Abstract
Objective: Microsurgery for the clipping of cerebral aneurysms requires a working knowledge of the anatomy of the cerebral vasculature and its relationship to landmarks on the surface of the brain and along the skull base. However, for more distally located aneurysms of the anterior cerebral artery (ACA), locating the lesion can prove frustrating and may require much more extensive interhemispheric dissection than is otherwise needed for proximal control, exposure of the aneurysm, and clip application. We report a case series of five patients in which frameless stereotaxy and CT angiographic data sets were used to minimize the extent of surgery required to clip distal ACA aneurysms.
Clinical presentations: Five patients were found to have distal ACA aneurysms during the work-up of subarachnoid hemorrhage or other neurologic symptoms. The patients comprised two with subarachnoid hemorrhage, one with dizziness, one with stroke, and one with migraines and polycystic kidney disease. Each patient was found to have an aneurysm at the pericallosal/callosal marginal junction.
Intervention: All five patients underwent a right parasagittal craniotomy and clipping of a distal ACA aneurysm. The location of the craniotomy and subsequent interhemispheric dissection were guided by CT angiographic data sets and computer-assisted frameless stereotaxy.
Conclusion: Frameless stereotaxy using a CT angiographic data set is a useful adjunct to routine microsurgery in the clipping of distal ACA aneurysms. Its use obviates the need for extensive interhemispheric dissection, allows the surgeon to gain proximal control and expose the aneurysm more efficiently, and should minimize complications related to unwitting aneurysm exposure.
Introduction
Frameless stereotaxy has found widespread and routine use in neurosurgery. Brain biopsies, the resection of brain tumors, the treatment of hydrocephalus, and even spinal surgery have made use of this technology with great success. However, frameless stereotaxy has played almost no role in the treatment of cerebral aneurysms. Cerebrovascular surgeons rely on their knowledge of vascular and skull-base anatomy to navigate the Sylvian fissure and Circle of Willis, largely obviating the value of frameless stereotaxy and computer-assisted image-guided surgery for the majority of aneurysm procedures. Aneurysms of the more distal vascular tree are an exception to this general rule. Although very little has appeared in the neurosurgical literature on this topic, surgery on distally located mycotic aneurysms Citation[1], Citation[2], berry aneurysms Citation[3–6], and intraventricular aneurysms Citation[7] can be greatly aided by the use of frameless stereotaxy.
One example of where frameless stereotaxy can be helpful is the treatment of more distally located aneurysms of the anterior cerebral artery (ACA). Without the benefit of frameless stereotaxy, locating these lesions can prove frustrating and may require more extensive interhemispheric dissection than is otherwise needed for proximal control, exposure of the aneurysm, and clip application. We report five cases in which frameless stereotaxy and CT angiographic data sets were used to facilitate the surgical exposure required to clip distal ACA aneurysms.
Clinical presentation
Case 1
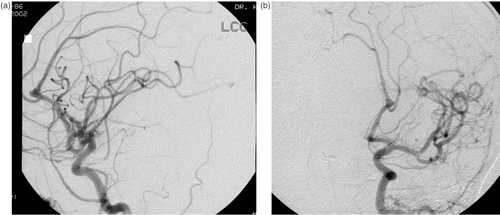
A 56-year-old Caucasian female suffered a subarachnoid hemorrhage and underwent clipping of a right posterior communicating artery aneurysm at an outside hospital. Her postoperative course included placement of a ventriculoperitoneal shunt that subsequently became infected. She was transferred to our institution, and her shunt infection was treated. Review of her angiographic images revealed that in addition to the treated posterior communicating artery aneurysm, the patient also harbored unruptured aneurysms of the right middle cerebral artery (MCA) and the distal left ACA ().
Figure 1. A digital subtraction angiogram of the left common carotid artery demonstrates an aneurysm at the bifurcation of the left A2 artery. (a) Lateral view. (b) Oblique view.
