Abstract
Objective: This report describes the design, development, and testing of a novel compact surgical assistant robot to control the orientation and insertion depth of a laparoscopic endoscope during minimally invasive abdominal surgery. In contrast to typical endoscope manipulators, the described robot is particularly compact and lightweight, is simple to set up and use, occupies no floor or operating table space, and does not limit access to the patient in any way.
Materials and Methods: The sterilizable endoscope manipulator is sufficiently small and lightweight at 625 g and 110 mm in diameter that it can be placed directly on the abdomen of the patient without interfering with other handheld instruments during minimally invasive surgery. It consists of an annular base, a clamp to hold an endoscope trocar, and two joints which enable azimuth rotation and inclination of the endoscope about a pivot point at the incision. The endoscope insertion depth is controlled by a cable winding acting against a compression spring on the endoscope shaft. Voice recognition and miniature keypad user command interfaces are provided, and the manipulator motors are backdriveable for manual repositioning.
Results: Endoscope camera trajectory-following accuracy and response-time results were measured using an optical localizer. Experimental results are given comparing the current prototype with the previous cable-driven prototype. The endoscope manipulator and its user interface were tested and evaluated by several surgeons during a series of minimally invasive surgical training procedures on cadavers and animals.
Conclusions: The endoscope manipulator described has been shown to be a viable, practical device with performance and functionality equivalent to those of commercially available models, yet with greatly reduced size, weight, and cost.
Introduction
Minimally invasive surgical procedures, in contrast to conventional open surgery, are performed entirely using long, thin instruments through trocars up to 12 mm in diameter inserted in miniature “keyhole” incisions. These laparoscopic surgical procedures offer considerable advantages over open surgery by minimizing trauma and recovery time for the patient due to the small size of the incisions. However, minimally invasive procedures require increased skill on the part of the surgeon, due to the length of the instruments and the constraint that the shaft of the instrument must pass through a pivot point at the trocar incision. In addition, an endoscope with an attached camera must be inserted into the abdomen and held in a given pose to visualize the instrument tips and internal tissues of the patient on an external video screen during surgery. An assistant is typically required to hold the endoscope and video camera while the surgeon manipulates the laparoscopic instruments with both hands.
To avoid the requirement for additional surgical staff, the human assistant can be replaced by a robotic endoscope manipulator. It is a simple task for a robotic assistant to move an endoscope to a desired orientation and insertion depth inside the abdomen, as the insertion point of the endoscope at its keyhole incision is fixed so that only three degrees of freedom are required. Speed and accuracy requirements for an endoscope robot to match the performance of a human assistant are low, as the orientation and tip position of a handheld endoscope vary over at least several millimeters and degrees due to tremor and fatigue during procedures that may last several hours. Sensitive force control is unnecessary, as the endoscope does not come into contact with internal tissues.
We have developed a compact and lightweight laparoscopic endoscope manipulator for use as a surgical assistant during minimally invasive surgery. This manipulator is dramatically easier to set up and use, and is less costly than currently available alternatives due to its simplicity and small size.
Significance of contribution
Our endoscope manipulator development has been based on the different approach of a small, lightweight robot that is placed directly on the abdomen of the patient and attached to the side of the operating table. The small size and abdominal placement of the robot ensure that the abdomen is completely accessible from all directions during surgery, except within a 50-mm radius of the endoscope trocar. Motions of the surgeon's hands and surgical instruments are never obstructed by the mechanism of the manipulator, which is not the case with endoscope manipulators based on conventional robot arms. Other operating room (OR) staff and equipment need not be rearranged to accommodate our manipulator, as it does not occupy any space next to the patient.
By comparing our newly developed endoscope manipulator to those currently available, we show that a compact manipulator can have functionality equivalent to that of a large manipulator arm and performance superior to that of human assistants, yet also provide significant advantages in terms of ease of setup and use, and be more easily integrated into the OR environment, while using a simpler mechanism that could be produced at greatly reduced cost.
Current endoscope manipulator robots
Several robot systems have previously been developed for laparoscopic endoscope manipulation during surgery Citation[1–4]. These robots generally consist of a large, heavy base and a serial or parallel linkage arm to hold the endoscope. The AESOP (Computer Motion, Davis, CA) Citation[5], Citation[6] and EndoAssist (Armstrong Healthcare, High Wycombe, England) Citation[7] are two examples of currently commercially available endoscope manipulator devices that have undergone clinical validation studies and have obtained various regulatory approvals. In addition, minimally invasive complete telerobotic surgical systems include the Da Vinci (Intuitive Surgical Systems, Sunnyvale, CA) Citation[8] and Zeus (Computer Motion) systems.
Clinical evaluation studies Citation[9–12], primarily undertaken using the EndoAssist and AESOP systems, have been generally favorable, indicating that the impact of using a robotic assistant in laparoscopic surgery is not significant with regard to the safety or efficacy of the procedure. Furthermore, the endoscope image is more stable, and execution times may be reduced.
