Abstract
Objective: Exact drilling into the ischemic areas of necrotic lesions of the femoral head remains a challenging procedure, particularly in obese patients. This study was conducted to evaluate the precision of fluoroscopically based drilling and the associated radiation exposure in an in-vitro model of adiposis.
Materials and Methods: In an in-vitro model of necrotic lesions in adiposis, 20 sawbones were drilled under the guidance of an intraoperative navigation system (VectorVision®, BrainLAB, Munich, Germany) and 20 more were drilled conventionally under fluoroscopic control only.
Results: A statistically significant difference was found with respect to the distance from the drill tip to the desired mid-point of the lesion, with a mean distance of 0.56 mm for the navigated group and 1.15 mm for the control group. Furthermore, a significant difference was found in the number of drilling corrections required, as well as the radiation exposure time: The navigated group required a mean of 0.35 second drillings or corrections of the drilling direction, compared to 2.45 for the control group, and the duration of radiation exposure was less than 1 second for the navigated group and 3.85 seconds for the control group.
Conclusions: Drilling guided by the VectorVision® navigation system shows high precision, even under difficult circumstances such as those encountered in adiposis, with a marked reduction in the duration of radiation exposure.
Introduction
Necrotic lesions of the femoral head can affect different parts of the hip joint. Osteochondritis dissecans of the femoral head is a rare disease of the young adult, affecting smaller parts of the hip joint. However, separation of the necrotic part into the joint can result in symptomatic blockade with severe pain, and secondary osteoarthritis may develop if this is left untreated Citation[1–4].
Osteonecrosis is a serious disease affecting larger portions of the femoral head, mainly in patients between 30 and 50 years of age, and may progress to a devastating extent. The femoral head is the predominant site of osteonecrosis in the adult population Citation[5], Citation[6]. The disease progresses through stages which can be distinguished on the basis of extent, location and diagnostic criteria, as in the systems devised by Ficat and ARCO (Association Research Circulation Osseous) Citation[7], Citation[8].
The effectiveness of conservative treatment remains uncertain, and a surgical approach is usually demanded, the extent of surgery depending on the stage of the disease Citation[1–4], Citation[9–12]. Later stages of the disease and osteoarthropathy usually have to be treated by arthroplasty Citation[9], Citation[13]. In the early stages, however, many authors suggest core decompression by exact drilling into the ischemic areas Citation[6], Citation[9], Citation[13]. This is said to improve symptoms by releasing pain through the relief of intraosseous pressure from the accompanying edema, and furthermore to improve the remodeling of the ischemic regions by breaking open sclerotic bone and thus enabling blood circulation and revascularization. This can be done with or without the additional insertion of bone grafts Citation[9], Citation[14].
The precision of the drilling is essential to the outcome in core decompression. On the one hand, the lesion has to be decompressed effectively. On the other hand, however, damage to the femoral cartilage and weakening of the femoral neck by multiple drillings must be strictly avoided. Therefore, the experience and spatial imagination of the surgeon is crucial, as well as the visualization itself. The latter is mostly performed by using an image intensifier–a standard piece of operating room equipment. Most such intensifiers visualize just one plane at a time, and this can result in the need for a series of intraoperative X-rays and thus increased X-ray exposure not only for the patient, but also for the OR team. Exact drilling is a challenging procedure in any case, but especially in obese patients where the surrounding tissues make spatial orientation even more difficult, so that drilling corrections are often necessary. This in turn leads to increased radiation exposure, as well as increased risk of fracture due to weakening of the bone.
As reported earlier, using the VectorVision® navigation system (BrainLAB, Munich, Germany), the precision of core decompression in an in-vitro model of osteonecrosis of the femoral head could be clearly improved, with a concurrent reduction in radiation exposure time Citation[15]. The aim of the present study was to evaluate the precision of fluoroscopically based drilling in an in-vitro model for percutaneous drilling in adiposis, as well as the expected reduction in radiation exposure time.
Materials and methods
Navigation system
VectorVision® Spine 5.5 (BrainLab, Munich, Germany) enables fluoroscopically based intraoperative navigation. It is based on an optical tracking unit, which detects reflecting marker spheres with an infrared camera (). The system is controlled via a draped touch-screen monitor.