Her right MCA aneurysm was repaired without complications via her previous right frontotemporal craniotomy. She returned approximately six weeks later for elective repair of her left ACA aneurysm. Although this aneurysm was small (5 mm), the family agreed to an aggressive approach given the rupture of the posterior communicating artery aneurysm. Preoperative work-up included a CT angiogram that was used as the data set for frameless stereotaxy-assisted surgery. The CT angiogram was obtained on a Toshiba Aquilion Multi-Slice scanner (Toshiba Corporation, Tokyo, Japan) with collimation of 0.5 mm, pitch of 5 mm/s, gantry rotation time of 0.75 s, and reconstruction interval of 0.5 mm. The patient received a 120-ml contrast bolus at 3 ml/s with a delay of 15 s.
After induction of general anesthesia, the patient was placed supine on the operating table, and her head was fixed in a radiolucent Mayfield head holder. The CT angiographic data set was imported into the surgical navigation equipment (Surgical Navigation Network, Cedara Software, Mississauga, Ontario, Canada), and the location of the patient's head was registered. The surgical navigation system was then used to plan a right parasagittal craniotomy based on the midline.
Following the craniotomy, the navigation system was used to plan a trajectory through the interhemispheric fissure to the aneurysm (). Retractors were placed to hold the falx medially and the right frontal lobe laterally. Because the optimal trajectory for the dissection was identified based on the data from the surgical navigation system, the amount of retraction was kept to a minimum. The interhemispheric fissure was then opened, and the cingulate gyri were separated. The callosal marginal arteries, pericallosal arteries, and the corpus callosum were identified sequentially. The pericallosal arteries were then followed anteriorly. Dissection of the pericallosal arteries was facilitated by intraoperative localization of the aneurysm with the surgical navigation system. The proximal A2 segments were identified bilaterally at the genu of the corpus callosum, and the left A2 was prepared for temporary clip placement for proximal control. Additional dissection demonstrated the aneurysm distal to the bifurcation of the left A2 segment (). Under high magnification, the proximal and distal aspects of the neck of the aneurysm were cleared. A 45° fenestrated clip was placed across the neck of the aneurysm. Intraoperative angiography demonstrated obliteration of the aneurysm without stenosis of the parent vessel. The patient's postoperative course was unremarkable, and she was discharged home without new neurological deficit.
Case 2
A 47-year-old African-American female was admitted to our institution after suffering a subarachnoid hemorrhage. Four-vessel cerebral angiography revealed a 3-mm right pericallosal/callosal marginal aneurysm. This lesion was not amenable to coil embolization, and the patient agreed to proceed with clipping of the aneurysm the next day. A CT angiogram was performed to obtain a CT angiographic data set.
After induction of general anesthesia, the patient was placed supine on the operating table with her head fixed in a radiolucent Mayfield head holder. The CT angiographic data was imported into the surgical navigation equipment, and the location of the patient's head was registered. The surgical navigation system was then used to plan a right parasagittal craniotomy based on the midline. Following the craniotomy, the brain appeared to be tense. Kocher's point was identified using CT wand guidance, and a ventriculostomy catheter was passed into the lateral ventricle with subsequent relaxation of the brain.
Dissection proceeded along the interhemispheric fissure to the top of the cingulate gyrus. Following the trajectory provided by the neuronavigation system, the pericallosal arteries on the right and left side were identified and were followed anteriorly. The right vessel containing the aneurysm was followed until a single large callosal marginal branch was identified. The aneurysm was seen in front of this branch at a smaller vessel origin, and the interhemispheric fissure was further split in order to follow this vessel forward. The neck of the aneurysm was brought into view. The dome of the aneurysm was missing any recognizable vessel wall components and consisted only of a clot, and further dissection around it was deemed exceedingly risky. Given this, a fenestrated 5-mm straight Yasargil titanium aneurysm clip was used with the callosal marginal artery incorporated into the fenestration. The smaller vessel with the aneurysm arising from it was taken with the clip. The patency of the A2 vessel as well as that of the major callosal marginal artery was confirmed by intraoperative angiography. The patient's postoperative course was unremarkable, and she was discharged home without new neurological deficit.
