's solution (LR), human serum albumin (HSA), autologous shed blood (ASB), and hemoglobin vesicles (HbV) on pulmonary capillary wedge pressure (PCWP) in anesthetized dogs subjected to 50% exsanguination. Value is expressed as a ratio to that of the baseline. There were significant differences between the LR group and the HSA, ASB, and HbV groups (η) and between the HSA and HbV and ASB groups (*). (○: LR group; □: HSA group; × : ASB group; △: HbV group).
Cardiac Output
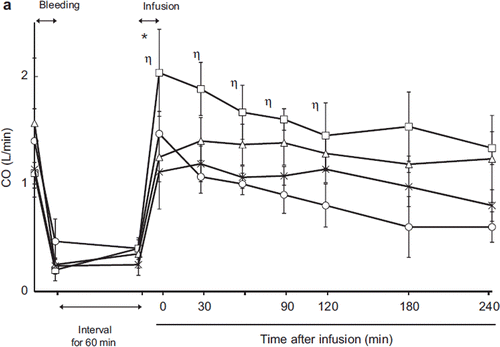
The pre-exsanguination cardiac output (CO) was 1.4 ± 0.3 L/min in the LR group, 1.1 ± 0.1 L/min in the HSA group, 1.14 ± 0.26 L/min in the ASB group, and 1.57 ± 0.6 L/min in the HbV group (). There were no significant differences between the groups. Exsanguination decreased CO to 0.20.47 L/min. Post- exsanguination, CO was 0.40 ± 0.10 L/min in the LR group, 0.4 ± 0.1 L/min in the HS group, 0.25 ± 0.1 L/min in the ASB group, and 0.35 ± 0.13 L/min in the HbV group. After resuscitation, cardiac output increased in every group (2.03 ± 0.4 L/min in the LR group, 1.47 ± 0.21 L/min in the HSA group, 1.11 ± 0.34 L/min in the ASB group, and 1.25 ± 0.23 L/min in the HbV group). The increase was greater in the HSA group than in the other groups, and there was a significant difference between the HSA and HbV groups (p = 0.04) immediately after resuscitation. After resuscitation, CO was stable in and not significantly different between the HbV, ASB, and HSA groups. In the LR group, CO decreased with time and was significantly lower than in the HbV group 90 minutes post-resuscitation.
Figure 2a. Effects of Lactated Ringer's solution (LR), human serum albumin (HSA), autologous shed blood (ASB), and hemoglobin vesicles (HbV) on cardiac output (CO) in anesthetized dogs subjected to 50% exsanguination. There were significant differences between the LR and HSA groups (η) and between the HSA and HbV groups (*). ( : LR group; □: HSA group; × : ASB group; △: HbV group).
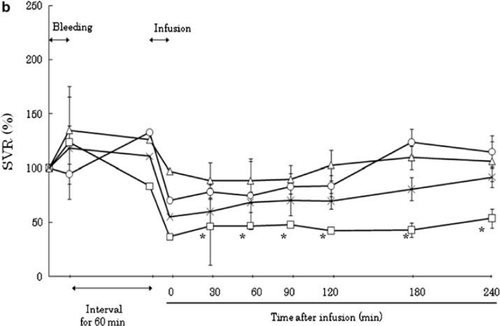
Vascular Resistance
After shock was established, the systemic vascular resistance (SVR) rose in every group (, ). Resuscitation restores the circulation volume, which in turn decreases the SVR. After resuscitation, the SVR decreased to 42.7–53.8% of its pre-exsanguination level in the HSA group and to 88.8–109.8% of its pre- exanguination level in the HbV group. In the LR group, SVR first decreased after resuscitation to 70.4% of initial pressure and then gradually increased to 115.0%. In the ASB group, SVR increased gradually after resuscitation from 55.1 ± 9.5% to 92.0 ± 8.4% of its pre-exanguination level. This gradual increase in SVR may have been due to the lack of maintenance fluid administration after resuscitation.
Figure 2b. Effects of Lactated Ringer's solution (LR), human serum albumin (HSA), autologous shed blood (ASB), and hemoglobin vesicles (HbV) on systemic vascular resistance (SVR) ratio in anesthetized dogs subjected to 50% exsanguination. There were significant differences between the HSA and HbV groups after resuscitation. (○; LR group, □; HSA group, × ; ASB group, △; HbV group ).
The effect of resuscitation fluid on pulmonary vascular resistance (PVR) seemed independent from that on SVR. The PVR was stable after resuscitation in all groups except for LR. In the LR group, PVR increased gradually after resuscitation, although there was no significant difference from the other groups.