AESOP
AESOP is a three-degree-of-freedom (3-DOF) robot endoscope manipulator with a SCARA-type architecture. The wrist of the robot contains a 2-DOF passive gimbal joint where the endoscope is held. The base of the robot is clamped to a rail on the side of the operating table. The robot must be draped in sterile plastic during surgery. Minimum and maximum height limits must be set before use, but no other calibration or registration procedure is necessary. The endoscope is held by a magnetic clamp which releases if external forces or torques are too large. The current AESOP 3000 model is available with a voice control system to command motion in all directions, variable speeds, and preset positions. Earlier control systems used pedals.
Various clinical trials have been performed with AESOP Citation[9], Citation[10]. A study of 26 laparoscopic colectomies done with either AESOP or a human assistant showed no significant difference in duration of patient stay or postoperative complications, and surgeons preferred the stability of the image of the robot-supported endoscope Citation[13]. The average operative time required was 235 min with a human assistant and 213 min with AESOP.
EndoAssist
EndoAssist Citation[12] is a floor-standing robot designed as an endoscope manipulator surgical assistant. It consists of an articulated arm and a wheeled base. The robot base is 520 mm wide, 812 mm deep, and extends from 1470 to 2170 mm high.
EndoAssist has 4 actuated joints that correspond to the degrees of freedom of the endoscope, and a passive pin joint where the endoscope is attached. The kinematics of this joint configuration produce a remote center of motion pivot point which must be manually aligned with the incision point of the trocar by rolling the robot base to the correct location and adjusting the height of the vertical axis. Two laser diode spots on the robot aid the manual registration of the pivot point at the incision. The end of the manipulator arm is detachable and autoclaveable, and the arm itself must be enclosed in a sterile bag or drapes during surgery. Generally, the arm holding the endoscope is sufficiently long and high that it does not interfere with the surgeon's instruments, but a curved arm is also available for use in those cases where the straight arm impedes the free motion of the instruments. The developers claim that a freestanding robot with a wheeled base is easier to set up than a robot mounted on the operating table.
The surgeon controls the movement of the EndoAssist manipulator by motions of the head. A headband must be worn with transmitters which are localized by a receiver by the video monitor. The robot only responds to head-movement commands while a footswitch is depressed.
For safety, the end-effector contains a force sensor and the robot is stopped if the force on the endoscope exceeds a given threshold. The passive pin joint and the configuration of the active joints constrain the endoscope to pass through the incision point even in the case of joint failure. Stepper motors with redundant encoders are used to minimize the possibility of uncommanded motion errors.
Clinical evaluation of EndoAssist Citation[12] was performed in a randomized series of 100 laparoscopic cholecystectomies. Procedures undertaken with manually held endoscopes required an average of 75 min, while procedures using EndoAssist required 66 min, resulting in a time saving of 9 min and 10%. The standard deviation of the procedure time was also reduced from 22 to 16 min.
Compact endoscope manipulator motivation
Although current endoscope manipulators perform well, they resemble industrial robots and are generally large, heavy, complex, and expensive. In the limited space of a typical crowded OR, the base of a conventional robot occupies a considerable amount of space next to the patient, and the robot arm holding the endoscope may restrict full access to the abdomen of the patient. We believe that these are the primary obstacles currently limiting the adoption of endoscope manipulators in surgery.
It would therefore be advantageous to use a much more lightweight, simple, and unobtrusive endoscope manipulator which would be easier to set up and use in conventional ORs. The novel features of our endoscope manipulator are that it is sufficiently small and lightweight that it can be placed directly on the abdomen of the patient, where it does not restrict access to the patient or motions of the surgeon's arms and instruments; the device is completely sterilizable; and it does not require any setup or initialization procedure other than placement on the abdomen and attachment to the operating table.
Materials and methods
The principal considerations in the design of our endoscope manipulator have been compact size, so as not to interfere with other handheld instruments during surgery; low weight to permit placement of the device on the abdomen; and simple and reliable setup and operation.
Our earliest endoscope manipulator prototype used four cables in parallel to control orientation and a fifth cable acting against a return spring to control the insertion depth Citation[14], with McKibben artificial pneumatic muscles Citation[15] being used for cable actuation. Later endoscope manipulator prototypes used motorized rack-and-pinion drives in place of the pneumatic muscle actuators and a small mechanism with two rotational joints driven by pairs of antagonistic cables in place of the arrangement of four cables working together in parallel Citation[16].
Current prototype
Preliminary testing of early prototypes indicated the need for modifications to improve the performance, reliability, and practicality of the endoscope manipulator. Pneumatic artificial muscles are difficult to control and have a slow response time compared to motors. Cable actuation through flexible sleeves leads to reduced reliability and accuracy, so the current prototype is actuated directly by miniature motors integrated into the robot mechanism.
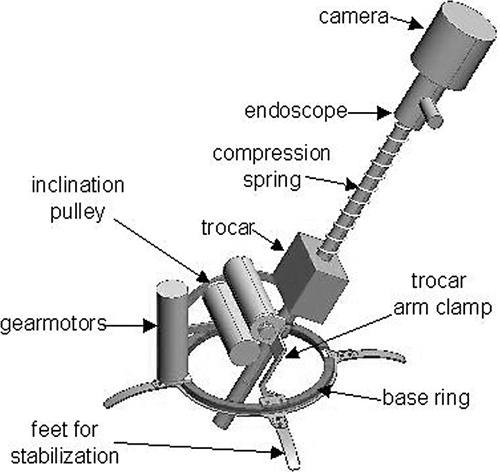
In this mechanism, the endoscope trocar is rigidly clamped to a small pivot arm attached to a rotating ring on the base of the manipulator. The axes of the two rotation joints intersect at the endoscope insertion-point incision. The insertion of the endoscope is controlled by a cable wound on a spool pulling against a compression spring on the endoscope shaft. Rotation of the endoscope axis is currently unactuated, although the camera can be rotated manually if desired.
