Abstract
Context/objective: Previous studies have demonstrated that various subtypes of the metabotropic glutamate receptors (mGluRs) are expressed in the dorsal root ganglion (DRG) of the peripheral nervous system (PNS), implicating that glutamate potentially contributes to sensory transmission through these receptors. While mGluR expression has been investigated largely in the DRG, the present study focused on mGluR expression on neurons and satellite glial cells (SGCs) of the trigeminal ganglion (TG). Materials and methods: To address the presence of mGluRs in rat TG neurons and their corresponding SGCs, the trigeminal ganglia from six adult male Wistar rats were isolated and immunohistochemistry and immunocytochemistry were performed. The expression of mGluR1α-, mGluR2/3- and mGluR8 on TG neurons and SGCs was investigated in tissue slices and isolated cells. Results: 35.1 ± 6.0% of the TG neurons were positive for mGluR1α, whereas 39.9 ± 7.7% and 55.5 ± 6.3% were positive for mGluR2/3 and mGluR8, respectively. Immunoreactive neurons expressing mGluRs were mainly medium- to large sized, with a smaller population of small-sized neurons showing immunoreactivity. The SGCs showed immunoreactivity toward mGluR1α and mGluR8, but not mGluR2/3, both in the tissue and in isolated cells. Conclusions: Findings from the present study showed that trigeminal neurons express mGluR1α, mGluR2/3 and mGluR8, while SGCs only express mGluR1α and mGluR8. This novel evidence may advance investigations on a possible role of mGluRs in relation to trigeminal pain transmission within the craniofacial region.
Introduction
Craniofacial pain conditions (e.g. headaches or temperomandibular disorders) are common disorders with a high negative impact on quality of life of the patients and are a socioeconomic burden to the society (Citation1,Citation2). The trigeminal ganglion (TG) is suggested to play a key role in the development of craniofacial pain since sensory information from peripheral nociceptors of the craniofacial region passes through this structure to the brainstem, where the signals are projected to the higher cortical centers (Citation3,Citation4). Sensitization of these peripheral nociceptors can be caused by the release of proinflammatory agents or recurrent noxious stimuli, which can further lead to sensitization of central nociceptors in the brainstem, resulting in transformation of acute to chronic pain (Citation5,Citation6). Disturbances in the levels of the neurotransmitter glutamate have been proposed to contribute to the development of peripheral sensitization which in turn can facilitate or maintain craniofacial pain (Citation7–9).
Glutamate interacts with ionotropic (iGluRs) and metabotropic glutamate receptors (mGluRs) (Citation10,Citation11). Activation of group I mGluRs (mGluR1 and mGluR5) can increase neuronal excitation through calcium mobilization, initiated by phospholipase C (Citation12,Citation13). In contrast, activation of group II (mGluR2 and mGluR3) and group III (mGluR4, mGluR6, mGluR7 and mGluR8) mGluRs decrease neuronal excitation by inhibiting adenylyl cyclase (AC) resulting in reduction of intracellular cyclic adenosine monophosphate (cAMP) levels (Citation13,Citation14). The expression of mGluRs has been demonstrated on neurons and glial cells in several structures of the central nervous system (CNS) (Citation15–18). In addition, in sensory ganglia, such as the dorsal root ganglion (DRG), mGluRs have been found on both neurons and glial cells, indicating a possible contribution in pain transmission (Citation19–22). In support of this notion, the injection of group I mGluR agonists 2-methyl-6-(phenylethynyl)-pyridine (MPEP) and (S)-3,5-dihydroxyphenyl-glycine resulted in the development of allodynia and hyperalgesia in rats (Citation22,Citation23). In addition, the application of selective and potent group II mGluR agonists (LY354740, LY379268 and LY389795) reduced pain behavior and mechanical allodynia in both rat inflammatory and neuropathic pain models (Citation24). Furthermore, injection of the non-specific group III mGluR agonist L(+)-2-amino-4-phosphonobutyric acid (L-AP4) significantly reduced pain behavior in rats in an inflammatory pain model (Citation25).
In contrast to DRG, TG has been less studied with regard to neuronal and glial expression of mGluRs. Hence, the aim of the present study was to investigate the expression of mGluR1α, mGluR2/3 and mGluR8 on both neurons and satellite glial cells (SGCs) of the rat TG to provide basis for potential modulation of craniofacial nociception. Understanding the contributory role of SGCs in pain processing is progressing and findings from this study could assist in identifying novel targets for pain management.