Case 3
A 65-year-old Caucasian female evaluated for dizziness by her primary care doctor was discovered to have a left cavernous carotid aneurysm using MRI/MRA. A subsequent angiogram at an outside hospital revealed that, in addition to the carotid aneurysm, she also had a 3-mm ACA aneurysm at the right pericallosal/callosal marginal artery bifurcation. A repeat angiogram one year later demonstrated growth of this aneurysm, and the patient agreed to proceed with elective clipping of the ACA aneurysm. Preoperative work up included a CT angiogram that was used as the data set for frameless stereotaxy-assisted surgery.
After induction of general anesthesia, the patient was placed supine on the operating table with her head fixed in a radiolucent Mayfield head holder. The CT angiographic data was imported into the surgical navigation equipment, and the location of the patient's head was registered. The surgical navigation system was then used to plan a right parasagittal craniotomy.
After exposure of the interhemispheric fissure, dissection proceeded along the falx until a large callosal marginal branch on the right side was identified. The interhemispheric fissure was then dissected along the course of this vessel to provide adequate exposure of the aneurysm. Because CT angiographic data was available to localize the aneurysm, we were able to minimize the extent of interhemispheric dissection. After interhemispheric dissection was completed, the large callosal marginal branch was traced back to its bifurcation with the pericallosal vessel.
A large branch of an azygous right A2 was then identified. This was then traced back to its bifurcation, where the aneurysm was clearly identified just anterior to the genu of the corpus callosum. Further dissection was undertaken both anteriorly and posteriorly to provide adequate exposure of the aneurysm. A retractor was then placed on the falx to provide further exposure. Following this, final dissection was undertaken of the fundus and neck of the aneurysm itself. The large azygous right A2 was clearly identified and cleared, and a site was prepared for temporary clipping if necessary. Both branches of this large A2 were then clearly identified. The neck of the aneurysm was cleared and examined. A 15-mm 45° angled Yasargil clip was used to clip the aneurysm, and intraoperative angiography revealed no residual aneurysm and no stenosis of the parent vessels. The patient's postoperative course was unremarkable, and she was discharged home without new neurological deficit.
Case 4
A 60-year-old Caucasian female admitted for stroke was found to have a 5-mm ACA aneurysm at the junction of the left pericallosal and callosal marginal arteries. Because the patient had a family history of aneurysmal subarachnoid hemorrhage, the patient agreed to proceed with elective clipping of the aneurysm. Preoperative work-up included a CT angiogram that was used as the data set for frameless stereotaxy-assisted surgery.
After induction of general anesthesia, the patient was placed supine on the operating table, and her head was fixed in a radiolucent Mayfield head holder. The CT angiographic data was imported into the surgical navigation equipment, and the location of the patient's head was registered. The surgical navigation system was then used to plan a right parasagittal craniotomy.
After exposure of the interhemispheric fissure, the frontal lobe was separated from the falx. There was a falx fenestration with significant adhesions requiring considerable time to release. After removing the adhesions, a single pericallosal artery was identified and dissected posteriorly to determine its destination. After confirming its destination as the left frontal lobe, the dissection proceeded anteriorly. Significant adhesions were encountered between the frontal lobes deep to the falx anteriorly. During the extensive dissection of this area, the surgical navigation system allowed for intraoperative localization of the aneurysm in order to avoid encountering it prematurely.
We were able to identify the A2 portion of the left anterior cerebral artery and its bifurcation into the pericallosal and callosal marginal branches. At this bifurcation, a multilobulated aneurysm was encountered. The aneurysm dome was fully exposed in order to see clearly the origin of the callosal marginal artery. The proximal A2 artery was prepared for temporary clipping. With this temporary clipping, we were able to accomplish complete exposure of the aneurysm dome. With the aneurysm identified and exposed, a side-opening Yasargil aneurysm clip was applied across the neck of the aneurysm. An intraoperative angiogram demonstrated patency of all the surrounding vessels and occlusion of the aneurysm. The patient's postoperative course was unremarkable, and she was discharged home without new neurological deficit.