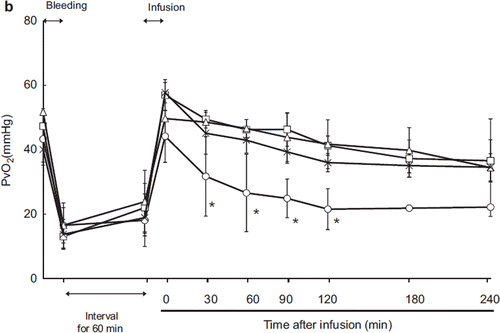
Arterial (PaO2) and Mixed Venous (PvO2) Oxygen Tension
Animals were intubated and ventilated mechanically; therefore, PaO2 remained consistently above 80 mmHg at every time point (). In contrast, mixed venous PvO2 decreased after exsanguination and recovered after resuscitation in every group (). However, in the LR group, the SvO2 declined gradually after resuscitation and was significantly different from that of the HbV group.
Figure 3a. Effects of Lactated Ringer's solution (LR), human serum albumin (HSA), autologous shed blood (ASB), and hemoglobin vesicles (HbV) on PaO2 in anesthetized dogs subjected to 50% exsanguination. There were no significant differences between the HbV group and the other groups. (○: LR group; □: HSA group; × : ASB group; △: HbV group).
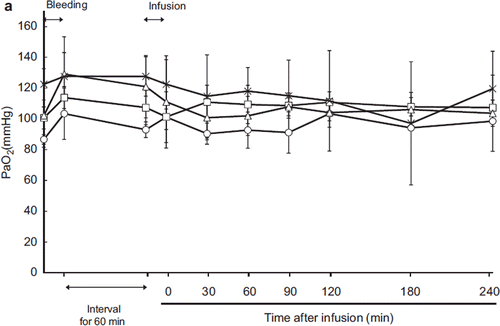
Figure 3b. Effects of Lactated Ringer's solution (LR), human serum albumin (HSA), autologous shed blood (ASB), and hemoglobin vesicles (HbV) on PvO2 in anesthetized dogs subjected to 50% exsanguination. There were significant differences between the LR group and the other groups 30 minutes after resuscitation (*). (○: LR group; □: HSA group; × : ASB group; △: HbV group).
Tissue Oxygen Tension of Renal Cortex and Regional Oxygen Saturation of Brain and Rectus Abdominis Muscle
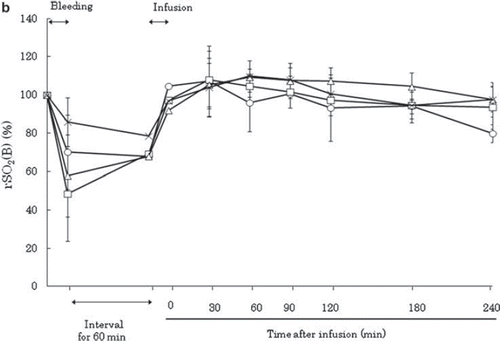
The renal cortical oxygen tension decreased after exsanguination and recovered after resuscitation (). After resuscitation, the PtO2 recovered to the baseline level in every group and there were no significant differences among the groups. The regional oxygen saturation was the mixed value of tissue blood flow and oxygen saturation (). During shock, brain rSO2 decreased but recovered and was maintained at the baseline level after resuscitation in all groups.
Figure 4a. Effects of Lactated Ringer's solution (LR), human serum albumin (HSA), autologous shed blood (ASB), and hemoglobin vesicles (HbV) on PtO2 of the renal cortex in anesthetized dogs subjected to 50% exsanguination. There were no significant differences between the HbV group and the other groups. (○: LR group; □: HSA group; × : ASB group; △: HbV group).
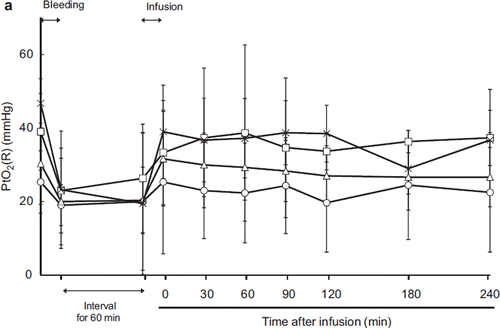
Figure 4b. Effects of Lactated Ringer's solution (LR), human serum albumin (HSA), autologous shed blood (ASB), and hemoglobin vesicles (HbV) on regional oxygen saturation of the brain (rSO2 (B)) in anesthetized dogs subjected to 50% exsanguination. The value was expressed as a ratio to the baseline. There were no significant differences between the HbV group and the other groups. (○: LR group; □: HSA group; × : ASB group; △: HbV group).