Materials and methods
Immunohistochemistry
For immunohistochemistry preparations, three adult male Wistar rats (Taconic, DK; body weight 250–300 g) were deeply anesthetized with a mixture of Hypnorm (Vetapharma, UK), midazolam (Hameln pharma, GER) and sterile water (25/25/50% v/v; 0.2 mL/0.1 kg) and transcardially perfused through the left ventricle with 100 mL isotonic saline (0.9% NaCl) (Fresenius Kabi, DK), followed by 45 mL of 4% formaldehyde (CellPath Ltd, UK). The trigeminal ganglia were removed and post-fixed in 4% formaldehyde at 4 °C overnight, and stored in MilliQ water. Before cryosectioning, the tissue was dehydrated in a 20% sucrose solution at 4 °C overnight, and then transferred to 40% sucrose solution for three days. The ganglia were then embedded in Tissue-Tek embedding medium (Sakura Finetek, NL) in cryomolds and frozen to optimal cutting temperature (−20 °C) for 24 h. Tissue sections were transversely cut (10 µm) using a cryostat, and subsequently placed on poly-lysine-coated glass slides (Sigma-Aldrich, St. Louis, MO, USA). In order to avoid the possibility of investigating the same sections of the TG, only every fifth section was mounted.
The tissue sections were incubated in 5% bovine serum albumin (BSA) (Europa Bioproducts Ltd, UK) containing 0.2% triton X-100 (Sigma-Aldrich, St. Louis, MO, USA) for 1 h at room temperature (RT) and then washed three times in PBS (GIBCO, UK). The samples were then incubated at 4 °C overnight in rabbit polyclonal anti-mGluR1α (1:200; Abcam, UK), rabbit polyclonal anti-mGluR2/3 (1:250; Abcam, UK), or rabbit polyclonal anti-mGluR8 (1:50; Abcam, UK). After rinsing in PBS, the slides were incubated with donkey polyclonal secondary antibody to rabbit IgG (Anti-rabbit Alexa Fluor® 555) (1:500; Abcam, UK) for 1.5 h, followed by final washes in PBS. Counterstaining was performed with nuclear stain Hoechst (1:3000) for 10 min followed by two washes in PBS and one in MilliQ water. Finally, slides were mounted with coverslips, using Fluorescent Mounting Medium (DAKO, DK).
Immunocytochemistry
For immunocytochemistry preparations, three adult male Wistar rats (Taconic, DK; body weight 250–300 g) were anesthetized with a mixture of Hypnorm (Vetapharma, UK), midazolam (Hameln pharma, GER) and sterile water (25/25/50% v/v; 0.2 mL/0.1 kg) and euthanized by cervical dislocation and further decapitation. The trigeminal ganglia were removed and transferred to a collagenase solution (5 mg/mL) (Sigma-Aldrich, Copenhagen, DK) for 15 min at 37 °C, followed by centrifugation at 1200 revolutions per minute (RPM) at RT. The collagenase was aspirated and 0.125% trypsin (Invitrogen, DK) was added for 10 min at 37 °C. The solution was then centrifuged at 1200 RPM for 5 min. Trypsin was aspirated and the pellet was re-suspended in 5 mL Ham’s F12 medium (Invitrogen, DK) supplemented with 10% endotoxin-free, heat-inactivated fetal calf serum (GIBCO Life Technologies, Invitrogen, CA) and 1% penicillin and streptomycin. Through manual mechanical dissociation, the solution was made homogenous and the resulting cell suspension was added to an uncoated 25 cm2 culture flask and incubated for 3 h at 37 °C. After decantation, 5 mL fresh complete growth medium was added and the medium was replaced every second day. After a week, the SGCs were seeded at a density of 2000 cells/well in uncoated eight-well chamber slides and two days later, the SGCs were washed in PBS and fixed in 4% paraformaldehyde for 20 min on ice.
The cells were incubated in 5% BSA with 0.2% Triton X-100 for 1 h at RT, and rinsed thrice in PBS. Cells were then incubated in the primary antibodies at 4 °C overnight: rabbit polyclonal anti-mGluR1α (1:200; Abcam, UK), rabbit polyclonal anti-mGluR2/3 (1:200; Abcam, UK), or rabbit polyclonal anti-mGluR8 (1:50; Abcam, UK). In order to confirm that the investigated cells were indeed SGCs, mouse anti-glutamine synthetase antibody (mouse anti-GS) (1:250; Abcam, UK) was employed and used as a marker for SGCs. After three washes in PBS, the SGCs were incubated in secondary antibodies for 1.5 h at RT: donkey polyclonal secondary antibody to mouse IgG (Anti-mouse Alexa Fluor® 488) (1:500; Abcam, UK); or Anti-rabbit Alexa Fluor® 555 (1:500; Abcam, UK). Subsequently, the cells were rinsed in PBS and counterstained with nuclear stain Hoechst (1:3000) for 10 min followed by two washes in PBS and one in MilliQ water. Finally, the slides were mounted with coverslips using Fluorescent Mounting Medium (DAKO, DK).