Case 5
A 53-year-old Caucasian female with lifelong migraine headaches and a history of polycystic kidney disease developed an increase in frequency of her headaches. Work-up included an MRI/MRA that showed an 8-mm pericallosal aneurysm. The aneurysm was confirmed by four-vessel angiography. Treatment options, including coiling, were discussed with the patient, and she opted for surgery. Preoperatively, a CT angiogram was obtained in order to acquire a data set for frameless stereotaxy-assisted surgery.
The patient was brought to the operating room and underwent induction and intubation. The patient's head was placed in a radiolucent Mayfield skull clamp in standard fashion for a right anterior parasagittal craniotomy. The patient's head was registered to the neuronavigational system, and the accuracy of the registration was verified. The neuronavigation system was used to plan the craniotomy and the trajectory to the aneurysm.
Using microscopic visualization and the neuronavigation system, the interhemispheric fissure was dissected beyond the falx and between the mesial aspects of the frontal lobes. Several large callosal marginal branches were followed to identify the major callosal marginal artery on the right side, and this was then traced back to the pericallosal arteries and the corpus callosum. The right pericallosal artery was then traced forward, exposing both the right and left pericallosal arteries. Following exposure of the vessels at the genu, no aneurysm could be seen arising from the right aspect of the right pericallosal artery aneurysm, so further exploration of the left pericallosal artery revealed a right-pointing aneurysm arising from the left pericallosal artery. The aneurysm displaced the right pericallosal artery posteriorly, thereby giving the appearance on angiogram that the aneurysm actually arose from the right side. Careful dissection was undertaken to expose the proximal and distal necks of the aneurysm. Then, under direct visualization, a bayoneted straight Yasargil aneurysm clip was placed across the neck of the aneurysm. This reconstituted the left pericallosal artery adequately. Intraoperative angiography revealed no residual aneurysm and reasonable filling of both distal anterior cerebral arteries. The patient's postoperative course was unremarkable, and she was discharged home neurologically intact.
Discussion
There are only a few papers in the neurosurgical literature regarding the use of frameless stereotaxy during microsurgery for the repair of distal cerebral aneurysms Citation[1–7]. This technology is of limited use when dealing with aneurysms in and near the Circle of Willis, as the anatomy of the cerebral vasculature and skull base supplies adequate landmarks for safe and efficient surgical repair. This was demonstrated by Schmid-Elsaesser et al. in a series of 16 patients in which frameless stereotaxy and CT angiography were used during the clipping of unruptured internal carotid artery (ICA), middle cerebral artery (MCA), and anterior communicating artery (ACA) aneurysms Citation[6]. In this series, they found that the use of frameless stereotaxy did not significantly change their approach to ICA and ACA aneurysms. In contrast, frameless stereotaxy was used in superficial MCA aneurysms to guide the incision of the temporalis muscle (thus limiting subsequent atrophy after myofascial flaps) and to plan small (3 cm in diameter) craniotomies directly over the Sylvian fissure and the aneurysm.
Frameless stereotaxy clearly offers benefits when dealing with aneurysms located more distally along the cerebrovascular tree. For example, this technology is of obvious benefit when approaching mycotic aneurysms associated with systemic bacterial infectio Citation[1], Citation[2]. These small and often intraparenchymal lesions are ideal for frameless stereotactic localization intraoperatively. The value of the application of this technology to distal berry aneurysms located in the subarachnoid space is less appreciated and, to our knowledge, only one peer-reviewed article has appeared on the subject Citation[5].