The trocar clamp is releasable by hand, so that the trocar can be fastened or unfastened easily during surgery if required. Feedback from surgeons led us to design the manipulator mechanism so as to maximize its range of motion in inclination, accommodate various trocar shapes and sizes, and enable the robot to be detached easily from the trocar without removing the trocar from the patient. The insertion cable attaches to the endoscope head by a hook which may be easily detached to remove the endoscope from the trocar for replacement or cleaning of the lens.
The current prototype is pictured in and shown schematically in . It is described in reference 17. The robot is fully sterilizable; all the components can withstand standard autoclave cycles. The robot base is wide enough to pass over all standard endoscope trocars, and the robot may be removed while leaving the trocar in the patient. The trocar clamp accommodates trocars up to 12 mm in diameter. To eliminate the Bowden cables and sleeves and remote motorized rack-and-pinion actuation of the previous prototypes, powerful miniature brushless motors (Faulhaber 2036U024B K1155, Faulhaber Gmbh, Schönaich, Germany) were built into the robot mechanism. Linear Hall-effect sensors on the brushless motors provide angular position feedback to the controllers, as autoclave-resistant shaft position encoders are unavailable.
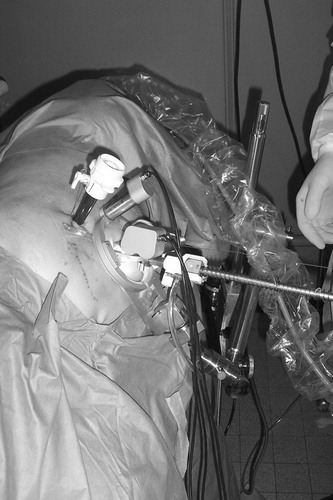
The endoscope robot may be attached to the patient and operating table by different means depending on the patient and procedures to be performed. For large patients in the dorsal position, the robot may be fastened directly to the abdomen with adhesive sheets and/or sutures on the stabilizing feet on the base ring. For patients lying on their sides, and when maximum stability is necessary, the base ring may be clamped to the side of the table by an articulated arm, as shown in .
Motor control
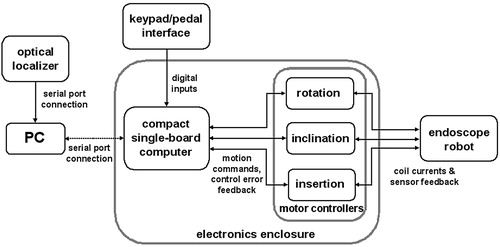
Compact single-axis controllers (MCBL 2805, Faulhaber) provide proportional-integral (PI) error feedback control of the brushless motors with Hall-effect sensor feedback. The motor controllers are programmed to operate in position-control mode to accurately hold a desired position and switch to velocity-control mode when motion commands are given. Input and control gains, minimum and maximum speeds, maximum currents, and other controller parameters are fully configurable and can be written to the motor controller EEPROMs.
The motion commands to the motor controllers are generated by a compact single-board computer (BL2100, Zworld, Davis, CA) with multiple analog and digital inputs and outputs. Use of the single-board computer eliminates the need for a PC interface to the motor controllers and considerably simplifies the setup and initialization of the robot. The power supply, single-board computer, and motor controllers are contained in a 340 × 150 × 120 mm enclosure, so that the entire robot system is easily portable. A schematic of the robot control system is shown in . The PC and its serial port connection to the robot controller are only necessary for use of the optical localizer or the voice-command recognition system.
Command interfaces
An effective user command interface for the endoscope robot must enable the surgeon to reposition the endoscope without any assistance and without releasing either of the instruments held in each hand. As motion commands can be given in both directions for each of the three DOFs of the device, the command interface must provide a minimum of six binary inputs plus an emergency stop command. Variable speeds for coarse-fine positioning would require additional commands.
The motor drives of the endoscope manipulator are backdriveable so that the endoscope may be positioned by hand whenever the current to the motors is switched off. This feature simplifies the initial setup of the robot system when the initial endoscope incision is made.
Manual positioning of the robot is direct, simple, and intuitive, and is the easiest means for initial positioning of the robot before other instruments are introduced into the abdomen and when the surgeon has at least one hand free.