Image processing and data analysis
For the immunohistochemistry analysis, five non-overlapping images were captured for each slide at 200× and 300× magnification using a Nikon AZ100 upright fluorescence microscope. For adjustment of the signal-to-noise ratio the images were processed in ImageJ (Version: 1.46 r, NIH, Bethesda, MD, USA) and neuron diameter and color intensity were recorded. The percentage and diameter of immunoreactive neurons for each of the receptor subtypes are presented as the grand mean ± standard error of the mean. Based on the diameter of the neuronal cell bodies, the neurons were classified as either being small (<25 µm), medium (25–35 µm) or large sized (>35 µm) neurons, in accordance with a previously published study (Citation26). The color intensity was measured in order to estimate the immunoreactivity of the neurons by the following criteria: a neuron was considered positive if the mean color intensity of that neuron surpassed a calculated threshold of background intensity (mean of three random background measurements + two standard deviations). Since it is not possible to clearly distinguish the boundaries of individual SGCs in tissue slices using fluorescence microscopy, SGCs were considered positive if SGC nuclei were located within the intense immunoreactive area of positive neurons. All the images were studied in a blinded fashion to avoid any bias.
For the immunocytochemistry, images were captured at 100× magnification using a Nikon AZ100 upright fluorescence microscope, and staining for the anti-rabbit mGluR subtype (red color) was used to confirm or refute the expression of the receptor.
Results
Expression of mGluRs on neurons in TG tissue
For mGluR1α, 35.1 ± 6.0% of the neurons was found to be positive (B and C). The mean diameter of these neurons was 33.5 ± 0.7 µm, distributed as 17.1% small-sized, 38.1% medium-sized and 44.8% large-sized neurons (A). Similarly, 39.9 ± 7.7% of the trigeminal neurons were found to be positive for mGluR2/3 (F and G), and the mean diameter of these neurons was estimated to be 33 ± 0.8 µm (19.70% small-sized, 40.15% medium-sized and 40.15% large-sized neurons; B). In the TG sections stained with mGluR8, 55.5 ± 6.3% of the neurons were positive for this receptor (B and C). Immunoreactive neurons were distributed as 5.7% small-sized, 41.1% medium-sized and 53.2% large-sized neurons with a mean diameter of 35.9 ± 0.7 µm (C).
Figure 1. Size distribution of immunoreactive trigeminal neurons. Assessment of neuronal diameters (µm) of mGluR1α (A), mGluR2/3 (B) and mGluR8 (C) populations showing that neurons being immunoreactive for each of the three receptors are mainly medium- (25–35 µm) to large-sized neurons (>35 µm). mGluR: metabotropic glutamate receptor.
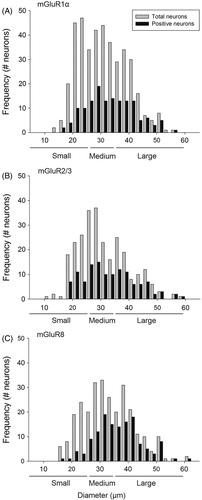
Figure 2. Expression of mGluR1α and mGluR2/3 in the TG. Labeling TG sections with rabbit anti-mGluR1α (B) and rabbit anti-mGluR2/3 (F), followed by counterstaining with Hoechst (A, E), revealed both positive (stars) and negative (cross) neurons (exemplified in 2C and G). Higher magnification of two positive neurons are shown for each mGluR staining (D, H, mGluR1α and mGluR2/3, respectively), where SGCs (arrowheads) were located inside the immunoreactive area (arrow) of the mGluR1α staining (D), indicating that trigeminal SGCs express mGluR1α. In contrast, SGCs (arrowheads) were located outside the immunoreactive area (arrow) of the mGluR2/3 staining (H), indicating that these SGCs do not express mGluR2/3. Magnification = 200× (A, B, C, E, F and G). Scale bars = 50 µm. mGluR, Metabotropic glutamate receptor; TG, trigeminal ganglion; SGCs, satellite glial cells.
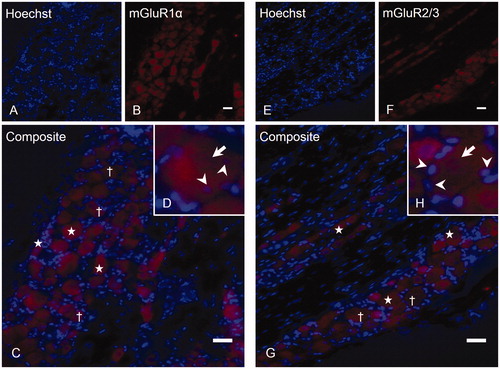
Figure 3. Expression of mGluR8 on trigeminal neurons and SGCs. TG sections were immunostained with rabbit anti-mGluR8 (B) followed by nuclear stain Hoechst (A). A merge (C) is shown, exemplifying positive (stars) and negative (cross) neurons. The same section at 300× magnification (D) shows the characteristic surrounding of neurons (arrows) by SGCs (arrowheads). (E) A positive neuron at higher magnification, where SGCs (arrowheads) are located within the immunoreactive area (arrow), indicating that these SGCs express mGluR8, whereas (F) shows another positive neuron, with SGCs (arrowheads) located outside the immunoreactive area, indicating that these SGCs do not express mGluR8. Magnification = 200× (A, B and C) and 300× (D). Scale bars = 50 µm. TG, Trigeminal ganglion; mGluR: metabotropic glutamate receptor; SGCs: satellite glial cells.
