Abstract
During the process of peripheral nerve regeneration, a single neuron can regenerate and maintain more than one collateral in a regenerative distal stump. Furthermore, some of the new shoots can mature gradually through remyelination and grow into the remote target organ to play a physiological function. Our study found that when neonatal nerve fibers are subjected to a second injury, the regenerative distal stump can regenerate and maintain more than one collateral in the second regenerative distal stump. The neonatal nerve contributed to the functional recovery of the nerve, but the restoration of nerve function was not complete.
Keywords::
Introduction
During peripheral nerve regeneration, a single neuron can regenerate and maintain more than one collateral in a regenerative distal stump. Furthermore, some of the new collaterals can mature gradually, through remyelination, and grow into remote target organs, where they perform their physiological function. However, it is unknown whether neonatal nerve fibers are capable of sprouting one or more collaterals following secondary injury, and whether these fibers have the ability for self-restoration. It is also unknown whether neonatal nerves contribute to the function recovery of injured nerves. Understanding what is happening in these situations will help give us a better insight into the mechanisms of peripheral nerve regeneration, and may even help us find new ways to reconstruct peripheral nerves.
In this study, we used the rat common peroneal nerve (CPN) and tibial nerve (TN) transactions as models for peripheral regeneration. The right CPN was transected and the distal and proximal stumps were bridged at the breakpoint using a biodegradable chitin nerve conduit. Three months after implantation, following which the peroneal nerve had regenerated, we used this nerve as a donor to repair the distal TN that was also transected. In this animal model, we can observe a number of different factors, including the regeneration ability of the TN, the amplification rate of a single nerve fiber while regenerating, the functional activity of the new fiber, and factors affecting nerve regeneration.
Materials and Methods
Materials
Hollow cylindrical biodegradable chitin conduits (hereafter referred to as conduits) were fabricated by Peking University People's Hospital and the Chinese Textile Academy (China Patent No: 01136314.2). Conduit size: Tube length 6 mm, thickness 1mm, inner diameter 2 mm.
Animals and grouping
Male Sprague-Dawley rats, weighing 200–250 g, were maintained under specific pathogen-free laboratory conditions. The rats were divided into two groups at random, experimental, and control groups, with n =8 per group. All animals were maintained in accordance with the guidelines of the National Animal Welfare Act.
Surgical procedures
Initial surgery
Surgical procedures of experimental animals were performed under a binocular surgical microscope using a microsurgical technique. Rats were anesthetized by intra-peritoneal injection of sodium pentobarbital into the abdomen (35 mg/kg). After anesthesia, the right lower limbs were shaved and sterilized. An incision was made in the skin along the long axis of the rear femur, following which the intramuscular septum was blunt dissected, and the sciatic nerve and its two main branches (the CPN and the TN) were exposed. Then, the initial nerve injury models were established by transecting the right CPN at 5 mm below the sciatic nerve bifurcation point. The distal and proximal nerve stumps were then bridged at the breakpoint using the conduit. 0-0 nylon microsutures were used to fix the epineurium within the conduit. The gap between the two nerve stumps was kept at 2 mm. Subsequently, the muscle incision was sutured and the wound was closed using 4-0 nylon sutures. The control group animals were anesthetized and the sciatic nerve and its two main branches (the CPN and the TN) were exposed without surgery, following which the wound was closed in the same way as the experimental group.
Secondary surgery
Three months after the initial surgery, the experimental animals were operated on as follows: The right CPN and TN were exposed and the CPN and TN were both transected with the TN being cut 1 mm under the first breakpoint. Following this, the proximal CPN and distal TN were bridged using a conduit, with the proximal CPN as the donor to repair the transection in the distal TN (). Concurrently, the distal CPN and proximal TN were ligated and fixed to adjacent muscle tissue. The wound incision was then sutured with 4-0 nylon sutures. Three months after the second surgery, a 2-cm segment of nerve near the second breakpoint was harvested and fixed in 4% paraformaldehyde.
Figure 1. Schematic diagram of experimental animal models. (A): The common peroneal nerve was transected 5 mm below the sciatic nerve bifurcation point and the distal and proximal nerve stumps were bridged at the breakpoint using the conduit. Three months after the first surgery, the common peroneal and tibial nerves were transected 1 cm below the first breakpoint and the proximal common peroneal nerve was used as the donor to repair the distal tibial nerve after it was also transected. Schematic diagram of control group animal models (B): The control group animals were anesthetized and the sciatic nerve was exposed without surgery and the wound was closed. Three months after the first surgery, the common peroneal and tibial nerves were transected and the proximal common peroneal nerve and the distal tibial nerve were bridged using the conduit.
