Abstract
We report the case of a patient with bilateral hypothenar hammer syndrome. The therapeutic decision was to resect a thrombosed segment of the distal ulnar artery then reconstruct it using a forearm venous graft. The original aspect of this case concerns the microsurgical technique employed: All vascular sutures were made by separate nylon 10/0 stitches using a da Vinci S® surgical robot. No particular problems were observed postoperatively and, except for some cold-related pain, the patient no longer experienced any symptoms at 6 months post-surgery. This clinical case shows that robots may be employed for some specific tele-microsurgical procedures.
Introduction
The hypothenar hammer syndrome was first described in 1772 Citation[1]. The syndrome is related to repeated microtrauma to the heel of the hand resulting from its use as a hammer, which is likely to cause endothelial lesions and false aneurysms with occlusion due to arterial thrombosis Citation[2].
Conservative treatment (cessation of microtrauma and smoking, coupled with anticoagulant therapy) does not prevent the risk of digital micro-emboli Citation[3], for which reason most authors recommend surgery, i.e., resection of the pathological arterial area followed by reconstruction of the defect by venous grafting Citation[4–6]. This intervention is traditionally performed using an operative microscope.
Telesurgery is frequently used in cardiovascular, urological, gynaecological and gastro-intestinal surgery Citation[7], Citation[8]. Remarkable improvements have been associated with the use of this technique, including reduced surgical duration, improved safety and accuracy of surgical gestures, less bleeding, and improved comfort for the surgeon Citation[9], Citation[10]. No clinical telesurgical case has yet been reported for limb microsurgery; however, many experimental studies have been published describing vascular suturing by tele-microsurgical techniques Citation[11–16].
We report the first clinical case of vascular tele-microsurgery in the context of venous grafting of a wrist ulnar artery in a patient with bilateral hypothenar hammer syndrome.
Case report
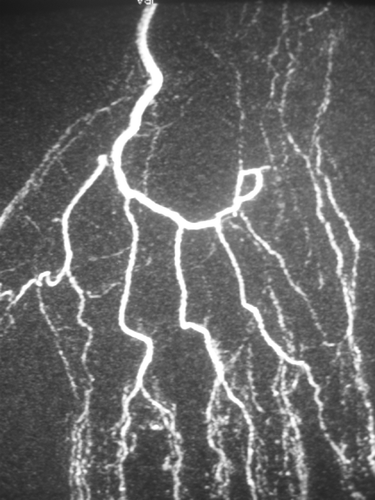
The patient was a left-handed, non-smoking, 39-year-old bricklayer who presented with bilateral pain at the ulnar portion of his left and right wrists and hands, paresthesias of the two ulnar fingers on the left hand only, and bilateral vasomotor disorders when exposed to cold. Further investigations had to be performed due to the overuse of his hands as hammers. Electromyography showed no abnormality, but the angio-MRI revealed a segmental thrombosis of the ulnar artery at the ulnar canal level (). Hypothenar hammer syndrome was diagnosed, and surgery was indicated and scheduled with the patient's agreement.
Figure 1. Angio-MRI showing the lack of permeability of the ulnar artery at the level of the tunnel of Guyon.
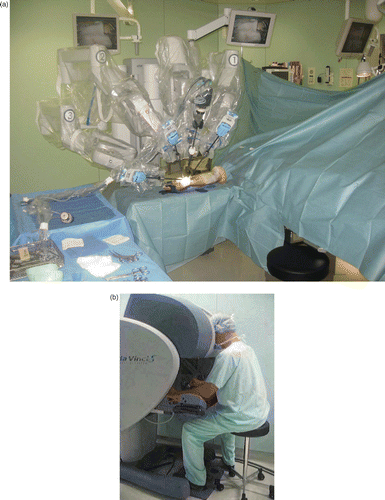
The surgical procedures were identical on both sides and were performed five months apart. Surgery was carried out under general anesthesia with the patient in the supine position. The upper limb was placed on an “arm table” with an inflatable tourniquet placed at the base of the limb for 170 minutes for the left arm and 180 minutes for the right arm. Prior to patient positioning, a da Vinci S® telemanipulation system (Intuitive Surgical) was put in place. The robot was fitted with three articulated arms, two of them supporting Black Diamond microsurgical forceps and the third holding Pott scissors ().
Figure 2. Installation of the da Vinci S® robot. (a) The patient cart is fitted with a sterile cover and is located opposite the conventional surgeon's position. The robot arms are obliquely directed in such a way that the instruments are placed in the same direction as those of a conventional operator. No operative assistance is necessary. (b) The surgeon's console is placed several meters distant from the patient, on the anesthetist's side. The surgeon handles the instruments remotely, without sterile clothing. His head is placed inside the safety system which allows the release of the instruments.
After establishing anterior access to the tunnel of Guyon, extended to reach the wrist, the first step in the procedure consisted of performing neurolysis of the ulnar nerve and its sensory and motor terminal branches, and investigating the ulnar artery. An arterial segment 5 cm (left side) or 4 cm (right side) in length was thrombosed with dilation downstream.