Ht and Hb Concentration
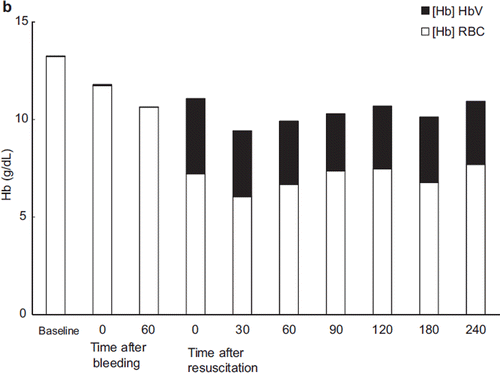
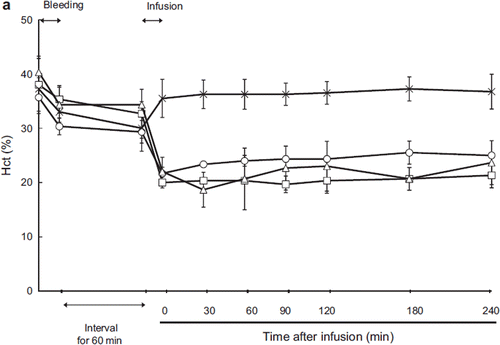
Ht decreased after resuscitation in all groups except ASB (LR: 21.7 ± 1.2, HSA: 20.0 ± 1.0, ASB: 36 ± 3.5, HbV: 22.0 ± 2.6). After resuscitation, Ht gradually increased in the LR group (21.7 ± 1.2% immediately after resuscitation to 25.5 ± 2.1% 180 minutes after resuscitation) (). In the HSA group, Ht did not change after resuscitation (20.0 ± 1.0% to 21.3 ± 2.3%). For accurate measurement of the Hb concentration in the HbV group, the blood was centrifuged and the HbV dispersed in the plasma component was measured separately. The change in Hb concentration is shown in . The Hb derived from RBC was 7.2 ± 1.33 g/dL immediately post- resuscitation and decreased to 6.0 ± 0.69 g/dL at 30 minutes post-resuscitation, then gradually increased to 7.7 ± 1.2 g/dL at 240 minutes post-resuscitation. The concentration of Hb derived from HbV was 3.86 ± 0.4 g/dL immediately after resuscitation and decreased to 3.24 ± 0.14 g/dL at 30 minutes after resuscitation, but there were no significant change in total Hb concentration.
Figure 5a. Effects of Lactated Ringer's solution (LR), human serum albumin (HSA), autologous shed blood (ASB), and hemoglobin vesicles (HbV) on hematocrit (Ht) in anesthetized dogs subjected to 50% exsanguination. There were no significant differences between the HbV group and the HSA and LR groups. (○: LR group; □: HSA group; × : ASB group; △: HbV group).
Acid-base Balance
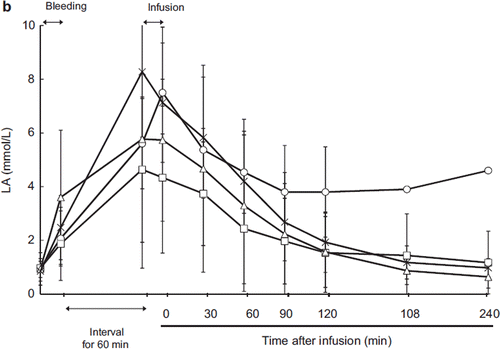
Blood pH decreased and serum lactate increased after exsanguination. After resuscitation, the pH level was restored in every group with no significant differences between groups (, ). The serum lactate level also decreased after resuscitation in the ASB, HSA, and HbV groups. In the LR group, lactate did not recover to the baseline level and serum lactate actually increased 90 minutes after resuscitation, although we cannot evaluate the statistical difference between the other groups because one animal died 150 minutes after resuscitation.
Figure 6a. Effects of Lactated Ringer's solution (LR), human serum albumin (HSA), autologous shed blood (ASB), and hemoglobin vesicles (HbV) on pH in anesthetized dogs subjected to 50% exsanguination. There were no significant differences between the HbV group and the other groups. (○: LR group; □: HSA group; × : ASB group; △: HbV group).
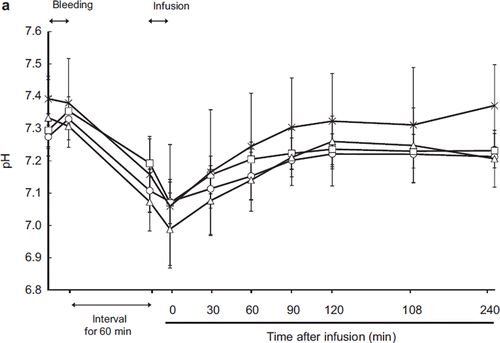
Figure 6b. Effects of Lactated Ringer's solution (LR), human serum albumin (HSA), autologous shed blood (ASB), and hemoglobin vesicles (HbV) on serum lactate level in anesthetized dogs subjected to 50% exsanguination. There were no significant differences between the HbV group and the other groups. ( ○: LR group; □: HSA group; × : ASB group; △: HbV group).
Methemoglobin (metHb)
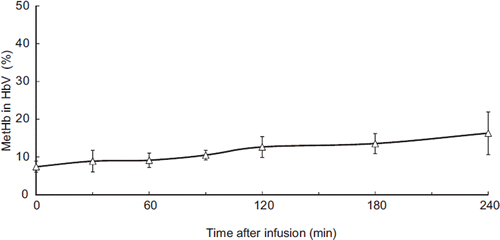
After plasma collection, a portion of the sample was ultracentrifuged and the HbV compartment recovered. The methemoglobin (metHb) component was measured after the liposomes were solubilized and increased with time, constituting 7% of total Hb immediately after resuscitation and 16.3% at the end of the experiment (240 min after resuscitation) (). This phenomenon could have been caused by the oxidative stress of hemorrhagic shock.