Specimens
Forty sawbones (Sawbones Europe, Malmö, Sweden) were used in these experiments. Each sawbone was prepared by drilling the femoral head with a conical-shaped drill, centrally implanting a radiopaque cement sphere measuring 5 mm in diameter (mimicking the size of an osteochondrotic lesion), and closing the remaining defect with gypsum. The femoral sawbones, together with the pelvis and tibia, were then fitted tightly into a surrounding custom-made foam brace of 20 cm thickness which mimicked an obese leg (). Twenty heads of femoral sawbones were drilled under the guidance of the VectorVision® intraoperative navigation system, while 20 others served as controls and were drilled using a conventional technique under fluoroscopic control in two planes only. The sawbones were randomly assigned to the two groups. Each drilling was performed by the same surgeon, the drilling method again being randomly assigned.
Navigation technique.
The models of obese legs were positioned on a standard operating table in a manner mimicking a patient in supine position ( and ). A reference array with passive reflecting marker spheres was rigidly attached to the lateral surface of each sawbone with a Schanz screw. Visualization of the femoral head with the cement spheres and gypsum was carried out, creating images in the anteroposterior and axial planes using a C-arm fluoroscope connected to the navigation system. The C-arm itself is equipped with a device with reflecting markers to define the spatial orientation of the C-arm relative to the navigation system. After the two fluoroscopic images have been acquired, the image intensifier is moved out of the operating field and no further intraoperative images are required.
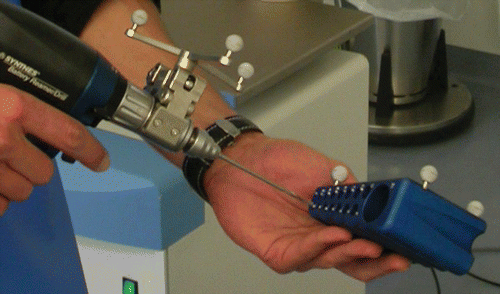
A standard long drill is equipped with a marker-clamp and measured with a special calibration tool (BrainLAB, Munich, Germany) in order to inform the navigation system of the length, diameter and position of the tip of the instrument (). Next, the surgical instrument is visualized on the touch-screen monitor. By online control of the navigation system, the drill is placed in the desired position (). The virtual connection of the position of all the reference markers enables the orientation of the drilling guide in the two fluoroscopic planes simultaneously.
Conventional technique
Twenty identically prepared and positioned obese leg models were drilled, with the drilling direction controlled by the same C-arm fluoroscope and image-intensifier in two planes.
Assessment
The time required for the procedure was measured starting from the positioning of the bones and ending with final removal of the bone. X-ray exposure time could be read directly on the image-intensifier after creating the images. A period of one second was taken into account as the exposure time of the navigated group, though in fact less than 1 second was needed for the acquisition of the two images. The number of drilling corrections required was documented for each drilling procedure. After computer assisted drilling of the femoral head, the positioning of the drill was controlled by reference to two perpendicular fluoroscopic images of the proximal femur sectioned with the drill left in situ to enable measurement of the distance between the mid-point of the target and the tip of the drill ().
Statistical analysis
Statistical analysis was performed using the t-test for the distance to the target and the Mann-Whitney rank-sum test for the overall procedure duration, the radiation exposure time, and the number of drilling corrections needed, in each case comparing the navigated group to the control group. A p < 0.05 was considered to indicate statistical significance.
Results
Precision
Regarding the distance from the tip of the drill to the desired mid-point of the cement sphere, the mean precision of the drilling showed a statistically significant difference, with a mean of 0.56 mm for the navigated group and 1.15 mm for the non-navigated control group (p < 0.001). A mean of 0.35 second drillings or corrections of drilling direction (range: 0 to 1) were necessary in the navigated group, compared to a mean of 2.45 drilling corrections (range: 0 to 5) in the control group (p < 0.001). No cement sphere was missed completely; however, with regard to the distance from the drill tip to the desired mid-point of the sphere, just two targets were missed by more than 1 mm in the navigated group, compared to 11 in the control group; and none was missed by more than 1.5 mm in the navigated drillings, compared to 6 in the control group (Tables , ).
Figure 6. Grouping of results into zones based on distance from the target. Bars 1–4 represent zones I–IV of the navigated group; bars 5–8 represent zones I–IV of the control group.
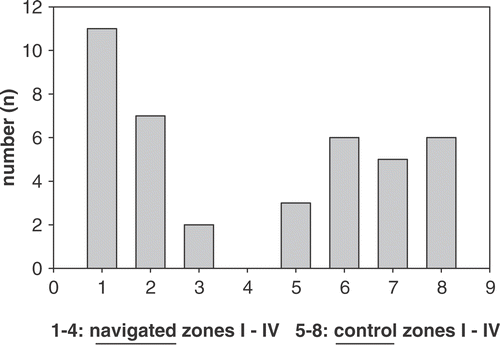
Table I. Results for navigated group.
Table II. Results for control group.
Table III. Distance of drill from the target (the lesion mid-point).