A voice-command recognition system has been implemented as a user command interface in combination with a single pedal which must be depressed to enable motion of the endoscope camera. Voice recognition as a command interface for an endoscope manipulator is already available for the AESOP 3000 manipulator. We used freely available commercial development software packages (Microsoft Speech SDK 5.1 with SpeechStudio Suite) to implement our interface. This speech recognition system is user-independent, aside from having different modes for male or female speakers. The following vocabulary of commands was used:
Move left/Move right/Move up/Move down/Zoom in/Zoom out
Step left/Step right/Step up/Step down/Step in/Step out
Switch on/Switch off
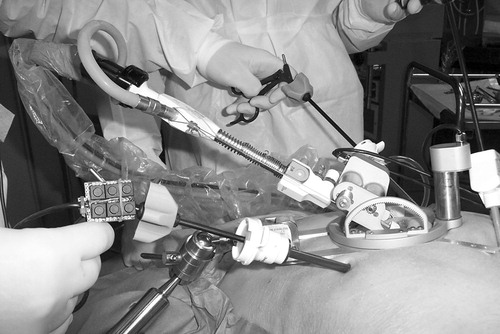
We also provide a miniature keypad which may be clipped onto the handle of any manual laparoscopic instrument next to the rotating wheel to be operated by the index finger of the user, as pictured in . Although this user command interface is not completely hands-free, we observe that while a laparoscopic instrument is held in the hand, the index finger is generally free to move, typically to rotate the tip of the instrument with a rotation wheel.
An automatic positioning system Citation[18] was implemented using an external optical localizer (POLARIS, Northern Digital, Inc., Waterloo, Ontario, Canada) to track motions of the instrument tips and move the endoscope to center the camera image on the instrument tips when desired. For large displacements, the endoscope shaft would be withdrawn before reorientation to avoid sweeping through the volume of the abdomen and potentially contacting internal tissues. As this system requires an additional registration procedure, the additional hardware of the localizer system, and a line of sight between the instrument position marker LEDs and the localizer, it is less practical for use in an OR environment.
Kinematics
The two axes of rotation of the manipulator mechanism intersect at the trocar insertion incision when the manipulator is placed on the abdomen. Passive rotational joints, such as those in the AESOP and EndoAssist manipulators, that constrain motion of the endoscope to pass through the trocar incision are unnecessary in this design.
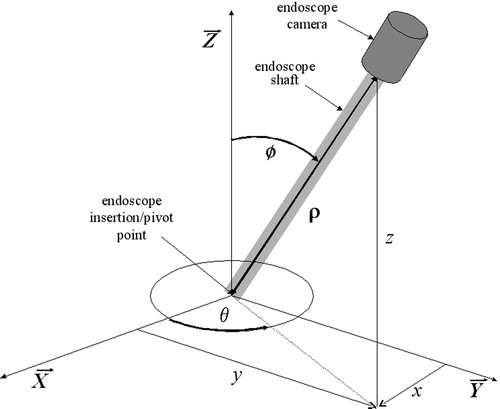
The kinematics of the current manipulator are simple and straightforward, as the actuator position variables directly determine the position of the endoscope camera expressed in spherical coordinates (θ, ϕ, ρ):where q1, q2 and q3 are the positions of the three actuators, Rθ and Rϕ are the radii of the geared horizontal base ring and vertical semicircle which are acted upon by the motors, Rm is the radius of the motor gears, and b is the length of the endoscope.
The motions of the three motors directly correspond to vertical, horizontal, and zoom motions of the endoscope camera image, so that no kinematic calculations are necessary during operation. No homing position or initialization sequence is needed, and absolute sensing of the motor shaft output angle is unnecessary. Although the rotation of the endoscope shaft is unactuated, the endoscope camera image remains correctly oriented during all motions of the endoscope.
Due to the more complex kinematics of the AESOP and EndoAssist manipulators, setup procedures must be performed before use to correctly configure their workspace limits to the anatomy of each patient and the procedure to be performed. Kinematic calculations must also be performed in real time by the controller during motion of these manipulators.
The workspace required by an endoscope manipulator depends on the patient and the procedure to be performed. Although the typical workspace for cholecystectomy is limited, other procedures may require placing the endoscope tip anywhere inside the abdominal cavity. Most laparoscopic procedures are performed using an endoscope with a 300-mm shaft through a 100-mm trocar, so our endoscope robot has a standard translation range of 200 mm for endoscope insertion. This translation can be extended to an arbitrary range by unwinding the translation cable and using a longer spring on the endoscope shaft if a longer endoscope is needed for a given procedure and patient. The necessary range in inclination may extend from vertical to near horizontal for visibility of the abdominal wall near the endoscope incision. However, it is difficult to approach 10° from horizontal due to the thickness of the abdominal wall at the incision. The endoscope can be rotated a full 360° inclined up to 80° away from vertical using our manipulator, so the workspace of the endoscope tip inside the abdomen is a spherical cone with a 160° vertex angle and a 200-mm radius, where the tip of the cone is located at the endoscope trocar incision point.
The relations between the endoscope configuration expressed in Cartesian and spherical coordinates are:which are inversible as:
The vertical configuration of the endoscope corresponds to a kinematic singularity as the value of θ is undefined for ϕ = 0. The two coordinate systems are shown with a schematic of the endoscope in .
If desired, noncontact 3D magnetic or optical localization systems could be used with the endoscope manipulator to provide absolute position feedback rather than the motor position feedback currently used. The localizer 3D coordinates would have to be registered to the endoscope pivot point to convert the localizer Cartesian (x, y, z) coordinates to the spherical/actuator (θ, ϕ, ρ) coordinates using Equation 3.