Distal ACA aneurysms account for approximately 5% of aneurysms in operative series Citation[8]. These aneurysms are ideally suited to the application of frameless stereotaxy combined with CT angiographic data. The use of frameless stereotaxy for surgical navigation allows the surgeon to confidently locate the aneurysm and appropriately size the craniotomy. In addition, knowledge of the location of the aneurysm can prevent the surgeon from inadvertently compromising the aneurysm wall during dissection prior to the establishment of proximal control. As illustrated in Case 4, extensive adhesions can make the dissection of the aneurysm challenging, and the information available to the surgeon from the surgical navigation equipment can expedite what is potentially a tedious and dangerous dissection. Furthermore, the surgeon will be able to minimize the extent of frontal lobe retraction during the approach. This is especially important for distal ACA aneurysms, as they are frequently embedded in the frontal lobe arachnoid and are at risk for rupture with extensive parenchymal retraction. Although the use of frameless stereotactic guidance will add to the set-up time before incision, total operating room time may actually be reduced when one considers its ability to accurately direct the surgical exposure.
Conclusions
Well-executed microsurgical technique and detailed anatomical knowledge are essential for safe and effective repair of distal ACA aneurysms. Surgeons with considerable experience of the repair of these aneurysms may find little benefit from frameless stereotactic guidance. However, surgeons less familiar with these aneurysms may find that frameless stereotaxy using a CT angiographic data set is a useful adjunct to microsurgery in the clipping of distal ACA aneurysms. Its use obviates the need for extensive interhemispheric dissection, allows the surgeon to gain proximal control and expose the aneurysm more efficiently, and should minimize complications related to unwitting aneurysm exposure or excessive frontal lobe retraction.
References
- Harris A, Levy E, Kanal E, Pollock A, Cahill AM, Omalu BI, Albright AL, Pollack A, Cayhill AM. Infectious aneurysm clipping by an MRI/MRA wand-guided protocol. A case report and technical note. Pediatr Neurosurg 2001; 35: 90–93
- Luders JC, Steinmetz MP, Mayberg MR. Awake craniotomy for microsurgical obliteration of mycotic aneurysms: Technical report of three cases. Neurosurgery 2005; 56(Suppl 1)ONS–201
- Albert FK, Wirtz CR, Forsting M, Jansen O, Polarz H, Mittermaier G, Kunze S. Image guided excision of a ruptured feeding artery “pedicle aneurysm” associated with an arteriovenous malformation in a child: case report. Comput Aided Surg 1997; 2: 5–10
- Frazee JG, King WA. Endoscopy and stereotaxy for aneurysms. Neurosurg Clin N Am 1998; 9: 869–878
- Origitano TC, Anderson DE. CT angiographic-guided frameless stereotactic-assisted clipping of a distal posterior inferior cerebellar artery aneurysm: Technical case report. Surg Neurol 1996; 46: 450–453
- Schmid-Elsaesser R, Muacevic A. Holtmannspotter M, Uhl E, Steiger, HJ. Neuronavigation based on CT angiography for surgery of intracranial aneurysms: Primary experience with unruptured aneurysms. Minim Invas Neurosurg 2003; 46: 269–277
- Ali MJ, Bendok BR, Getch CC. Gottardi-Littell NR, Mindea S, Batjer HH. Surgical management of a ruptured posterior choroidal intraventricular aneurysm associated with moyamoya disease using frameless stereotaxy: Case report and review of the literature. Neurosurgery 2004; 54(4)1019–1024
- Weir B. Specific sites and results of series: Distal anterior cerebral artery aneurysms. Aneurysms affecting the nervous system, B Weir. Williams and Wilkins, Baltimore 1957; 464–468
![Figure 2. Preoperative targeting of the distal left ACA aneurysm using a CT angiographic data set loaded into the surgical navigation system. The aneurysm is seen at the center of the crosshairs in the coronal, sagittal, and axial orthogonal planes. [Color version available online.]](/cms/asset/d463ea23-69d2-4c74-a04f-a76bb549f93a/icsu_a_289841_f0002_b.gif)
![Figure 3. Intraoperative localization of the distal left ACA aneurysm. Left: The exposed aneurysm and the frameless stereotactic pointing device. Right: The trajectory view confirming the location of the aneurysm on the CT angiographic data set. [Color version available online.]](/cms/asset/5f2ffe1f-96ce-494f-82ed-cbd7e0e59652/icsu_a_289841_f0003_b.gif)