The second step consisted of performing the resection, up to the healthy area, of this thrombosed arterial segment, sampling a vein from the anterior face of the proximal forearm and inverting it, then placing it between the arterial ends that were to be anastomosed.
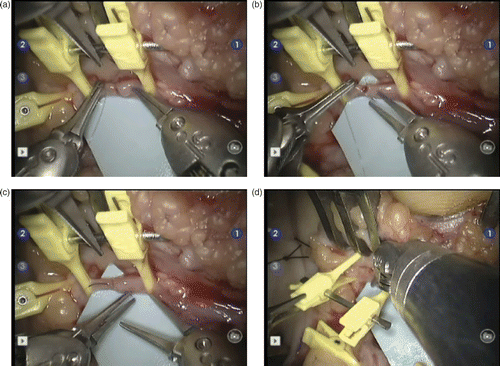
The final step was the proximal and distal vascular anastomosis using the da Vinci S® robot. Six separate nylon 10/0 stitches were sutured on each of the two anastomoses (). The patency test performed after tourniquet release was positive in both cases.
Figure 3. The operating surgeon's view of the procedure on the control screens. The operative field corresponds to the distal anastomosis of the inverted venous graft (at right) with the distal end of the ulnar artery (at left). The vascular clamp is maintained by Pott scissors (at top). (a) The Black Diamond forceps on the right exerts a slight pressure on the wall of the venous graft to facilitate passing the needle and nylon 10/0 thread through the lumen of the ulnar artery distal end with the other Black Diamond forceps on the left. (b) The forceps on the right is about to grab the end of the needle which is still held by the left forceps. (c) The forceps on the left regains its hold on the end of the needle before knot tightening. (d) Once the anastomosis is achieved, the left forceps exerts some pressure on the clamp to release the distal end of the ulnar artery. The right forceps exerts a slight pressure on the venous graft wall to facilitate clamp removal.
No particular postoperative after-effects were reported following a 15-day period of immobilization in a wrist splint. The vasomotor disorders disappeared immediately after surgery, and progressive regression of the paresthesias in the left ulnar nerve area was observed after 2 months. Only some infrequent cold-related pain episodes were still being experienced at 6 months post-surgery.
Discussion
Surgical treatment is recommended by most authors for hypothenar hammer syndrome to prevent thrombo-embolic complications that may result in multi-digit amputations Citation[4–6]. The use of an operative microscope rather than magnifying glasses is also recommended, so as to obtain an anastomosis of the highest quality. In fact, any imperfection in the nylon 10/0 sutures may promote a thrombosis of the venous graft with related iatrogenic complications. The use of tele-microsurgery may help improve the quality of these microsurgical sutures.
Tele-microsurgery has several specific properties that facilitate microsurgical gestures. The first of these properties is the good quality of the peroperative vision. Unlike the conventional microscope that provides only a limited view of the operative field, the da Vinci S® telemanipulation system allows the operation to be performed efficiently with or without optical magnification. In contrast, a conventional microscope must be displaced in order to obtain an overall view of the operative field. The three-dimensional vision of the da Vinci S® robot, generated by two light sources and two cameras, allows a 25x enlargement that may be doubled in models equipped with a digital zoom. The quality of the high-definition 3D vision is such that it compensates for the lack of tactile retrocontrol Citation[9].
The second specific advantage is the elimination of physiological tremor Citation[17]. This was of particular value in our clinical case, allowing the suturing to be performed with considerable ease. This quality is likely to prove even more valuable in emergency operations that are often carried out at night after a long working day.
The third advantage is the increased accuracy and precision of the operative gestures Citation[18]. The da Vinci S® robot is fitted with a system that enables the operator's gesture to be de-coupled from that of the robot, allowing the scale of 1/1 (as in conventional microsurgery) to become a reduction of 1/3 or even 1/5 (i.e., a 5-cm motion of the operator corresponds to a 1-cm motion of the robot).
The fourth advantage is the number of available instruments. In conventional microsurgery, the operating surgeon has only two hands and sometimes needs help from an assistant whose gestures are not under the surgeon's direct control. In tele-microsurgery, with the da Vinci S® version used in this case, the operating surgeon effectively has three hands available to him, and some versions of the robot offer up to 6 hands. Of course, the surgeon cannot mobilize more than two instruments simultaneously, but he may fix an instrument in a defined position in the operative field without any tremor, something that is not feasible with a human operating assistant.
The fifth specific advantage is the extreme mobility of the distal articulation of the machine arm that supports the robot instrument. This allows pronosupination motions of 360° in a very restricted area, whereas natural pronosupination by a human operator is limited to 180° in a much wider area. This property is very useful when the surgeon has to work in a small operative field or in positions with poor ergonomics Citation[19]. In the present clinical case, the distal anastomosis would have been barely accessible in the context of conventional microsurgery because of the depth of its location, whereas the instruments of the robot had no difficulty in moving within this restricted and ergonomically poor area.