Oxygen Delivery and Oxygen Consumption
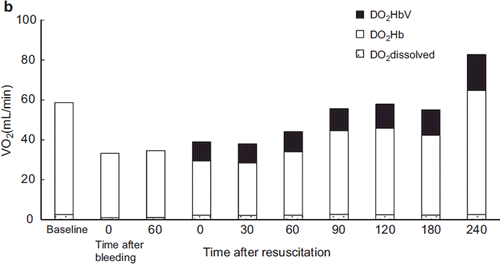
After resuscitation, oxygen delivery improved substantially in each group except for the LR group, in which the recovery of oxygen delivery was only about half that of the other groups (). The change in oxygen delivery in the albumin group was almost identical to that of the ASB and HbV groups. Although the Hb concentration was less than 7 g/dL (6.03–6.27 g/dL) after resuscitation (about 55–57% that of the ASB group), the increased cardiac output compensated the oxygen delivery. In the HbV group, 33.2% (30 min after resuscitation) to 25.8% (240 min after resuscitation) of the oxygen delivery was mediated by HbV itself.
Figure 8a. Contribution of HbV to oxygen delivery. HbV delivered 33–26% of the tissue oxygen supply. (Black bar: oxygen delivered by HbV; blank bar: oxygen delivered by RBC).
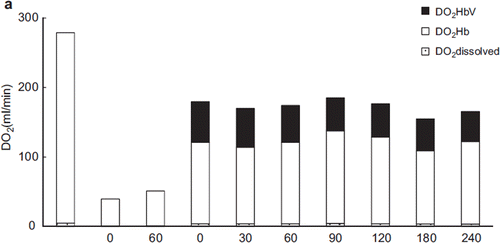
Furthermore, in the HbV group the contribution of HbV-derived oxygen to the total oxygen consumed was calculated as 25.4% (30 min after resuscitation) to 20.1% (240 min after resuscitation) ().
Serum Chemistry
We found a significantly increased albumin-to-globulin (A/G) ratio in the HSA and HbV groups. This is not surprising as we administered albumin as a resuscitation fluid in these groups ().
Table 1. Results of serum chemistry analysis.
Aspartate aminotransferase (AST) and alanine aminotransferase (ALT) were increased in all groups, indicating severe liver damage due to hemorrhagic shock. The increases in AST and ALT were significant in the ASB and HbV groups. Cholinesterase (ChE) is an important enzyme for indicating the liver functional reserve. In the HbV group, ChE decreased significantly compared to the baseline value, which may indicate that HbV influenced liver function.
The change in lactate dehydrogenase (LDH) was similar to those of AST and ALT. In the LR and HbV groups, the differences between baseline and 240 min post-resuscitation values were not statistically significant.
In the LR and HSA groups, the creatinine (Cr) level increased at the end of resuscitation. In the LR group, decreased organ perfusion seemed to account for the significant increase. In the HSA group, Cr increased to 120% of baseline but remained within normal limits.
Creatine phosphokinase (CPK) increased at the end of experiment in the ASB and HbV groups, but there were no significant inter-group or within-group pre- and post-resuscitation differences (ASB: baseline; 419.8 ± 250.3 to 240 min; 773.0 ± 185.8, p = 0.06; HbV: baseline; 459.3 ± 132.8 to 240 min; 733.7 ± 180.4, p = 0.10).
Hb vesicles contain a fair amount of lipid, about five times as much as red blood cells. Although a fair amount of lipid was infused into the animals, the serum lipid component decreased at the end of the experiment. In a rat study, serum cholesterol increased 24 hours after resuscitation [Citation9]. HbV was thought to be still circulating in the blood, and metabolized HbV was small in volume in dogs.
The serum lipid component decreased at the end of experiment in all groups, presumably due to dilution by the resuscitation fluid.
DISCUSSION
We have developed hemoglobin vesicles as an artificial oxygen carrier that is a candidate red blood cell substitute [Citation10]. HbV are composed of concentrated Hb solution covered by a lipid bilayer [Citation4–6]. The efficacy and safety of this material have been tested largely in small animals [Citation11–14]. The results so far have been encouraging. However, we cannot evaluate the pulmonary circulation in small animal studies. Evaluation of the effect of HbV on the pulmonary circulation required that we use larger animals. We chose the canine model because the anatomy and metabolism are similar to those of humans.
We established a hemorrhagic shock model in Beagle dogs, examined the safety and efficacy of HbV as a resuscitation fluid, and evaluated the influence of fluid resuscitation on pulmonary circulation. It was difficult to establish a uniform hemorrhagic shock model in dogs because a dog's spleen contracts in response to blood loss, and splenic contraction supplies extra blood that contributes to the effect of the resuscitation fluid. Therefore, we surgically removed the spleen before the experiment, as previously reported, to produce a uniform hemorrhagic shock in Beagles [Citation15,Citation16].