Safety considerations
The primary safety consideration for any medical device is to minimize any possibility of harm to the patient. Potential harm to the surgeon operator is a secondary consideration. For our endoscope manipulator, the endoscope should be prevented from contacting internal tissues with forces high enough to cause tissue damage in case of failure or malfunction in the robot motors, sensors, controller, or command interface, or as a result of user error.
The low size and weight of our endoscope manipulator are an intrinsic safety benefit, as any collisions with internal organs will dissipate less energy. The maximum motor torques required are also reduced due to reduced gravity loads. To minimize the forces on internal tissues that can arise from endoscope contact, the peak and continuous currents that can be produced by the motor amplifiers have been limited to no more than what is sufficient for positioning the endoscope.
The maximum contact forces that can be generated against soft tissue by the endoscope tip when inserted in the abdomen are approximately 7 N along the axis of the endoscope and 15 N in radial directions. The torque limit on the inclination motor also prevents the fingers of the operator from being pinched.
The kinematics of the robot ensure that the motions of the endoscope always pass through the trocar incision. When the robot is strapped or sutured directly to the patient the robot moves with the patient, and when the robot is clamped to the table by an articulated arm there is sufficient compliance that the abdominal wall would not be significantly damaged in case of excessive patient motion.
The motion command interfaces of the endoscope manipulator are multiply redundant: If any difficulty is encountered with the voice recognition system, the miniature keypad on a surgical instrument may be used. In case of failure of the motors, sensors, or controllers, the disabled motors are backdriveable and the manipulator can be repositioned manually. Furthermore, the endoscope may be disconnected from the robot entirely by manually loosening a single miniature clamp, and the robot can be removed from the patient entirely without disturbing the trocar or endoscope. Breaking or disconnecting the cable winding which actuates the insertion of the endoscope causes the endoscope to be withdrawn from the abdomen by the compression spring on the shaft.
As electrocautery is commonly used in minimally invasive surgery, we tested the effects on the patient and robot of repeatedly touching a live electrocautery instrument to the endoscope manipulator during procedures on a cadaver. The robot and its electronic controller were undamaged, but the patient can be burned if the base of the robot contacts the abdomen along a single edge. This hazard can be eliminated by connecting all external metal parts of the robot mechanism directly to electrical ground.
Results
The performance parameters listed below are as measured for the final prototype. The trajectory-following accuracy of the endoscope robot was measured using an optical localizer (POLARIS, Northern Digital, Waterloo, Ontario Canada) and infrared LEDs fixed to the end of the endoscope. To confirm the utility of the endoscope manipulator, it was tested and evaluated by several surgeons during minimally invasive surgical training procedures on cadavers and animals.
Performance parameters
Measured performance parameters and programmed controller parameters for the endoscope manipulator were as follows:
The size and mass of the device given do not include the 450 mm length and 340 g mass of the endoscope and camera.Trajectory-following experiments
When the endoscope manipulator is under direct control of the surgeon by voice command or keypad control, only one motor is in motion at any given time, as the surgeon gives commands which correspond to vertical, horizontal, or zoom motion of the camera image. Commercial endoscope manipulators such as AESOP and others also respond to commands based on orthogonal motion of the camera image, but multiple motors must move in coordination due to the more complicated kinematics of these endoscope manipulators.
To enable the surgeon to move the endoscope easily to a desired location, the motion of the robot should be smooth at a constant velocity with minimal response delay and no oscillation or overshoot, so that the endoscope camera image moves smoothly and the surgeon can visually track objects in the moving image without difficulty. Absolute positioning accuracy is a less important consideration when all initiation and termination of the manipulator motion is under the direct control of the surgeon.