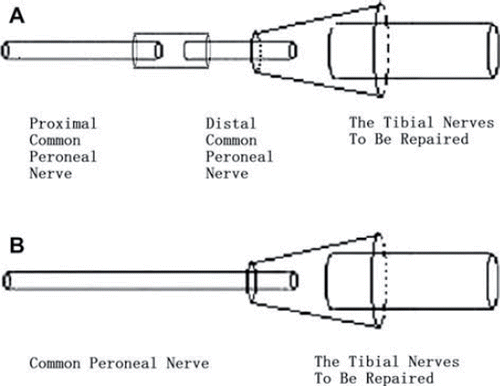
The control group animals were anesthetized and exposed as described earlier and the CPN and TN were transfected at 15 mm below the sciatic nerve bifurcation point, following which the proximal CPN and distal TN were bridged using a conduit (). Concurrently, the distal CPN and proximal TN were ligated and fixed to adjacent muscle tissue. The wound incision was sutured with 4-0 nylon sutures. Three months after the second surgery, a 2-cm segment of nerve adjacent to the second breakpoint was harvested and fixed in 4% paraformaldehyde.
Functional Evaluation
The tibial functional index (TFI) was chosen to assess functional recovery. Rat walking tracks were recorded in a confined walkway that had a dark shelter at the end of a corridor (8.2 × 42 cm). The bottom of the track was lined with strips of paper 10 × 50 cm. Tracks were obtained by dipping the hind feet into red ink before placing the animal at the entrance of the corridor. Three to four prints from both sides were obtained for each track. All rats were trained to walk the corridor prior to any surgical procedure, and a baseline walking track pattern was recorded. Paired footprint parameters for footprint length, distance from first to fifth toe (toe spread), and distance from second to fourth toe (intermediary spread) were recorded for the normal control foot (NPL, NTS, NIT) and the corresponding experimental foot (EPL, ETS, EIT) for each rat. Prints for measurement were chosen at the time of walking based on clarity and completeness at a point when the rat was walking briskly. TFI was calculated as follows: TFI = –37.2 (EPL – NPL)/NPL +104.4(ETS – NTS)/NTS + (EIT – NIT)/NIT– 8.8 (Zhang et al. Citation2005).
Electrophysiological Study
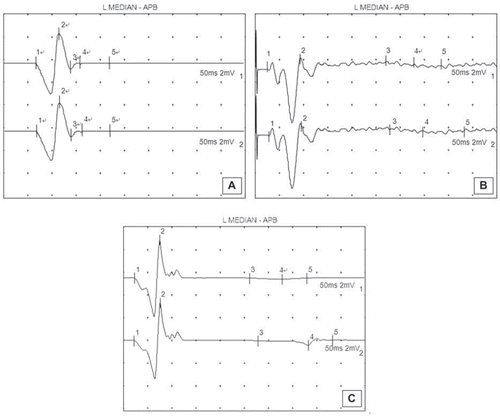
Electrophysiological assessment was performed three months after the second surgery, prior to sacrificing the animals. The repaired TN was exposed and stimulating bipolar electrodes were placed proximal and distal to the repair site in each group. The recording electrode was placed in the gastrocnemius muscle, while the grounding electrode was placed subcutaneously between the stimulating and recording electrodes. Rectangular pulses (duration 0.1 ms, 0.9 mA, 10 Hz, six continual stimuli) were used to stimulate the repaired TN. Compound muscle action potential was recorded and nerve conduction velocity (NCV; m/s) was obtained semi-automatically by dividing the distance between the two stimulating sites by the difference in onset latency. The NCV of the contralateral normal TN was similarly recorded (Jiang et al. Citation2007).
Histological Study
Osmium tetroxide stain
The entire nerve, including the proximal CPN and the distal TN, was removed en bloc from each rat. Tissues were harvested and fixed in 4% paraformaldehyde in 0.1 M phosphate buffer saline (PBS) for 12 h at 4°C. Following this, the nerves were rinsed twice in PBS and three tissue blocks (approx. 5 mm long) were cut, one proximal to the residual segment, one distal to the residual segment, and one from the residual segment. Each sample was then post-fixed in 1% osmium tetroxide for 12 h, dehydrated through a graded series of ethanol, and embedded in paraffin. Specimen sections were then stained with osmium acid, which stains the myelin sheath of axons, and quantified according to an unbiased counting rule. Finally, the total number of myelinated axons was estimated by multiplying the axonal density by the total cross-sectional area of the entire nerve in each animal (Jiang et al. Citation2007, Kou et al. Citation2011, Zhang et al. Citation2010).