In cases of hemorrhagic shock, proper hemostasis and restoration of circulation volume as well as transfusion to maintain oxygen transport are the keys to saving life [Citation1,Citation17]. Traditionally, large volumes of crystalloid fluids have been used to maintain the circulation volume and blood pressure during hemorrhagic shock (Advanced Trauma Life Support; ATLS). This ATLS guideline has been criticized for its lack of evidence and poor outcomes, and recently the trend in fluid resuscitation has shifted to hypertonic saline plus a plasma expander [Citation18–21], although several problems, such as coagulopathy and secondary insult to kidney function and head injury, have been noted [Citation22–24]. In this study, isovolemic resuscitation was performed. This resuscitation method restores circulating volume, which we thought most appropriate for evaluating the efficacies of resuscitation fluids.
HbV are small particles, 250 nm in diameter [Citation25]. HbV disperses in the plasma fraction and does not extravasate. As HbV is a particle in solution, HbV in saline does not exert colloid osmotic pressure. Therefore, we used 5% albumin in saline, which has the same colloid osmotic pressure as plasma, as a dispersion fluid.
MAP increased after resuscitation in every group. In the LR group, the recovery of MAP was poor and unstable. One animal died during the experiment (150 minutes after resuscitation). For isotonic crystalloid resuscitation, large-volume resuscitation has been thought necessary. Our result indicated that the permeability of the microcirculation increased after hemorrhagic shock, allowing LR to leak into the interstitial space and thus decreasing the circulating blood volume. The other three groups (ASB, HSA, and HbV) received colloidal fluids that were able to maintain the circulation volume. After resuscitation, MAP was stable in the HbV, HSA, and ASB groups. The LR and HbV groups were significantly different, but we could find no significant differences between the HbV and HSA groups or between the HbV and ASB groups.
In contrast to the MAP, the MPAP increased in the HbV group after resuscitation. The MPAP immediately after resuscitation was also significantly higher in the HbV group than in the other groups. This increase was rapidly resolved, and there were no significant differences between the HbV group and the other groups at later time points. The increase in MPAP was very brief, lasting less than 10 minutes. The highest pressure measured was 20 mm ± 5.6 mmHg. Since pulmonary hypertension is diagnosed in humans when MPAP is above 25 mmHg in the resting state [Citation26], we are inclined to describe this phenomenon as a transient rise in MPAP. This might be considered an effect of HbV infusion.
HbV were 250 nm in size and did not extravasate. Although HbV is able to bind NO, its binding kinetics are slow compared to those of the Hb molecule or a modified Hb-based oxygen carrier [Citation27]. Therefore, it seems unlikely that the transient increase in MPAP could be due to NO scavenging by the HbV. In this study, we used deoxy-type HbV for resuscitation. Rapid infusion of deoxy-HbV might cause transient hypoxia. Although HbV is oxygenated immediately after passing through the lung, hypoxic pulmonary vasoconstriction could occur before the majority of the HbV is oxygenated. We cannot disprove that the significant increase in PAP was due to NO scavenging; however, if NO scavenging is the cause of the transient rise in MPAP, the increase in MPAP might have been expected to be of longer duration. To clarify this point, we will have to perform another study focusing on NO metabolism.
PCWP (pulmonary capillary wedge pressure) recovered after resuscitation in every group. In the HSA group, PCWP increased after resuscitation to significantly higher than the baseline. Cardiac output in the HSA group was high after resuscitation because of compensation for low hemoglobin concentration. High cardiac output was necessary to deliver adequate oxygen to the tissues. An increase in left ventricular endo-diastolic pressure (which is almost identical to PCWP) could be followed by increased cardiac output.
Cardiac output (CO) showed consistent changes in every group (). After resuscitation, CO recovery was high in the HSA group and remained significantly higher than in the HbV group. In the LR group, the recovery of CO decreased with time, indicating volume loss of circulating blood due to fluid shift to the interstitial space.
Vascular resistance after resuscitation decreased compared to the baseline level. After resuscitation, SVR was significantly lower in the HSA group than in the HbV group (). In contrast, SVR was also lower in the LR group than in the HbV group until 120 minutes after resuscitation. In the HSA and LR groups, the resuscitation fluid had no oxygen-carrying capacity. Therefore, it was physiologically appropriate to increase the cardiac output and decrease the SVR in order to facilitate oxygen delivery and maintain tissue oxygenation. This appears to be an auto-regulatory mechanism in this model.
As respiration was maintained by a ventilator, PaO2 and PvO2 were quite consistent (, ). This result indicated that administration of HbV itself did not influence oxygenation in the lung. The PvO2 was decreased in the LR group relative to the other groups, indicating increased oxygen extraction by the peripheral tissues.