Expression of mGluRs on SGCs in TG tissue
There was minimal staining of mGluR1α (D) and no staining of mGluR2/3 (H) on SGCs in the TG preparations. For mGluR8, assessment of the locations of SGC nuclei in relation to the immunoreactive neurons showed that some (E), but not all (F) trigeminal SGCs were mGluR8 positive.
Expression of mGluRs on isolated, cultured SGCs
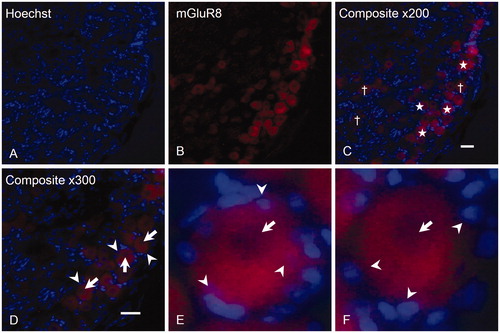
In order to confirm the immunohistochemistry findings, immunocytochemistry was performed. Staining for the specific SGC-marker, GS, showed that all SGCs were GS positive (C), confirming the establishment of SGC-enriched cultures.
Figure 4. Expression of GS by isolated SGCs in culture. In order to confirm the establishment of SGC-enriched cultures, an anti-GS antibody (B) was employed, showing that virtually all cells were GS positive (C). Magnification = 100× (A–C). Scale bar = 50 µm. GS, Glutamine synthetase; SGCs, satellite glial cells.
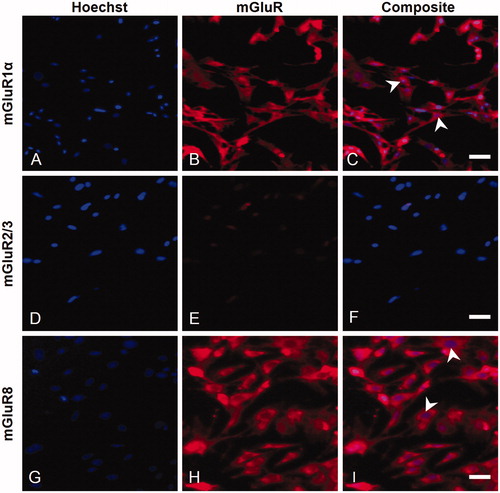
Assessment of mGluR1α in isolated cells indicated that SGCs did express this receptor (A–C). In contrast, no mGluR2/3 immunoreactivity was found on the isolated SGCs, suggesting that this receptor is not present on cultured SGCs (D–F). Similar to the mGluR1α results, mGluR8 staining revealed that trigeminal SGCs express mGluR8, which supports the immunohistochemistry findings (G–I).
Figure 5. Characterization of mGluR expression on isolated trigeminal SGCs in culture. Immunofluorescent labeling of trigeminal SGCs using rabbit anti-mGluR1α (B), rabbit anti-mGluR2/3 (E), or rabbit anti-mGluR8 (H), showed that mGluR1α and mGluR8, but not mGluR2/3, were expressed on cultured trigeminal SGCs. Arrowheads mark examples of SGC nuclei. Magnification = 100× (A–I). Scale bars = 50 µm. mGluR, Metabotropic glutamate receptor; SGCs, satellite glial cells.
Discussion
The current study investigated the expression of mGluR1α, mGluR2/3 and mGluR8 on neurons and SGCs in the TG of adult male Wistar rats. SGCs showed immunoreactivity toward mGluR1α and mGluR8, but not mGluR2/3. All three mGluR subtypes were expressed on neurons, mGluR8 being the most abundant. Immunoreactive neurons were predominantly medium to large sized, with a smaller population of small-sized neurons.
Expression of mGluRs on TG neurons and potential contribution to pain transmission
mGluR1α
In line with an earlier study (Citation27), TG neurons were found to express mGluR1α in the present study. Other lines of evidence have reported that rat DRG neurons express group I mGluRs (Citation9,Citation19,Citation22), but the percentage of mGluR1α immunoreactive neurons in the present study was somewhat higher than it was reported by Carlton and Hargett (Citation19) in DRG. This may indicate a differential expression pattern of mGluR1α in TG and DRG, but may also be a result of different rat species or quantification methods used.