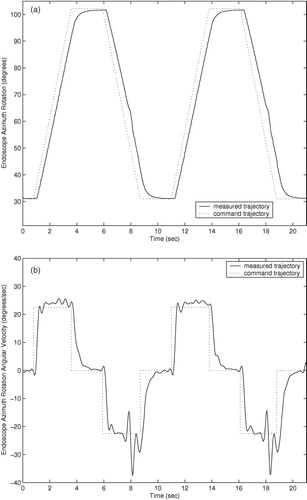
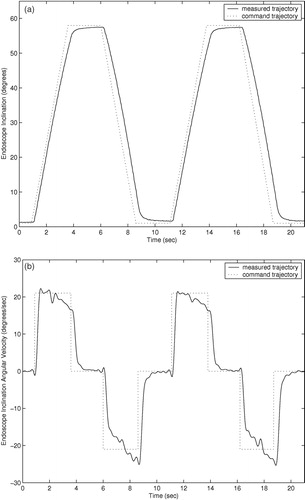
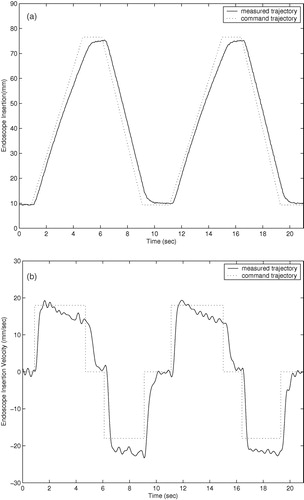
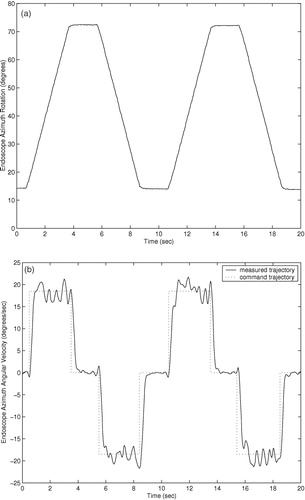
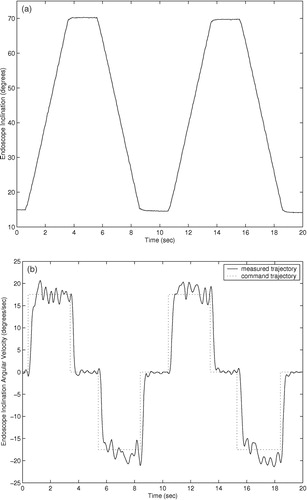
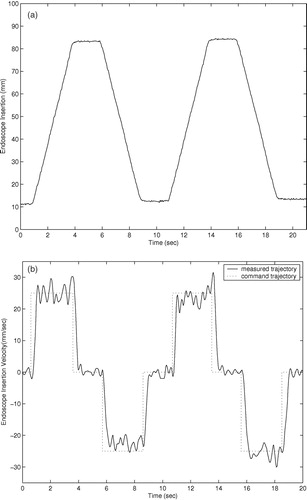
Trajectory responses of an earlier prototype using cable actuation are shown in . The trajectory responses of the current prototype with integrated actuators are shown in . The command trajectories shown consist of constant velocity motions in both directions for each degree of freedom of the two prototypes. The orientation and insertion depth of the endoscope was recorded during each trajectory using a POLARIS optical localizer and infrared LEDs fixed to the eyepiece of the endoscope. Rotation trajectories were executed with the endoscope fully extended. Measured position in millimeters and velocity in mm/second are shown for the insertion depth of the endoscope, and orientation and angular velocity are shown for the rotation and inclination. The position command trajectories in are not shown for clarity. The trajectory-following response of the cable-driven prototype is slower and less accurate than that of the final prototype with integrated motors due to stretching, hysteresis, and added friction in the cable actuators.
The cable-driven prototype shows settling times of approximately 1.0 second. This settling time poses difficulties in positioning the endoscope quickly and accurately under user control. The large velocity errors in the cable-driven rotation response of at 8 and 18 seconds are most likely due to wear in the cables causing added friction when the cable is at a given position. The velocity and angular velocity errors in the cable-driven inclination angle and insertion depth responses of and are due to the variable loads on the cables: The load on the inclination actuator from the weight of the endoscope camera is proportional to sin (φ), and the load on the insertion actuator is proportional to the compression of the endoscope shaft spring.
The settling times of the current prototype manipulator trajectory responses of are approximately 0.5 seconds, and position and angular errors are generally limited to less than 1.0 mm and 1.0° during motion. The improved accuracy and response time of the prototype with integrated motors, compared to the cable-driven prototype, allows the user to move the endoscope to a desired position more quickly and accurately using voice or keypad control commands. Although the required accuracy of an endoscope robot is a subjective measure on the part the surgeon, it is certain that errors of less than 1.0 mm and 1.0° cannot be achieved by handheld endoscopes due to human tremor and fatigue during operations that may last several hours.
The velocity and angular velocity responses exhibit small oscillating errors with a period of 2–3 Hz. These errors are due to resonant vibration of the manipulator and the endoscope camera when the manipulator is supported by a cantilevered bar 200 mm long and the camera extends an additional 200 mm when the the endoscope shaft is extended. To suppress these small vibrations would require either more massive and rigid mounting of the manipulator on the operating table, or much more sophisticated feedback sensing and control. However, these vibrations during motion were found to be imperceptible to surgeons while moving the endoscope.
Cadaver and animal testing
The endoscope manipulator has been used by surgeons to perform various minimally invasive surgical training procedures on cadavers and pigs. These sessions allow us to evaluate the performance of the manipulator in an OR environment, obtain feedback from the surgeons regarding setup and operation, and refine various details of the manipulator and its user interface in preparation for randomized clinical trials on patients. The voice-controlled endoscope manipulator system is shown in use in and , where the endoscope manipulator is clamped to the operating table by an articulated arm and the miniature keypad can be seen fastened to one of the surgical instruments.
The endoscope manipulator was tested and evaluated during pelvic and renal dissection procedures by a senior surgeon in urology and three interns. The cadaver was in the dorsal position for the pelvic dissection procedures and in the lateral position for the renal dissection procedures. In the dorsal position, two different methods were tested to attach the endoscope manipulator to the patient: In the first, the manipulator was attached to the table by an articulated arm and clamp, and in the second the feet of the manipulator were sutured directly to the skin of the abdomen. In the lateral position the articulated arm was used. After performing these surgical procedures, the four surgeons evaluated the aspects of the endoscope manipulator shown in .
Table I. Surgeons' evaluation of features of the endoscope manipulator.