Hematoxylin and eosin staining
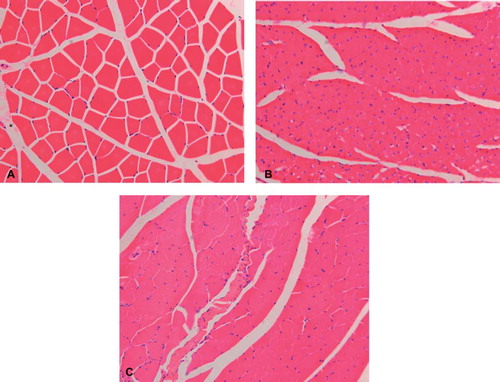
To study general morphology of the muscle, rats were sacrificed by intra-arterial overdose (2 ml) of sodium pentobarbital solution (30 mg/kg) and both sides of the gastrocnemius muscle were harvested, rinsed with PBS, and fixed in 4% paraformaldahyde solution for histological assessment. Fixed specimens were paraffin-embedded, cut into 5-mm sections, and stained with hematoxylin and eosin (H&E). Slides were examined under a light microscope (Zhang et al. Citation2010).
Statistical analysis
Results were expressed as mean ± standard deviation. Differences between the two groups were evaluated by one-way ANOVA, using SPSS version 13.0 software (SPSS Inc., Chicago, USA). A P value less than 0.05 was considered statistically significant.
Results
Morphology of the regenerated nerve
Three months after the second surgery, the TN of each rat (200 to 250 g) was exposed through a dorsal incision. The external appearance of the implanted conduits was smooth at the first suture site, and surrounded with blood capillaries, indicating good biocompatibility of the conduit. Fibrous connective tissue could also be seen adhered to the suture site of the second surgery, and the biodegradable chitin conduit was damaged. Using a surgical microscope, we saw that proximal nerve fibers had grown into the distal nerve stump through the conduits; however, the regenerated nerve fibers in the conduits of the second injury were thinner than the regenerated fibers of the first injury.
Myelinated nerve fiber quantification
Sciatic nerves, including the CPN and TN, were removed, formalin-fixed, stained with osmium tetroxide, paraffin-embedded, and sectioned. Images of transverse sections of the nerve are shown in . Under the microscope the proximal CPN, distal CPN, and distal TN were observed and quantified. The RDP (RDP = the number of the receptor nerve/the number of the donor nerves, RDP1 = the number of the distal CPN/the number of the proximal CPN, RDP2 = the number of the distal TN/the number of the distal CPN) of different groups at the different sites were also quantified. The relevant results are listed in . The number of distal TN and the RDP2 of the experimental group were lower than that of the control group, and both were significantly different (p <0.05).
Figure 2. Images of the transverse section of the nerve following osmium tetroxide staining (X400): The normal tibial nerve (A); the normal peroneal nerve (B); the donor nerve of the second recovery process in the experimental group (C); the receptor nerve of the second recovery process in the experimental group (D); the donor nerve of the second recovery process in the control group (E); the receptor nerve of the second recovery process in the control group (F).
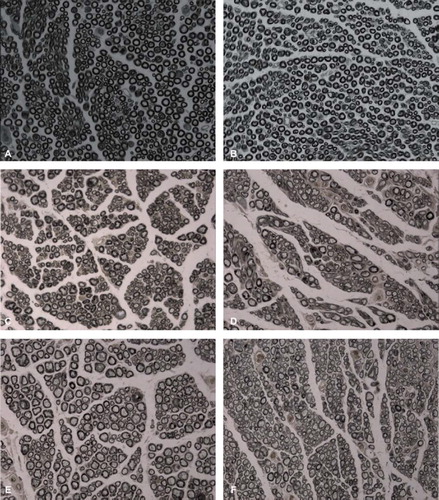
Table I. Number of the myelinated nerve fibers and RDP.
Electrophysiology
Three months after the second surgery, the surgical side peroneal nerve and TN trunk were exposed. The TN trunk was electrically stimulated, the gastrocnemius compound action potential was recorded, and nerve conduction velocity was measured using the latency difference method. The conduction velocity of normal TN is 48.4±4.1 m/s. The neonatal nerve conduction velocity of the control group (19.10±4.30 m/s) was higher than that of the experimental group (30.20±3.70 m/s), with a significant difference between the two groups (p <0.05). The waveform of gastrocnemius compound action potential after stimulation of the normal TN was smooth, with most forming single peaks (). However, the waveform of the gastrocnemius compound action potential after stimulation of neonatal TN was irregular, and formed multiple or overlapping peaks ().