In the present study, neuron sizes were taken into consideration since small- and medium-sized neurons usually facilitate nociceptive transmission (Citation28,Citation29), but it has also been demonstrated that large-sized neurons, i.e. Aβ-fibers, can be activated in allodynia (Citation30). Since mGluR1α activation leads to excitation of neurons, the expression of this receptor on small- and medium-sized TG neurons could signify a participation in nociceptive transmission. For instance, it has been reported that knocking down mGluR1 in rat lumbar spinal cord could reduce hyperalgesia dramatically (Citation31). Moreover, mGluR1 antagonists MPEP and 7-(hydroxyimino) cyclopropa[b]chromen-1 a-carboxylate ethyl ester (CPCCOEt) proved successful in blocking capsaicin-induced glutamate release, indicating that mGluR1 activation may influence nociception through modulation of glutamate release (Citation32). Recent evidences demonstrated that application of a group I mGluR selective agonist (DHPG) on DRG neurons could cause a significant increase in inward calcium currents (Citation9), which could enhance the release of glutamate from neurons. This has indeed been shown in rat hippocampus (Citation33), hence, it is likely that activation of group I mGluRs on sensory neurons could increase extracellular glutamate concentrations both at peripheral and central levels, which in turn could lead to nociceptor sensitization and enhanced craniofacial pain sensations (for review, see Coderre and Katz (Citation34)).
mGluR2/3
The current study demonstrated that mGluR2/3 is expressed on mainly medium- to large-sized neurons. Previously, group II mGluRs have been found in several locations throughout the CNS (Citation18,Citation35) and more recent reports have also demonstrated the presence of group II mGluRs in the DRG (Citation19,Citation21).
Group II mGluRs are predominantly located on presynaptic neurons (Citation11,Citation36) and activation on CNS neurons has been shown to inhibit AC, thereby reducing cAMP levels and neurotransmitter release (Citation37). The expression of mGluR2/3 on the TG neurons may propose a potential role for this receptor in pain transmission, since application of specific mGluR2/3 agonists (LY354740, LY379268 and LY389795) could lower behavioral nociceptive responses in the formalin-pain model in rats (Citation24). In addition, Yang and Gereau (Citation38) reported that plantar injection of a selective group II agonist, (2R,4R)-4-aminopyrrolidine-2,4-dicarboxylate, led to attenuation of pain behavior in prostaglandin E2-induced hyperalgesia. It could therefore be speculated that the activation of mGluR2/3 could result in reduced nociceptive transmission through the TG. This statement, however, needs investigation and remains a speculation as of now.
mGluR8
To our knowledge, this is the first report of mGluR8 expression on neurons in the rat TG. Several authors have reported the expression of group III mGluRs in various locations in both CNS (Citation16,Citation39,Citation40) and DRG in the PNS (Citation19–21).
In the present study, mGluR8 was found to be expressed by mainly large- and medium-sized TG neurons. Since earlier evidence has reported that application of different group III mGluR agonists (L-AP4 or (1S,3R,4S)-1-aminocyclo-pentane-1,3,4-tricarboxylic acid) could exert anti-allodynic and anti-nociceptive effects in neuropathic pain models (Citation41,Citation42) and inflammatory models (Citation42), the expression of mGluR8 on TG neurons might also be important in trigeminal pain transmission. Hence, group III mGluR agonists may prove beneficial in pain management.
Expression of mGluRs on TG SGCs and potential contribution to pain transmission
mGluR1α
Expression of mGluR1α on SGCs in the TG was found. This is in agreement with Carlton and Hargett (Citation19) who reported that SGCs of the DRG showed very minimal staining of mGluR1α, and more recently, Kung et al. (Citation9) demonstrated that SGCs contain functional mGluR1 in vitro.
Following unilateral spinal cord injury in rats, an upregulation of group I mGluRs expression on astrocytes has been described in (Citation43) which potentially could increase nociceptive transmission, e.g. by increasing the release of neurotransmitters (Citation33) or by downregulation of essential components in the glutamate homeostasis machinery, such as the glutamate aspartate transporter (GLAST) and/or glutamate transporter-1 (GLT-1) (Citation17). Both scenarios could lead to an increase in the amount of extracellular glutamate, potentially causing the sensitization of central nociceptors and result in elevated pain sensation. Whether the same mechanism occurs in trigeminal SGCs is not known at present time and needs further investigation. However, the expression of this distinct receptor subtype on SGCs could implicate a unique contribution to nociceptive transmission through the TG.
mGluR2/3
No expression of mGluR2/3 on SGCs was found in the present study. Group II mGluRs in the CNS have been shown to stay silent during physiological conditions, but become activated when glutamate levels increase (Citation44). In addition, it was demonstrated that following activation of mGluR2/3 on human and mouse astrocytes by (2S, 2′R, 3′R)-2-(2′,3′-dicarboxycyclopropyl)-glycine, an upregulation of GLAST and GLT-1 was observed (Citation17,Citation45). Together, this suggests that group II mGluRs are involved in balancing glutamate levels through increased uptake when endogenous glutamate levels are elevated (Citation46). However, the present study investigated the expression in intact rat ganglia, and it is not known whether development of craniofacial injury could alter the expression of group II mGluRs on trigeminal SGCs.
mGluR8
The present study showed for the first time that trigeminal SGCs in rat express mGluR8, which previously has been demonstrated in the rat DRG (Citation19,Citation21).