Procedure time
The mean procedure time was 4.78 min for the navigated group and 4.7 min for the non-navigated control group, which was not statistically significant (p = 0.646; Tables and ).
Radiation exposure time
The mean radiation exposure time was less than one second for the navigated group and 3.85 seconds for the non-navigated control group, which was statistically significant (p < 0.001), despite taking one second into account as the exposure time for the navigated group (Tables and ).
Discussion
The technique of core decompression was first described in 1964 by Arlet and Ficat Citation[7]. This procedure is still widely accepted and performed, although a disparity exists between the various reports on outcome; success rates range from 33% to 92%, with an overall success rate between 60% and 70% Citation[9], Citation11–13, 16–20. Meanwhile, it is generally accepted that core decompression should be performed only in the early stages of osteonecrosis, with the decision on whether to use this technique being further dependent on the size of the necrotic lesion Citation[9], Citation[11–13].
Accuracy in achieving the desired point and course is essential. Fractures after drilling, which are likely to occur more often following multiple drilling corrections, as well as penetration injuries to the cartilage and failure due to missing the lesion altogether have been described Citation[9], Citation[13], Citation[21]. Furthermore, in the conventional technique, determining the exact location of the drill or wire by multiple checks of the drilling course and depth results in considerable X-ray exposure for both the patient and OR staff. These side effects associated with drilling corrections gain particular importance in obese patients, where the surrounding tissues make spatial orientation more difficult. It is therefore desirable to minimize such side effects of the procedure, as well as reducing imaging time and radiation dosage.
Computer Assisted Orthopaedic Surgery (CAOS) has been shown to improve precision and also minimize X-ray exposure Citation[22–24]. Different approaches such as CT-based, fluoroscopically assisted or imageless methods are used to simultaneously generate different planes of the therapeutic object to be treated Citation[22]. As a fluoroscope is a standard piece of OR equipment and surgeons are well adapted to its visual informational content, fluoroscopic navigation was developed Citation[25–27]. This method does not require registration like the CT-based system, and reduces both imaging time and radiation dosage.
Regarding the precision of navigated drilling, which may be therapeutic in itself or the first crucial step in procedures paving the way for subsequent osteotomies or milling, only a few published studies exist, despite the proliferation and increasing use of such navigation systems Citation[15], Citation[21], Citation[23], Citation[24], Citation[28], Citation[29]. Various authors have reported clinically acceptable results for precision, with an average distance from the given target of 2 to 4 mm Citation[21], Citation[23], Citation[24], Citation[30] or even less Citation[15], Citation[29]. These distances are consistent with CT-based navigated drillings with further application of special individual fixing methods Citation[31]. Despite these reports, and keeping in mind the spreading use of different systems, one should note that the different methods and navigation systems used in these studies cannot necessarily be directly compared and generalized. As mentioned above, the precision of core decompression in an in-vitro model of osteonecrosis of the femoral head could be markedly improved with high reproducibility in hitting the target, as compared to conventional techniques Citation[15]. Drilling can be performed by visualization of the trajectory to the target on the navigation system screen.
To our knowledge, this is the first study dealing with drilling under difficult circumstances such as those associated with obesity. Regarding this challenging procedure, in the present study we found a distinct high precision or lesser average distance from the target with the navigated technique. No cement sphere was missed, and in only two navigated cases was the desired mid-point of the sphere missed by more then 1 mm, compared to 11 instances in the control group. A mean of just 0.35 second drillings or corrections of drilling direction were necessary in the navigated group; this is one of the most important findings, as multiple drilling corrections are thought to weaken the bone as mentioned previously. In contrast, a mean of 2.45 drilling corrections (range: 0 to 3) were needed in the control group. There was a clear tendency toward higher precision in the navigated group, with 11 out of 20 drill tips ending in a zone less than 0.5 mm from the midpoint, as compared to three out of 20 in the control group (, ). In comparison to our previously published study Citation[15], there was only a slight decrease in the mean deviation. However, the number of drillings that missed the target by more than 1 mm, as well as the number of drilling corrections, clearly increased.
We were able to markedly reduce radiation exposure time compared to that associated with conventional techniques, in which the drilling and every possible correction must be controlled in at least two different X-ray planes. In the navigated group, less than one second was needed to acquire the initial biplanar radiographs for calculation of the geometry construct, which was significantly less than the mean time needed in the conventional drilling group. Also, the additional time required in the navigated approach for placement of the reference tool, frames and diodes, as well as calibration of the drill, is negligible compared to the additional time required with the conventional method due to the need for corrections of drilling direction and control by X-ray in two planes.