We measured tissue oxygen tension at the renal cortex to evaluate the degree of hypoxia due to poor organ perfusion caused by hemorrhagic shock (). We also measured rSO2 (regional oxygen saturation) of the brain (). The kidney is one of the organs most vulnerable to hypoperfusion. The results showed that after exsanguination the PtO2 of the renal cortex decreased uniformly in every group but also recovered to be baseline after resuscitation in every group. HbV did not worsen the renal perfusion or oxygen metabolism.
The regional oxygen saturation (rSO2) of the brain recovered after resuscitation in all groups. These results indicated that HbV was an effective resuscitation fluid in terms of restoring the circulation of vital organs.
Hematocrit (Ht) changed in proportion to the hemorrhage and resuscitation (). The Ht level remained stable after resuscitation in every group. In the HbV group, the Hb concentration attributable to HbV was measured separately. The total hemoglobin concentration is shown in . The Hb concentration derived from HbV was 3.86 ± 0.4 g/dL immediately after resuscitation and decreased to 3.24 ± 0.14 g/dL 30 minutes after resuscitation. This decrease in Hb concentration was thought to be caused by phagocytotic elimination of HbV by the reticuloendothelial (RES) system, although the change in Hb concentration was not significant [Citation9].
Blood pH and lactate are indicators of anoxic metabolism [Citation28]. After resuscitation, the decrease of serum lactate was limited in the LR group because lactate was supplied in the resuscitation fluid (). Ninety minutes post-resuscitation, the LR group showed increased lactate, and one animal later died, indicating hypoperfusion and anoxic metabolism. In the ASB, HSA, and HbV groups, lactate decreased over time to the baseline level, indicating that HbV fluid was as capable of restoring tissue oxygen metabolism as ASB and HSA.
Methemoglobin concentration (MetHb) in HbV was measured after ultracentrifugation to recovers the HbV from the blood. The initial MetHb level in the HbV used for resuscitation was 5.0%. Immediately after resuscitation, MetHb constituted 7.4 ± 1.5% of the Hb, and the proportion steadily increased to 16.3 ± 5.7% 240 minutes after resuscitation (). The oxidization of Hb in HbV was attributed to oxidative stress during hemorrhagic shock and resuscitation. However, even in this oxidative environment, more than 80% of the injected HbV was functional as an oxygen carrier 4 hours after administration.
Oxygen delivery was calculated by measuring the Hb concentration of HbV after resuscitation and the O2 dissociation curve of HbV (). The proportion of the oxygen delivered to the tissues by HbV was 33% (immediately after resuscitation) to 26% (24 min post-resuscitation). When the impact of HbV was measured in terms of oxygen consumption, 25.0–20.1% of the oxygen consumed was delivered by HbV (). This result showed that HbV circulated during resuscitation and contributed to the oxygen delivered and consumed.
The influence on serum chemistry of HbV was evaluated. As HbV was dispersed in 5% albumin saline, the albumin increased after resuscitation similarly to that of the HSA group. The liver is one of the organs vulnerable to damage by hypoperfusion and hypoxia. From the results of hemodynamic observation, all groups experienced hemorrhagic shock. AST and ALT increased in the LR, HbV, and ASB groups. In the HbV and ASB groups, the end-experiment values were significantly increased from the baseline. In the HbV group, ChE decreased significantly after resuscitation. ChE is a short half-life serum enzyme that represents the protein synthesis function of the liver. This result showed that HbV suppresses hepatic protein synthesis after resuscitation of hemorrhagic shock. The liver functions as one of the RES organs, and HbV is phagocytosed by macrophages and further metabolized. This process might cause temporary suppression of liver function. ChE did not change after resuscitation in the rat, indicating a species difference in the effect of HbV [Citation9]. Further investigation of the recovery of liver function will require a longer study.
The increase of LDH indicates systemic or organ cellular damage. The LDH results in the LR and HbV groups seem to indicate severe cellular damage. Hypoperfusion is thought to be the cause of the organ dysfunction in the LR group [Citation29]. In the HbV group, the increased LDH might be a side effect. Further study is necessary to clarify the cause of the increased LDH and whether it is affected by dose and speed of administration.
Cardiac toxicity has been noted for a hemoglobin-based oxygen carrier [Citation30]. Our HbV is encapsulated by a lipid bilayer, which we suspected would avoid this effect of free hemoglobin molecules. In this study, CPK did increase in the HbV group, although not significantly. It was noteworthy that the ASB group also showed increased CPK. Since we splenectomized the animals before the experiment, the abdominal wall, including muscles, was damaged during laparotomy and splenectomy. This damage might be the source of the increased CPK, and an isozyme study to test this hypothesis should be considered.
The decreased lipid component is thought to be a dilutional effect of the resuscitation fluid. This study was designed to examine the short-term circulatory effect on Beagle dogs; another study should be planned to investigate the long-term effects.
Organs were recovered at the end of the experiment and examined histologically; we observed no significant changes except in the LR group, in which slight congestion of the liver was noted at 240 min after resuscitation.