The two surgeons who had experience using other endoscope manipulators preferred our compact device. The manipulator speeds, range of motion, and accuracy for all degrees of freedom were found to be acceptable by all the surgeons. A minor inconvenience in operating the endoscope manipulator system was noted during the surgical procedures: The optical fiber from the light source to the endoscope shaft had to be disconnected in order to remove the endoscope from the trocar whenever the lens was cleaned.
The off-the-shelf voice recognition system requires using a head-mounted microphone and a foot pedal. Users must pronounce clearly without excessive background noise for commands to be recognized, and the processing time of the software causes a perceptible delay between commands and the resulting motion. Nevertheless, the surgeons evaluating the system generally preferred using the voice command interface to the instrument-mounted miniature keypad. The keypad and voice command interfaces can be used in combination.
The use of a foot pedal was seen as a disadvantage. When multiple pedals are used to control electrocautery instruments, operating table position, and/or suction, surgeons must occasionally look away from the endoscope camera monitor to find the correct pedal without moving the tips of the surgical instruments out of the field of view of the endoscope.
Proposed clinical testing
An application to a local review board is in preparation to perform randomized clinical trials of our compact endoscope manipulator. One hundred digestive and urological minimally invasive procedures are to be performed over one year in three different surgical sites, with each procedure randomly assigned to be performed using either the endoscope manipulator or a human assistant to hold the endoscope. The preparation and operating time will be recorded for each procedure. Comments of the surgeons regarding the use and setup of the manipulator and the occurrence of any complications will also be recorded.
Given the results of similar clinical trials of the AESOP Citation[9], Citation[10] and EndoAssist Citation[12] endoscope manipulators, significant reductions in operating times are not necessarily expected. The primary purpose of the proposed clinical trials will be to establish that the performance of the compact endoscope manipulator is at least equivalent to that of a human assistant. The clinical benefits to be shown for the compact manipulator are the reduction in number of surgical staff required and the convenience for the operating surgeons.
Discussion
The development process behind this compact endoscope manipulator is based on extensive testing in the laboratory and during surgical procedures on cadavers, and was undertaken in close consultation with several surgeons. The trajectory-following experiments measured by the optical localizer show that the performance of the latest prototype is more than adequate for endoscope camera displacement during minimally invasive surgery. Further testing of the device while performing actual surgical procedures has confirmed its utility and practicality. Evaluation feedback from surgeons has been mostly positive with “excellent” to “good” ratings, aside from minor inconveniences with the endoscope and robot attachments that were easily corrected. The voice command interface of the endoscope manipulator was particularly welcomed.
Inconveniences encountered by the surgeons during the operation of the endoscope manipulator on the cadaver have been eliminated by minor modifications of the mechanism. A guide and hook attachment for the insertion actuation cable simplifies endoscope setup and eliminates the need to disconnect the light-source optical fiber when cleaning the lens, while a flat ring on the end of the spring reduced the friction on the endoscope.
Due to increasing concerns regarding prion diseases such as Creutzfeldt-Jakob disease (CJD), current sterilization protocols require that surgical instruments must be immersed in a caustic solution for 20–30 min in addition to the autoclave cycle. This requires the LER motors to be watertight as well as autoclavable so that the internal wiring and electronics of the motors are not damaged by leakage of the caustic solution. Waterproof joints are currently being added to the motor shafts to allow the manipulator to be immersed in liquids for up to 30 min.
Further discussions with hospital personnel responsible for operating room hygiene have also shown the necessity for further modifications of the manipulator mechanism for easier cleaning and disassembly Citation[19]. The possibility of bodily fluids penetrating the ball bearing and gear teeth inside the base of the LER is an important concern, as these areas are difficult to reach with a brush for cleaning even when the base is disassembled. An acceptable solution to prevent penetration of fluids during procedures is to enclose the base of the LER in a waterproof bag with a hole for the trocar. Another potential solution would be to remove the outer casing and a portion of the upper walls enclosing the gear teeth and ball bearing inside the base of the LER, thereby permitting sufficient access to the interior with a fine brush for cleaning.
A potential area of further development would be to modify the current manipulator by adding additional actuated degrees of freedom of rotation and gripping or cutting to manipulate laparoscopic instruments instead of an endoscope. Multiple manipulators could then be used together in a complete teleoperated surgical system at a fraction of the cost of current commercial telesurgery systems.
Conclusion
Although testing with surgeons has been limited, it has provided better understanding of human interface and cleaning issues, and enabled minor modifications to be made in advance of clinical trials.
In summary, we have developed an endoscope manipulator with considerable advantages over current alternatives due to its compact size, light weight, ease of use, and low cost. Our final prototype is fully sterilizable and ready for clinical trials once approval is granted by a review board, and we have implemented user interfaces for hands-free control of the robot.
Acknowledgments
The expertise of Gildas de Saint Albin of PRAXIM S.A. has significantly contributed to this project. The new prototype was designed and fabricated in cooperation with Alpes Instruments S.A. of Meylan, France. We also thank Dr. Olivier Skowron from the Centre Hospitalier Universitaire d'Annecy and Drs. Jean-Jacques Rambeaud, Francois Bocqueraz, and Pierre Cassi of the Centre Hospitalier Universitaire de Grenoble for their help in testing the endoscope manipulator. This work is supported under RNTL grants (Ministère de L'Industrie, ANVAR, MMM Project) and by PRAXIM, Inc.