Possible drawbacks to this approach are the bending of a wire or the tip of a thin drill, which could falsify the measured distances from the target, as only the lengths of wires or drills and their indirect orientation by the positioning of the drill guide are detected. Furthermore, as fluoroscopic visualization of the necrotic lesions is only possible in the later stages of the disease, a CT- or, even better, MRI-guided system should be used to treat the lesions in the earliest stages, but this is usually not available in most institutions.
Of course, our findings represent in-vitro conditions and therefore cannot be extrapolated directly to the in-vivo situation. However, they certainly show the clear trend towards the above-mentioned advantages, i.e., improved precision with reduced radiation exposure time, particularly under difficult circumstances such as those encountered in patients with severe obesity. However, clinical trials will need to be undertaken before final assessments can be made.
Conclusions
Hand-guided drilling under guidance from the VectorVision® navigation system shows high precision and accuracy, even in difficult circumstances such as those encountered in obese patients, and enables a considerable reduction in radiation exposure time. Particularly in obese patients, the additional time needed for installation and calibration of the navigation devices is negligible compared to the potential time demands associated with conventional techniques.
Acknowledgments
The authors would like to thank the BrainLAB Company (Heimstetten, Germany) for providing the VectorVision® navigation system.
References
- Hagemann L, Berger S, Philipps B, Ostertag H, Siebert CH. [Osteochondrosis dissecans of the hip in adults - differential diagnosis of free joint bodies - case report] [in German]. Z Orthop Ihre Grenzgeb 2006; 144: 301–304
- Linden B, Jonsson K, Redlund-Johnell I. Osteochondritis dissecans of the hip. Acta Radiol 2003; 44: 67–71
- Rowe SM, Yoon TR, Jung ST, Lee KB. Free osteochondral fragment caught in the acetabular fossa in the osteochondritis dissecans after Legg-Calve-Perthes’ disease - report of 2 cases. Acta Orthop Scand 2003; 74: 107–110
- Rowe SM, Moon ES, Yoon TR, Jung ST, Lee KB, Lee JJ. Fate of the osteochondral fragments in osteochondritis dissecans after Legg-Calve-Perthes’ disease. J Bone Joint Surg Br 2002; 84: 1025–1029
- Ohzono K, Saito M, Takaoka K, Ono K, Saito S, Nishina T, Kadowaki T. Natural history of nontraumatic avascular necrosis of the femoral head. J Bone Joint Surg Br 1991; 73: 68–72
- Soucacos PN, Beris AE, Malizos K, Koropilias A, Zalavras H, Dailiana Z. Treatment of avascular necrosis of the femoral head with vascularized fibular transplant. Clin Orthop Relat Res 2001; 386: 120–130
- Ficat RP. Idiopathic bone necrosis of the femoral head. Early diagnosis and treatment. J Bone Joint Surg Br 1985; 67: 3–9
- Schoutens A, Arlet J, Gardeniers J, Hughes SPF (editors). ARCO (Association Research Circulation Osseous) Committee on Terminology and Staging. Bone circulation and vascularization in normal and pathological conditions. The ARCO perspective for reaching one uniform staging system of osteonecrosis. Springer, New York; 1993; 375–380
- Steinberg ME, Larcom PG, Strafford B, Hosick WB, Corces A, Bands RE, Hartman KE. Core decompression with bone grafting for osteonecrosis of the femoral head. Clin Orthop Relat Res 2001; 386: 71–78
- Mont MA, Fairbank AC, Krackow KA, Hungerford DS. Corrective osteotomy for osteonecrosis of the femoral head. J Bone Joint Surg Am 1996; 78: 1032–1038
- Mont MA, Carbone JJ, Fairbank AC. Core decompression versus nonoperative management for osteonecrosis of the hip. Clin Orthop Relat Res 1996; 324: 169–178
- Yoon TR, Song EK, Chung JY, Park CH. Femoral neuropathy caused by enlarged iliopsoas bursa associated with osteonecrosis of femoral head - a case report. Acta Orthop Scand 2000; 71: 322–324
- Aigner N, Schneider W, Eberl V, Knahr K. Core decompression in early stages of femoral head osteonecrosis - an MRI-controlled study. Int Orthop 2002; 26: 31–35