In this pilot study, we evaluated the short-term safety and efficacy of HbV as a resuscitation fluid for hemorrhagic shock in Beagle dogs. The long-term effects of administering a large volume of HbV should be tested in another study.
CONCLUSIONS
HbV dispersed in a 5% albumin/saline solution was as effective as ASB or HSA in resuscitation of a 50% hemorrhagic shock resuscitation model in Beagle dogs. In this model, 50% of the circulation volume of resuscitation fluid was administered into the right femoral vein over 20 minutes. Rapid large-volume administration could be performed safely in Beagle dogs. We found a temporary increase in MPAP after resuscitation. The infused HbV was deoxy-form. Although oxygenation occurs immediately, hypoxic vasoconstriction could occur before most of the HbV was converted to the oxygenated form. We could not conclude that the significant increase in PAP was due to NO scavenging. MAP increased after resuscitation and remained stable in the HbV group. If the temporary increase in PAP is due to NO scavenging, it should have lasted longer and should have been reflected in the systemic pressure. We concluded that the transient increase of MPAP after infusion of HbV should be followed up in another study focused on NO metabolism. Long-term effects, including organ toxicity, should also be examined in follow-up studies.
Declaration of Interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
REFERENCES
- American College of Surgeons Committee on Trauma. (2008). Advanced Life Support for Doctors (ATLS) Student Course Manual, 8th ed., American College of Surgeons, Chicago.
- Rossaint, R., Bouillon, B., Cerney, W., Coats, T.J., Duranteau, J., Fernandez-Mondejar, E., Hunt, B.J., Komadina, R., Nardi, G., Neugebauer, E., Ozier, Y., Riddez, L., Schulz, A., Stahel, P.F., Vincent, J.L., Spahn, D.R. (2010). Management of bleeding following major trauma: an updated European guideline. Critical Care 14:R52.
- Finfer, S., Bellomo, R., Boyce, N., French, J., Myburgh, J., Norton, R. (2004). SAFE study investigators. A comparison of albumin and saline for fluid resuscitation in the intensive care unit. N Engl J Med 350:2247–56.
- Sakai, H., Hamada, K., Takeoka, S., Nishide, H., Tsuchida, E. (1996). Physical properties of hemoglobin vesicles as red cell substitutes. Biotechnol Prog 12:119–25.
- Takeoka, S., Ohgushi, T., Terase, K., Ohmori, T., Tsuchida, E. (1996). Layer-controlled hemoglobin vesicles by interaction of hemoglobin with a phospholipid assembly. Langmuir 12:1755–9.
- Sou, K., Naito, Y., Endo, T., Takeoka, S., Tsuchida, E. (2003). Effective encapsulation of proteins into size-controlled phospholipid vesicles using freeze-thawing and extrusion. Biotechnol Prog 19:1547–52.
- Izumi, Y., Sakai, H., Kose, T., Hamada, K., Takeoka, S., Yoshizu, A., Horinouchi, H., Kato, R., Nishide, H., Kobayashi, K., Tsuchida, E. (1997). Evaluation of the capabilities of a hemoglobin vesicle as an artificial oxygen carrier in a rat exchange transfusion model. ASAIO J 43:289–97.
- Sakai, H., Horinouchi, H., Masada, Y., Yamamoto, M., Ikeda, E., Takeoka, S., Kobayashi, K., Tsuchida, E. (2004). Hemoglobin-vesicles suspended in recombinant human serum albumin for resuscitation from hemorrhagic shock in anesthetized rats. Crit Care Med 32:539–45.
- Sakai, H., Horinouchi, H., Tomiyama, K., Ikeda, E., Takeoka, S., Kobayashi, K., Tsuchida, E. (2001). Hemoglobin-vesicles as oxygen carriers: Influence on phagocytic activity and histopathological changes in reticuloendothelial system. Am J Pathol 59:1079–88.
- Sakai, H., Sou, K., Horinouchi, H., Kobayashi, K., Tsuchida, E. (2008). Haemoglobin-vesicles as artificial oxygen carriers: Present situation and future visions. J Intern Med 263:4–15.
- Izumi, Y., Sakai, H., Hamada, K., Takeoka, S., Yamahata, T., Kato, R., Nishide, H., Tsuchida, E. (1996). Physiologic responses to exchange transfusion with hemoglobin-vesicles as an artificial oxygen carrier in anesthetized rats: Changes in mean arterial pressure and renal cortical oxygen tension. Crit Care Med 24:1869–73.
- Sakai, H., Tsai, A.G., Rohlfs, R.J., Hara, H., Takeoka, S., Tsuchida, E., Intaglietta, M. (1999). Microvascular responses to hemodilution with Hb-vesicles as red cell substitutes: Influences of O2 affinity. Am J Physiol Heart Circ Physiol 276:H553–62.