References
- Kobayashi E, Masamune K, Sakuma I, Dohi T, Hashimoto D. A new safe laparoscopic manipulator system with a five-bar linkage mechanism and optical zoom. Comput Aided Surg 1999; 4(4)182–92
- Taylor R H, Funda J, Eldridge B, Gomory S, Gruben K, LaRose D, Talamini M, Kavoussi L, Anderson J A. Telerobotic assistant for laparoscopic surgery. Computer Integrated Surgery: Technology and Applications, R H Taylor, S Lavallée, G C Burdea, R Mösges. MIT Press, Cambridge, MA 1995; 581–92
- Munoz V F, deGabriel J G, Fernandez-Lozano J, Garcia-Morales I, Molina-Mesa R, del Pulgar C P, Seron-Barba J, Azouaghe M. Design and control of a robotic assistant for laparoscopic surgery. Proceedings of 9th International Symposium on Intelligent Robotic Systems, ToulouseFrance, July, 2001
- Buess G F, Arezzo A, Schurr M O, Ulmer F, Fisher H, Gumb L, Testa T, Nobman C. A new remote-controlled endoscope positioning system for endoscopic solo surgery – the FIPS endoarm. Surgical Endoscopy 2000; 14: 395–9
- Geis W P, Kim H C, Brennan E J, Jr, McAfee P C, Wang Y. Robotic arm enhancement to accommodate improved efficiency and decreased resource utilization in complex minimally invasive surgical procedures. Medicine Meets Virtual Reality: Health Care in the Information Age, H Sieburg, S Weghorst, K Morgan. IOS Press, Amsterdam 1996; 471–81
- Sackier J M, Wang Y. Robotically assisted laparoscopic surgery: from concept to development. Computer Integrated Surgery: Technology and Applications, R H Taylor, S Lavallée, G C Burdea, R Mösges. MIT Press, Cambridge, MA 1995; 577–80
- Finlay P A. Clinical experience with a goniometric head-controlled laparoscopic manipulator. Proceedings of IARP Workshop on Medical Robots, ViennaAustria, October, 1996
- Guthart G S, Salisbury J K. The Intuitive™ telesurgery system: overview and application. Proceedings of IEEE International Conference on Robotics and Automation, San Francisco, April, 2000, 618–21
- Mettler L, Ibrahim M, Jonat W. One year of experience working with the aid of a robotic assistant (the voice controlled optic holder AESOP) in gynaecological endoscopic surgery. Human Reproduction 1998; 13: 2478–50
- Kavoussi L R, Moore R G, Adams J B, Partin A W. Comparison of robotic versus human laparoscopic camera control. J Urol 1995; 154: 2134–6
- den Boer K T, Bruijn M, Jaspers J E, Stassen L PS, van Erp W FM, Jansen A, Go PMNYH, Dankelman J, Gouma D J. Time-action analysis of instrument positioners in laparoscopic cholecystectomy. Surgical Endoscopy 2002; 16: 142–7
- Aiono S, Gilbert J M, Soin B, Finlay P A, Gordon A. Controlled trial of the introduction of a robotic camera assistant (EndoAssist) for laparoscopic cholecystectomy. Surgical Endoscopy 2002; 16: 1267–70
- Merola S, Weber P, Wasielewski A, Ballantyne G H. Comparison of laparoscopic cholectomy with and without the aid of a robotic camera holder. Surgical Laparoscopy, Endoscopy, and Percutaneous Techniques 2002; 12(1)46–51
- Berkelman P J, Cinquin P, Troccaz J, Ayoubi J-M, Létoublon C, Bouchard F. A compact, compliant, laparoscopic endoscope manipulator. Proceedings of IEEE International Conference on Robotics and Automation, Washington, DC, May, 2002, 1870–5
- Nickel V L, Perry M DJ, Garrett A L. Development of useful function in the severely paralyzed hand. J Bone Joint Surg 1963; 45A(5)933–52
- Berkelman P J, Cinquin P, Troccaz J, Ayoubi J-M, Létoublon C (2002) Development of a compact cable-driven laparoscopic endoscope manipulator. Proceedings of Fifth International Conference on Medical Image Computing and Computer-Assisted Intervention (MICCAI 2002), ToykoJapan, September, 2002, T Dohi, R Kikinis. Springer, Berlin, 17–24
- Berkelman P J, Boidard E, Cinquin P, Troccaz J. LER: The light endoscope robot. Proceedings of IEEE/RSJ International Conference on Intelligent Robots and Systems, Las VegasNevada, October, 2003, 2835–40
- Berkelman P J, Boidard E, Cinquin P. Automatic instrument tracking with a compact laparoscopic endoscope robot using an external optical localizer. Surgetica (Grenoble) September, 2002; 77–82
- Berkelman P J, Boidard E, Cinquin P, Troccaz J. From the laboratory to the operating room: Usability testing of LER, the light endoscope robot. Proceedings of Medical Robotics, Navigation, and Visualization, RemagenGermany, March, 2004