- Urbaniak JR, Harvey EJ. Revascularization of the femoral head in osteonecrosis. J Am Acad Orthop Surg 1998; 6: 44–54
- Beckmann J, Goetz J, Baethis H, Kalteis T, Grifka J, Perlick L. Precision of computer-assisted core decompression drilling of the femoral head. Arch Orthop Trauma Surg 2006; 126: 374–379
- Camp JF, Colwell Jr, CW. Core decompression of the femoral head for osteonecrosis. J Bone Joint Surg Am 1986; 68: 1313–1319
- Fairbank AC, Bhatia D, Jinnah RH, Hungerford DS. Long-term results of core decompression for ischaemic necrosis of the femoral head. J Bone Joint Surg Br 1995; 77: 42–49
- Smith SW, Fehring TK, Griffin WL, Beaver WB. Core decompression of the osteonecrotic femoral head. J Bone Joint Surg Am 1995; 77: 674–680
- Kristensen KD, Pedersen NW, Kiaer T, Starklint H. Core decompression in femoral head osteonecrosis. 18 Stage I hips followed up for 1–5 years. Acta Orthop Scand 1991; 62: 113–114
- Meyers MH. Osteonecrosis of the femoral head. Pathogenesis and long-term results of treatment. Clin Orthop Relat Res 1988; 231: 51–61
- Arand M, Schempf M, Kinzl L, Fleiter T, Pless D, Gebhard F. [Precision in standardized Iso-C-Arm based navigated boring of the proximal femur] [in German]. Unfallchirurg 2001; 104: 1150–1156
- Sugano N. Computer-assisted orthopedic surgery. J Orthop Sci 2003; 8: 442–448
- Ohnsorge JA, Portheine F, Mahnken AH, Prescher A, Wirtz DC, Siebert CH. [Computer-assisted retrograde drilling of osteochondritic lesions of the talus with the help of fluoroscopic navigation] [in German]. Z Orthop Ihre Grenzgeb 2003; 141: 452–458
- Ohnsorge JA, Schkommodau E, Wirtz DC, Wildberger JE, Prescher A, Siebert CH. [Accuracy of fluoroscopically navigated drilling procedures at the hip] [in German]. Z Orthop Ihre Grenzgeb 2003; 141: 112–119
- Slomczykowski M, Hofstetter R, Burquin I, Nolte LP, Synder M. [The method of computer-assisted orthopedic surgery based on two-dimensional fluoroscopy: the principles of action]. Chir Narzadow Ruchu Ortop Pol 1998; 63: 443–450
- Hofstetter R, Slomczykowski M, Sati M, Nolte LP. Fluoroscopy as an imaging means for computer-assisted surgical navigation. Comput Aided Surg 1999; 4: 65–76
- Nolte LP, Slomczykowski MA, Berlemann U, Strauss MJ, Hofstetter R, Schlenzka D, Laine T, Lund T. A new approach to computer-aided spine surgery: fluoroscopy-based surgical navigation. Eur Spine J 2000; 9(Suppl 1)S78–S88
- Gautier E, Bächler R, Heini PF, Nolte LP. Accuracy of computer-guided screw fixation of the sacroiliac joint. Clin Orthop Relat Res 2001; 393: 310–317
- Beckmann J, Goetz J, Bathis H, Kalteis T, Grifka J, Perlick L. Precision of computer-assisted core decompression drilling of the knee. Knee 2006; 13: 211–215
- Arand M, Hartwig E, Hebold D, Kinzl L, Gebhard F. [Precision analysis of navigation-assisted implanted thoracic and lumbar pedicle screws. A prospective clinical study] [in German]. Unfallchirurg 2001; 104: 1076–1081
- Rosenberger RE, Bale RJ, Fink C, Rieger M, Reichkendler M, Hackl W, Benedetto KP, Kunzel KH, Hoser C. [Computer-assisted drilling of the lower extremity. Technique and indications] [in German]. Unfallchirurg 2002; 105: 353–358
![Figure 1. Overall view of the assembly, showing the positioned leg model with attached reference markers, the fluoroscope with its marker device, the infrared camera, and the touch-screen monitor of the navigation system. [Color version available online.]](/cms/asset/189480ae-1cdb-401a-80d6-630425dddd83/icsu_a_288425_f0001_b.gif)
![Figure 2. Femoral sawbones with pelvis and tibia fitted into the custom-made, conical, soft synthetic brace. [Color version available online.]](/cms/asset/0603cbb9-b8cc-4885-8cb7-e845e7bd70c8/icsu_a_288425_f0002_b.gif)
![Figure 4. Real-time visualization of drilling on the associated touch-screen. [Color version available online.]](/cms/asset/a9c9dac1-e99c-408d-ab4b-86bd71f55c8a/icsu_a_288425_f0004_b.gif)
![Figure 5. Section through a proximal femur after drilling with the drill left in situ. [Color version available online.]](/cms/asset/725bf469-0a24-4b79-ac7d-74d1740f1451/icsu_a_288425_f0005_b.gif)