Several lines of evidence support an autoreceptor function for group III mGluRs in the CNS, i.e. they lower the amount of glutamate released in, e.g. rat hippocampus (for review see Cartmell and Schoepp (Citation47)). In addition, the functional role of group III mGluRs on CNS glial cells was investigated, where Yao et al. (Citation48) demonstrated an upregulation of GLAST following activation of group III mGluRs on astrocytes. Reducing the expression of this transporter on trigeminal SGCs by RNA interference was later shown to cause facial allodynia, indicating that a reduction in glutamate uptake by SGCs may lead to enhanced pain sensations (Citation49). It could therefore be speculated that group III mGluR8 on SGCs partakes in a delicate balance between glutamate release and uptake, and disruption of this SGC-mediated glutamate balance in the TG could counterbalance glutamate levels toward neuronal hyperexcitability and enhanced nociception. Manipulating mGluR8 activity in the TG could therefore possibly reduce excessive amounts of extracellular glutamate, thereby preventing the sensitization of peripheral and/or central nociceptors. The pharmacological properties of mGluR8 on SGCs, however, still remain to be elucidated in order to determine its functional importance with regard to craniofacial pain transmission.
Besides, one should consider that SGCs express a wide variety of other receptors with diverse functional roles. For instance, purinergic receptors, in particular P2X7, have been studied in neuron-to-glia signaling (Citation50). Recently, it has been shown that SGCs of DRG express all three types of iGluRs (Citation9). Furthermore, group III mGluRs have been demonstrated to negatively modulate transient receptor potential vanilloid-1-induced nociception by inhibiting AC, indicating a role in pain conditions (Citation21). Taken together, investigating potential interactions between neurons and glia, underlying pathways, and potential consequences of such interactions, requires further clarifications at cellular levels.
Clinical perspective
Selective targeting of the glutamatergic transmission may potentially facilitate the development of new drugs for pain control. It is known that activation of group I mGluRs and elevated levels of glutamate (Citation51) is associated with the development of hyperalgesic conditions (Citation23). Hence, selective blockade of this group of mGluRs might reverse the condition and offer novel candidates in pain management. Indeed, a mGluR5 negative allosteric modulator, ADX-10059, has been attempted against migraine; however, severe side-effects such as dizziness and visual disturbances inhibited further development (Citation52). In contrary, the inhibitory nature of groups II and III mGluRs are associated with regulating extracellular glutamate levels. For instance, the group II mGluR positive allosteric modulator 1-methyl-2-((cis-(R,R)-3-methyl-4-(4-trifluoromethoxy-2-fluoro)phenyl)piperidin-1-yl)methyl)-1Himidazo[4,-b]pyridine has been shown to reduce phasic glutamate release under restraint–stress conditions in rat prefrontal cortex (Citation53). Thus, neuronal and glial mGluRs can be selectively targeted to inhibit the glutamate release or increase the glutamate uptake. Novel approaches such as positive/negative allosteric modulators for specific targeting might be promising as potential candidates for pain management in the future.
Limitations
It was not possible to clearly separate single SGCs and their boundaries in tissue slices using fluorescence microscopy. This limitation in the methodology yielded a subjective assessment for SGC immunoreactivity. The immunohistochemistry results revealed both positive and negative SGCs, in contrast to results obtained in isolated cells, which showed that virtually all SGCs were immunoreactive for mGluR1α and mGluR8. This may indicate a relatively low sensitivity of the present immunohistochemical assessment. However, it should also be considered that trigeminal SGCs have been shown to undergo phenotypic changes in culture (Citation54), which may raise the question whether the strong response in isolated cells of mGluR stainings was a result of culture artifacts. Nonetheless, both studies employed in the present study seem to be in overall agreement with regard to SGC expression of mGluRs.
Conclusions
The present study characterized the expression of mGluRs within rat TG and provided novel evidence on expression of mGluR8 on trigeminal SGCs. The expression of mGluR8 on SGCs suggests a potential novel target for future research in craniofacial nociception. Overall, findings from the present study support a possible role of peripheral mGluRs in modulation of nociception within the TG.
Declaration of interest
This study was supported by a research grant from the Danish Research Council to Parisa Gazerani in 2010.
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this paper.
Acknowledgements
Special thanks to Tamana Afzali, Christina Bleis and Lyaila Islambekovna Akisheva for technical assistance and to Andreas Kiesbye Øvlisen for editorial assistance.