Tibial nerve functional index
The operative procedures were all well tolerated and all wounds healed nicely. No animals died in either group. No trophic ulcerations formed on the operated legs and no autotomy occurred in the control group; however, in the experimental group, two rats had trophic ulcerations on the operated legs and autotomy was observed in three rats. This indicated that there was poor recovery of neurological function in the experimental group. As expected, toe spread was normal in the control group, but was not observed in the experimental group. The TFI of the control group was −75.4±5.7 and the TFI of the experimental group was not detected, indicating dysfunction of the neonatal nerve in the experimental group.
Muscle histology
Compared with the contralateral normal muscle, there were different degrees of gastrocnemius muscle atrophy in the operated side. This atrophy was most obvious in the experimental group. Transverse sections of normal H&E staining in muscle fibers showed a clear boundary, with uniform staining along the length and diameter of the fiber (). Muscle fiber diameter of the operated side in the experimental and control groups were less than normal muscle. Furthermore, the diameter was significantly smaller than normal muscle (). However, muscle atrophy in the control group was significantly better than the experimental group. This indicates that regeneration after the second surgery was not as effective as the initial repair.
Discussion
Peripheral nerve injury repair is limited by poor curative rates, especially poor curative effects of limb defects and root avulsion injuries. Thus, studies in nerve regeneration are a hot topic in tissue engineering and regenerative medicine. In this project we focused on whether newly regenerated fibers have the ability to sprout one or more collaterals that can self-repair when neonatal nerve fibers are subjected to a secondary injury. The most important finding of this study was that the distal stump of the injured nerve regenerated and maintained more than one collateral in the secondary regenerative distal stump; however, the function of the restored limb was poor and there was a high rate of autophagy. This showed that the function of neonatal nerve fibers after secondary regeneration is poor.
During peripheral nerve regeneration, amplification of damaged nerves can occur. Baoguo Jiang et al. (Citation2007) confirmed the existence of peripheral nerve bifurcation during the regeneration process (Sun et al. Citation2002, Jiang and Zhang Citation2008, Zhang et al. Citation2011). It has been shown that axons extending many collaterals can grow into target organs. Sprouts from the node of Ranvier extend tubes through the basal lamina in the proximal segment that traverse the narrow gap of connective tissue between the proximal and distal stumps, finally entering the distal nerve segment. Thus, a single neuron can regenerate and maintain more than one collateral in the regenerative distal stump.
In this study, we used the rat CPN and TN transections as the models for peripheral nerve injury. Furthermore, we used the proximal CPN as the donor to repair the distal CPN. Three months later, when the CPN had regenerated, we used it as the donor to repair the distal TN that was also transected. As the difference in diameter between the receptor and donor nerves is poor, we used a biological conduit to bridge the injured nerve (Sun et al. Citation2002, Jiang and Zhang Citation2008). This conduit facilitate was sutured into place, thereby reducing the epineurial suture tension as compared to traditional epineurial suturing. The chamber formed by the conduit helps promote nerve regeneration (Zhang et al. Citation2011).
As can be seen from the results of this study, a neural amplification phenomenon occurs in the self-regeneration and restoration of peripheral nerve injury, which we term secondary amplification. However, the secondary amplification ratio is smaller than the primary amplification. The capacity of nerve regeneration is subject to many factors, such as neuronal metabolism, axoplasmic transport, and local axonal nutrition (Rich et al. Citation1987, Makwana and Raivich Citation2005, Yin et al. Citation2007).
In addition, it is well known that secondary degeneration can cause axonal injury to spread from the initial site to other neurons. According to previous studies, there are two major factors that affect neuronal regeneration: the organism's age and the distance between the injury site and the neuron (Jiang and Zhong Citation1997). Experimental studies in mouse models showed that if the sciatic nerve is transected in a four-week old mouse, 10% of the corresponding segments of the spinal cord anterior horn motor neuron die (Jiang and Zhong Citation1997). We hypothesize that when neonatal nerve fibers are subjected to a secondary injury, the rough endoplasmic reticulum, Golgi complex, and other structures of the neuron will be exposed to different degrees of damage. Nutrient synthesis of corresponding organelles will be also affected, resulting in secondary regenerating nerve fibers, as well as the donor nerve, receiving fewer nutrients after injury than the first restoration process. This reduces the capacity for regeneration in the secondary injury, which in turn reduces the ratio of secondary amplification compared to the first restoration process.