Tele-microsurgery also presents some specific disadvantages. The first of these is the lack of proprioceptive retrocontrol. In the present case, it was not possible to estimate the strength of closure of the dissection forceps Citation[14], Citation[16], so there was a risk of twisting or fracturing the needle used for suturing if it were to be simultaneously manipulated by the two forceps. To prevent this, we decided to always handle the needle with only one Black Diamond forceps at a time. To preserve the suture thread and prevent its rupture, knot-tightening also required visual control Citation[20].
The second disadvantage is the expense of telesurgical systems. However, some reports have emphasized paradoxical reductions in costs, related to the reduced bleeding and the improvement of some operative procedures Citation[10].
The third disadvantage is the lengthening of the duration of the surgical procedure, which is an important restricting factor in limb surgeries where the patient is fitted with an inflatable tourniquet. When first working with this system, setting up the robot requires approximately 45 minutes, though this may be reduced by half once the operating team is well trained. Moreover, in the present clinical case, the robot installation was carried out prior to the patient's arrival in the operating room. Altogether, the overall duration of the surgical procedure was increased by approximately 15 minutes for each side.
In conclusion, the use of telesurgery in the presented clinical case did not result in any marked improvement compared with conventional microsurgery, but we believe it will be possible in the future to define some specific indications for telesurgery owing to its exceptional properties (such as the elimination of physiological tremor, facilitation of the simultaneous use of several instruments, and improvement of the surgeon's gestures).
References
- Lorelli DR, Shepard AD. Hypothenar hammer syndrome: An uncommon and correctable cause of digital ischemia. J Cardiovasc Surg 2002; 43: 83–85
- Conn J, Bergan JJ, Bell JL. Hypothenar hammer syndrome: Post traumatic digital ischemia. Surgery 1970; 68: 1122–1128
- Koman LA, Urbaniak JR. Ulnar artery insufficiency: A guide to treatment. J Hand Surg 1981; 6A: 16–24
- De Monaco D, Fritsche E, Rigoni G, Schlunke S, von Wartburg U. Hypothenar hammer syndrome. Retrospective study of nine cases. J Hand Surg 1999; 24B: 731–734
- Rothkopf DM, Bryan DJ, Cuadros CL, May JW. Surgical management of ulnar artery aneurysms. J Hand Surg 1990; 15A: 891–897
- Vayssairat M, Debure C, Cormier JM, Bruneval P, Laurian C, Juillet Y. Hypothenar hammer syndrome: Seventeen cases with long-term follow-up. J Vasc Surg 1987; 5: 838–843
- Cohn LH. Future directions in cardiac surgery. Am Heart Hosp J 2006; 4: 174–178
- Zorn KC, Orvieto MA, Gong EM, Mikhail AA, Gofrit ON, Zagaia GP, Shalhav AL. Robotic radical prostatectomy learning curve of a fellowship-trained laparoscopic surgeon. J Endourol 2007; 21: 441–447
- Blavier A, Gaudissart Q, Cadière GB, Nyssen AS. Perceptual and instrumental impacts of robotic laparoscopy on surgical performance. Surg Endosc 2007; 65: 80–91
- Nelson B, Kaufman M, Broughton G, Cookson MS, Chang SS, Herrell SD, Baumgartner RG, Smith JA, Jr. Comparison of length of hospital stay between radical retropubic prostatectomy and robotic assisted laparoscopic prostatectomy. J Urol 2007; 177: 929–931
- Katz R, Rosson G, Taylor J, Singh N. Robotics in microsurgery: Use of a surgical robot to perform a free flap in a pig. Microsurgery 2005; 25: 566–569
- Katz RD, Taylor JA, Rosson GD. Robotics in plastic and reconstructive surgery: Use of a telemanipulator slave robot to perform microvascular anastomoses. J Reconst Microsurg 2006; 22: 53–57
- Stephenson ER, Sankholkar S, Ducko CT, Damiano RJ. Successful endoscopic coronary artery bypass grafting: An acute large animal trial. J Thorac Cardiovasc Surg 1998; 116: 1071–1073
- Taleb C, Nectoux E, Liverneaux P. Telemicrosurgery: A feasibility study in a rat model. Chir Main 2008; 28: 104–108
- Taleb C, Nectoux E, Liverneaux P. Limb replantation with two robots: A feasibility study in a pig model. Microsurg 2009; 29: 232–235
- van der Hulst R, Sawor J, Bouvy N. Microvascular anastomosis: Is there a role for robotic surgery?. J Plast Reconstr Aesthet Surg 2007; 60: 101–102
- Smith A, Smith J, Jayne DG. Telerobotics: Surgery for the 21st century. Surgery 2006; 24: 74–78
- Taylor RH, Jensen P, Whitcomb L, Barnes A, Kumar R, Stoianovici D, Gupta P, Kavoussi L. A steady-hand robotic system for microsurgical augmentation. Inter J Robotics Res 1999; 18: 1201–1210
- Liverneaux P, Nectoux E, Taleb C. The future of robotics in hand surgery. Chir Main 2009; 28(5)278–285
- Saraf S. Role of robot assisted microsurgery in plastic surgery. Indian J Plastic Surg 2006; 39: 57–61