References
- Scrivani SJ, Keith DA, Kaban LB. Temporomandibular disorders. N Engl J Med 2008;359;2693–705
- Jensen R, Stovner LJ. Epidemiology and comorbidity of headache. Lancet Neurol 2008;7:354–61
- Sessle BJ. Acute and chronic craniofacial pain: brainstem mechanisms of nociceptive transmission and neuroplasticity, and their clinical correlates. Crit Rev Oral Biol Med 2000;11:57–91
- Durham PL, Garrett FG. Emerging importance of neuron-satellite glia interactions within trigeminal ganglia in craniofacial pain. Open Pain J 2010;3:3–13
- Sessle BJ. Recent insights into brainstem mechanisms underlying craniofacial pain. J Dent Educ 2002;66:108–12
- Cairns BE. Pathophysiology of TMD pain – basic mechanisms and their implications for pharmacotherapy. J Oral Rehabil 2010;37:391–410
- Gazerani P, Wang K, Cairns BE, et al. Effects of subcutaneous administration of glutamate on pain, sensitization and vasomotor responses in healthy men and women. Pain 2006;124:338–48
- Svensson P, Cairns BE, Wang K, et al. Glutamate-evoked pain and mechanical allodynia in the human masseter muscle. Pain 2003;101:221–7
- Kung LH, Gong K, Adedoyin M, et al. Evidence for glutamate as a neuroglial transmitter within sensory ganglia. PLoS One 2013;8:e68312
- Nakanishi S. Molecular diversity of glutamate receptors and implications for brain function. Science 1992;258:597–603
- Conn PJ, Pin JP. Pharmacology and functions of metabotropic glutamate receptors. Annu Rev Pharmacol Toxicol 1997;37:205–37
- Abe T, Sugihara H, Nawa H, et al. Molecular characterization of a novel metabotropic glutamate receptor mGluR5 coupled to inositol phosphate/Ca2+ signal transduction. J Biol Chem 1992;267:13361–8
- Pin JP, Galvez T, Prezeau L. Evolution, structure, and activation mechanism of family 3/C G-protein-coupled receptors. Pharmacol Ther 2003;98:325–54
- Tanabe Y, Nomura A, Masu M, et al. Signal transduction, pharmacological properties, and expression patterns of two rat metabotropic glutamate receptors, mGluR3 and mGluR4. J Neurosci 1993;13:1372–8
- Biber K, Laurie DJ, Berthele A, et al. Expression and signaling of group I metabotropic glutamate receptors in astrocytes and microglia. J Neurochem 1999;72:1671–80
- Valerio A, Rizzonelli P, Paterlini M, et al. mGluR5 metabotropic glutamate receptor distribution in rat and human spinal cord: a developmental study. Neurosci Res 1997;28:49–57
- Aronica E, Gorter JA, Ijlst-Keizers H, et al. Expression and functional role of mGluR3 and mGluR5 in human astrocytes and glioma cells: opposite regulation of glutamate transporter proteins. Eur J Neurosci 2003;17:2106–18
- Petralia RS, Wang YX, Niedzielski AS, Wenthold RJ. The metabotropic glutamate receptors, mGluR2 and mGluR3, show unique postsynaptic, presynaptic and glial localizations. Neuroscience 1996;71:949–76
- Carlton SM, Hargett GL. Colocalization of metabotropic glutamate receptors in rat dorsal root ganglion cells. J Comp Neurol 2007;501:780–9
- Li JL, Ohishi H, Kaneko T, et al. Immunohistochemical localization of a metabotropic glutamate receptor, mGluR7, in ganglion neurons of the rat; with special reference to the presence in glutamatergic ganglion neurons. Neurosci Lett 1996;204:9–12
- Govea RM, Zhou S, Carlton SM. Group III metabotropic glutamate receptors and transient receptor potential vanilloid 1 co-localize and interact on nociceptors. Neuroscience 2012;217:130–9
- Walker K, Reeve A, Bowes M, et al. mGlu5 receptors and nociceptive function II. mGlu5 receptors functionally expressed on peripheral sensory neurones mediate inflammatory hyperalgesia. Neuropharmacology 2001;40:10–9
- Zhou S, Komak S, Du J, Carlton SM. Metabotropic glutamate 1alpha receptors on peripheral primary afferent fibers: their role in nociception. Brain Res 2001;913:18–26
- Simmons RM, Webster AA, Kalra AB, Iyengar S. Group II mGluR receptor agonists are effective in persistent and neuropathic pain models in rats. Pharmacol Biochem Behav 2002;73:419–27
- Fisher K, Coderre TJ. The contribution of metabotropic glutamate receptors (mGluRs) to formalin-induced nociception. Pain 1996;68:255–63
- Zhao X, Tang Z, Zhang H, et al. A long noncoding RNA contributes to neuropathic pain by silencing Kcna2 in primary afferent neurons. Nat Neurosci 2013;16:1024–31
- Araki T, Kenimer JG, Nishimune A, et al. Identification of the metabotropic glutamate receptor-1 protein in the rat trigeminal ganglion. Brain Res 1993;627:341–4
- Harper AA, Lawson SN. Conduction velocity is related to morphological cell type in rat dorsal root ganglion neurones. J Physiol 1985;359;31–46
- Lawson SN. Phenotype and function of somatic primary afferent nociceptive neurones with C-, Adelta- or Aalpha/beta-fibres. Exp Physiol 2002;87:239–44