In our study, the TIF of the experimental group rats could not be detected, indicating poor recovery of muscle function. During peripheral nerve injury repair, one of the causes of target organ dysfunction is the denervation of nerves, which causes the target muscle to weaken, causing atrophy and fibrosis. In this study we saw a progressive loss of muscle innervation, with atrophy occurring rapidly, and a gradual disappearance of muscle striations, which was then replaced by fat and fibrous tissue. Therefore, the reduction of muscle atrophy was key to achieving good regeneration in the treatment of peripheral nerve injury (Li et al. Citation2011).
Outgrowth of collateral sprouts from both afferent and motor axons is a natural process that arises during development of and regeneration in the peripheral nervous system. When a nerve is injured, the proximal stump of damaged axons sends out numerous sprouts to reach endoneurial tubes in the distal nerve stump. In this process, some sprouts remain viable, while others may go astray, eventually being eliminated. The residual axons in the proximal stump and the endoneurial tubes, where the regenerative axons can grow into the distal nerve stump, may greatly influence the number and quality of regenerative axons that grow into the target organ, and thus determine the reconstructive effect (Yin et al. Citation2011).
In this study, our sampling time was three months post-neurorrhaphy. At this stage, the regenerated nerve fibers have completely innervated the distal target organs, partially restoring the function of the target organ, but they are not yet fully matured. Through osmium tetroxide staining of the nerve tissue, we found that the diameter of the regenerated myelinated nerve fiber varies at the time of sampling. As some small diameter nerve fibers can be seen, we need to observe nerve fiber regeneration over a longer time period.
Based on our results, we can use a relatively minor nerve, whose function is not as important as the damaged nerve, as a donor to repair the more important distal nerve, by secondary nerve amplification. The function of the distal nerve and target organs can then be partially restored. The results of this study suggest the feasibility of this method of nerve repair, but the reliability of this treatment requires further clinical validation.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Jiang B, Yin X, Zhang D. 2007. Maximum number of collaterals developed by one axon during peripheral nerve regeneration reinnervation effects. European Neurology 58:12–20.
- Jiang B, Zhang P. 2008. The electrophysiology analysis of biological conduit sleeve bridging Rhesus monkey median nerve injury with small gap. Artif Cells Blood Substit Immobil Biotechnol 36:457–463.
- Jiang L, Zhong S. 1997. The mechanism of peripheral nerve repair research. Chinese Journal of Hand Surgery 13:55–58.
- Kou Y, Zhang P, Yin, X. 2011. Influence of different distal nerve degeneration period on peripheral nerve collateral sprouts regeneration. Artif Cells Blood Substit Immobil Biotechnol 39:223–227.
- Li M, Xue C, Gu X. 2011. Ultrastructural changes after denervation of soleus and extensor digitorum longus. Medical Journal of Communications 25:107–109.
- Makwana M, Raivich G. 2005. Molecular mechanisms in successful peripheral regeneration. FEBS Journal 272:2628–2638.
- Pollin MM, McHanwell S, and Slatter CR. 1991. The effect of age on motoneurone death following axotomy in the mouse. Development 112:83–89.
- Rich KM, Luszczynski JR, Osborne PA, Johnson, EM. 1987. Nerve growth factor protects adult sensory neurons from cell death and atrophy caused by nerve injury. Journal of Neurocytology 16:261–268.
- Sun Y, Jiang B, Zhu Q. 2002. The experimental functional medical material in animal body. Spinning and Weaving Science Research 13:7–22.
- Yin X, Kou Y, Wang Y. 2011. Portion of a nerve trunk can be used as a donor nerve to reconstruct the injured nerve and donor site simultaneously. Artif Cells Blood Substit Immobil Biotechnol 39:304–309.
- Yin XF, Zhang DY, Jiang BG. 2007. Alterations in the expression of ATP-sensitive potassium channel subunit mRNA after acute peripheral nerve and spinal cord injury. European Neurology 57:4–10.
- Zhang C, Zhang P, Wang Y. 2010. Early spatiotemporal progress of myelinated nerve fiber regenerating through biological chitin conduit after injury. Artiicial Cells, Blood Substitutes, and Biotechnology 38:103–108.
- Zhang P, He X, Zhao F. 2005. Bridging small-gap peripheral nerve defects using biodegradable chitin conduits with cultured schwann and bone marrow stromal cells in rats. Journal of Reconstructive Microsurgery 21:565–571.
- Zhang P, Yin X, Jiang B. 2011. The experimental research of nerve fibers compensation amplification innervation of ulnar nerve and musculocutaneous nerve in rhesus monkeys. Artif Cells Blood Substit Immobil Biotechnol 39:39–43.