- Yoshizu, A., Izumi, Y., Park, S.I., Sakai, H., Takeoka, S., Horinouchi, H., Ikeda, E., Tsuchida, E., Kobayashi, K. (2004). Hemorrhagic shock resuscitation with an artificial oxygen carrier hemoglobin-vesicle (HbV) maintains intestinal perfusion and suppresses the increase in plasma necrosis factor alpha (TNFα). ASAIO J 50:458–63.
- Terajima, K., Tsueshita, T., Sakamoto, A., Ogawa, R. (2006). Fluid resuscitation with hemoglobin vesicles in a rabbit model of acute hemorrhagic shock. Shock 25:184–9.
- Jahr, J.S., Lurie, F., Bezdikian, V., Driessen, B., Gunther, R.A. (2006). Measuring circulating blood volume using infused hemoglobin-based oxygen carrier (OxyglobinR) as an indicator: Verification in a canine hypovolemia model. American Journal of Therapeutics 15:98–101.
- Elliot, L.A., Ledgerwood, A.M., Lucas, C.E., McCoy, L.E., McGonigal, M., Sullivan, M.W. (1989). Role of Fluosol-DA 20% in prehospital resuscitation. Crit Care Med 17:166–72.
- Jones, A.E., Kline, J.A. Shock. (2009). In: Rosen's Emergency Medicine Concepts and Clinical Practice, 7th ed., Mosby, Philadelphia, 34–41.
- Stahel, P.F., Smith, W.R., Moore, E.E. (2009). Current trends in resuscitation strategy for the multiply injured patient. Injury, Int J Care Injured 40S4:27–35.
- Cerqueira Braz, J.R., Nascimento, P., Filho, O.P., Braz, L.G., Vane, L.A., Vianna, P.T.G., Rodorigues, G.R., Jr. (2004). The early systemic and gastrointestinal oxygenation effects of hemorrhagic shock resuscitation with hypertonic saline and hypertonic saline 6% Dextran-70: A comparative study in dogs. Anesth Analg 99:536–46.
- Bender, R., Breil, M., Heister, U., Dahmen, A., Hoeft, A., Krep, H., Fischer, M. (2007). Hypertonic saline during CPR: Feasibility and safety of a new protocol of fluid management during resuscitation. Resuscitation 72:74–81.
- Holcomb, J.B. (2006). Fluid resuscitation in modern combat casualty care: Lessons learned from Somalia. J Trauma 61:75–81.
- Morrison, C.A., Carrick, M.M., Norman, M.A., Scott, B.G., Welsh, F.J., Liscum, K.R., Wall, M.J., Jr., Mattox, K.L. (2011). Hypotensive resuscitation strategy reduces transfusion requirements and severe postoperative coagulopathy in trauma patients with hemorrhagic shock: Preliminary results of randomized controlled trial. J Trauma 70:652–63.
- Exo, J.L., Shellington, D.K., Bayir, H., Vagni, V.A., Janesco-Feldman, K., Ma, L., Hsia, C.J., Clark, R.S., Jenkins, L.W., Dixon, C.E., Kochanek, P.M. (2009). Resuscitation of traumatic brain injury and hemorrhagic shock with polynitroxylated albumin, Hextend, hypertonic saline, and lactated Ringer's: Effects on acute hemodynamics, survival, and neuronal death in mice. J Neurotrauma 26:2403–8.
- Chesnut, R.M., Gautille, T., Blunt, B.A., Baldwin, N., Eisenberg, H.M., Jane, J.A., Marmarou, A., Foulkes, M.A. (1993). The role of secondary brain injury in determining outcome from severe head injury. J Trauma 34:216–22.
- Sakai, H., Sou, K., Horinouchi, H., Kobayashi, K., Tsuchida, E. (2008). Haemoglobin-vesicles as artificial oxygen carriers: Present situation and future visions. J Intern Med 263:4–15.
- Lee, S.H., Rubin, L.J. (2005). Current treatment strategies for pulmonary arterial hypertension. J Intern Med 258: 199–215.
- Sakai, H., Okuda, N., Sato, A., Takeoka, S., Tsuchida, E. (2010). Hemoglobin encapsulation in vesicles retards NO- and CO-bindings and O2-release when perfused through narrow gas-permeable tubes. Am J Physiol Heart Circ Physiol 298:H956–65.
- Nguyen, H.B., Rivers, E.P., Knoblich, B.P., Jacobsen, G., Muzzin, A., Ressler, J.A., Tomlanovich, M.C. (2004). Early lactate clearance is associated with improved outcome in severe sepsis and septic shock. Crit Care Med 32: 1637–42.
- Sato, H., Tanaka, T., Kita, T., Tanaka, N. (2010). A quantitative study of lung dysfunction following haemorrhagic shock in rats. Int J Exp Pathol 91:267–75.
- Nathanson, C., Kern, S.J., Lurie, P., Banks, S.M., Wolfe, S.M. (2008). Cell free hemoglobin-based blood substitutes and risk of myocardial infarction and death: A meta analysis. JAMA 299:2304–12.