- Devor M. Ectopic discharge in Abeta afferents as a source of neuropathic pain. Exp Brain Res 2009;196:115–28
- Fundytus ME, Yashpal K, Chabot JG, et al. Knockdown of spinal metabotropic glutamate receptor 1 (mGluR(1)) alleviates pain and restores opioid efficacy after nerve injury in rats. Br J Pharmacol 2001;132:354–67
- Jin YH, Yamaki F, Takemura M, et al. Capsaicin-induced glutamate release is implicated in nociceptive processing through activation of ionotropic glutamate receptors and group I metabotropic glutamate receptor in primary afferent fibers. J Pharmacol Sci. 2009;109:233–41
- Parpura V, Haydon PG. Physiological astrocytic calcium levels stimulate glutamate release to modulate adjacent neurons. Proc Natl Acad Sci USA 2000;97:8629–34
- Coderre TJ, Katz J. Peripheral and central hyperexcitability: differential signs and symptoms in persistent pain. Behav Brain Sci 1997;20:404–19; discussion 35–513
- Tang FR, Sim MK. Pre- and/or post-synaptic localisation of metabotropic glutamate receptor 1alpha (mGluR1alpha) and 2/3 (mGluR2/3) in the rat spinal cord. Neurosci Res 1999;34:73–8
- Niswender CM, Conn PJ. Metabotropic glutamate receptors: physiology, pharmacology, and disease. Annu Rev Pharmacol Toxicol 2010;50:295–322
- Wang S, Chen X, Kurada L, et al. Activation of group II metabotropic glutamate receptors inhibits glutamatergic transmission in the rat entorhinal cortex via reduction of glutamate release probability. Cereb Cortex 2012;22:584–94
- Yang D, Gereau RW. Peripheral group II metabotropic glutamate receptors (mGluR2/3) regulate prostaglandin E2-mediated sensitization of capsaicin responses and thermal nociception. J Neurosci 2002;22:6388–93
- Azkue JJ, Murga M, Fernandez-Capetillo O, et al. Immunoreactivity for the group III metabotropic glutamate receptor subtype mGluR4a in the superficial laminae of the rat spinal dorsal horn. J Comp Neurol 2001;430:448–57
- Li H, Ohishi H, Kinoshita A, et al. Localization of a metabotropic glutamate receptor, mGluR7, in axon terminals of presumed nociceptive, primary afferent fibers in the superficial layers of the spinal dorsal horn: an electron microscope study in the rat. Neurosci Lett 1997;223:153–6
- Chen SR, Pan HL. Distinct roles of group III metabotropic glutamate receptors in control of nociception and dorsal horn neurons in normal and nerve-injured Rats. J Pharmacol Exp Ther 2005;312;120–6
- Goudet C, Chapuy E, Alloui A, et al. Group III metabotropic glutamate receptors inhibit hyperalgesia in animal models of inflammation and neuropathic pain. Pain 2008;137:112–24
- Gwak YS, Hulsebosch CE. Upregulation of Group I metabotropic glutamate receptors in neurons and astrocytes in the dorsal horn following spinal cord injury. Exp Neurol 2005;195:236–43
- Kew JN, Ducarre JM, Pflimlin MC, et al. Activity-dependent presynaptic autoinhibition by group II metabotropic glutamate receptors at the perforant path inputs to the dentate gyrus and CA1. Neuropharmacology 2001;40:20–7
- Gegelashvili G, Dehnes Y, Danbolt NC, Schousboe A. The high-affinity glutamate transporters GLT1, GLAST, and EAAT4 are regulated via different signalling mechanisms. Neurochem Int 2000;37:163–70
- Schoepp DD. Unveiling the functions of presynaptic metabotropic glutamate receptors in the central nervous system. J Pharmacol Exp Ther 2001;299:12–20
- Cartmell J, Schoepp DD. Regulation of neurotransmitter release by metabotropic glutamate receptors. J Neurochem 2000;75:889–907
- Yao HH, Ding JH, Zhou F, et al. Enhancement of glutamate uptake mediates the neuroprotection exerted by activating group II or III metabotropic glutamate receptors on astrocytes. J Neurochem 2005;92:948–61
- Ohara PT, Vit JP, Bhargava A, et al. Gliopathic pain: when satellite glial cells go bad. Neuroscientist 2009;15:450–63
- Chen Y, Zhang X, Wang C, et al. Activation of P2X7 receptors in glial satellite cells reduces pain through downregulation of P2X3 receptors in nociceptive neurons. Proc Natl Acad Sci USA 2008;105:16773–8
- Mills CD, Xu GY, McAdoo DJ, Hulsebosch CE. Involvement of metabotropic glutamate receptors in excitatory amino acid and GABA release following spinal cord injury in rat. J Neurochem 2001;79:835–48
- Marin JC, Goadsby PJ. Glutamatergic fine tuning with ADX-10059: a novel therapeutic approach for migraine? Expert Opin Investig Drugs 2010;19:555–61
- Hascup ER, Hascup KN, Pomerleau F, et al. An allosteric modulator of metabotropic glutamate receptors (mGluR(2)), (+)-TFMPIP, inhibits restraint stress-induced phasic glutamate release in rat prefrontal cortex. J Neurochem 2012;122:619–27
- Belzer V, Shraer N, Hanani M. Phenotypic changes in satellite glial cells in cultured trigeminal ganglia. Neuron Glia Biol 2010;6:237–43
