Abstract
Several theoretical models have shown that the range of motion of the hip joint is impaired in patients with femoroacetabular impingement, and that the acetabular cartilage is at risk of being damaged as a result of abnormal shear stresses, even during normal everyday activities. Computer aided technologies might add to the early diagnosis and adequate treatment of such lesions. This paper describes the technique, theories and algorithms we have developed for patient-specific detection, analysis and computer aided surgery of femoroacetabular impingement.
Currently available models applicable to femoroacetabular impingement offer modeling based on collision analysis of a constrained hip joint. Such an approach implies that neither the femur nor the acetabulum can be analyzed completely separately for the presence of structural lesions responsible for the impingement problem. Moreover, a constrained model does not allow for comprehensive prediction of the possible locations and extent of secondary cartilage lesions (so-called contre-coup lesions) of the posterior acetabulum opposite the anterior impingement site. We report a new technique for the subject-specific morphological analysis of the proximal femur, acetabulum and hip joint. The technique offers a number of advantages compared to currently used techniques for the diagnosis and evaluation of hip impingement, and has direct orthopaedic applications as it allows computer aided planning and minimally invasive surgery for patients with femoroacetabular impingement.
Introduction
Femoroacetabular impingement (FAI) is a mechanical hip disorder defined as early and/or repetitive contact between osseous prominences of the acetabulum and/or the proximal femur, potentially resulting in damage to the hip joint cartilage and labrum in young adults. Depending on the clinical and radiographic findings, two types of impingement are distinguished Citation[1]: Pincer-type impingement is described as the acetabular cause of FAI and is characterized by focal or general over-coverage of the femoral head resulting in early abutment against the femoral neck in end-range of motion activities. Cam-type impingement is the femoral cause of FAI and is characterized by the presence of an aspherical extrusion at the femoral head–neck junction. Most patients present a combination of these two mechanisms Citation[2]. Depending on the source, the prevalence of these lesions – including asymptomatic cases - has been reported to be as high as 17% Citation[1]. Cam-type prominences appear to have a particularly destructive effect on acetabular cartilage as they have the potential to intrude into the hip joint and thereby increase von Mises stresses and contact pressure at the labrum-cartilage junction and at the subchondral bone of the acetabulum, with subsequent delamination and tearing of the acetabular cartilage. Finite element analysis has provided evidence that in cam-type lesions this mechanism operates even during normal everyday activities, such as sitting and standing Citation[3], Citation[4].
Computer simulation of hip motion based on collision-detection algorithms was introduced more than 10 years ago, providing a means of visualizing the impingement problem and of developing surgical treatment tailored to the patient's needs. In combination with surgical navigation, this might improve the surgical success rate for FAI patients in the future. Unfortunately, most of the available bony restraint models merely provide a means of analyzing the theoretical range of motion (ROM) and fail to analyze the femur and acetabulum separately for the presence of structural lesions. Neither do they provide an insight into the extent of the secondary lesion at the opposite posterior acetabulum, the so-called contre-coup lesion Citation[2], Citation[5]. Our purpose in this study was to identify and describe femoroacetabular contact, depending on the shape of the cam lesion and its position in the ROM. Based on this information, patient-specific recommendations and computer-assisted tools for minimally invasive arthroscopic treatment are provided.
Methods
Morphological analysis of the proximal femur
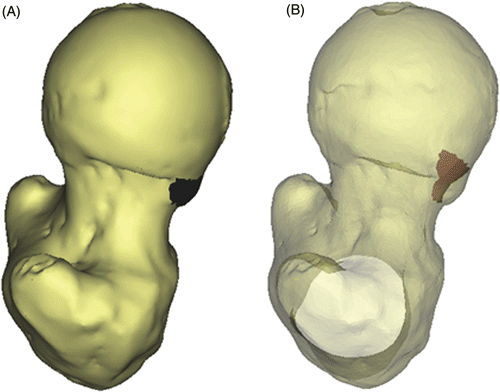
In non-dysplastic hips, the femoral head can be approximated as a sphere, the femoral neck being elliptical in cross section and roughly constant in size in the proximal part of the neck Citation[6]. Samples of data points outside the head-neck area representing the patient-specific morphology of the femoral head and neck were acquired by means of spherical and elliptical least squares fitting, respectively. A tolerance interval was calculated to represent 98% of the included vertices, with a probability of 0.98 (alpha level 0.01) to define the subject-specific geometry of the femoral head and neck Citation[7]. Using this information, the femoral head-neck junction was evaluated and any clusters of protruding points defining the cam lesion were identified. Based on the surface area and the connectivity of the surface nodes following a Delaunay triangulation, random noise was distinguished from a true cam-type lesion, and the size, extent and location of the lesion was defined at the femoral head-neck region. Additionally, as for each protruding point the minimum distance to the fitted femoral head and neck was known, a reshaping to a normal morphology as performed during surgical osteochondroplasty was suggested and volumetric data of the lesion were obtained ().
Evaluation of ROM and hip joint cartilage at risk
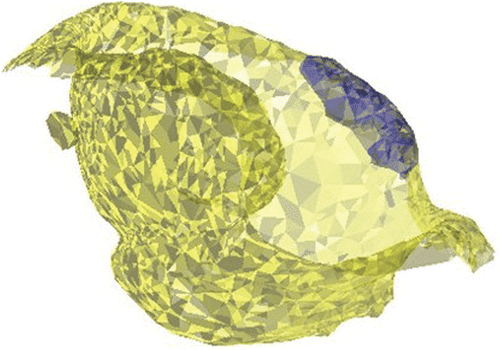
Simulation of the hip ROM is commonly performed based on collision detection between the femoral neck and acetabular rim Citation[8], Citation[9]. In FAI, extensive intra-articular cartilage lesions are usually found in the presence of cam-type impingement, suggesting intrusion of the non-spherical femoral head into the joint, with concomitant damage to the conflict area and opposite posterior acetabulum, called the contre-coup lesion Citation[2], Citation[5]. The extent of acetabular cartilage at risk for shear damage cannot be assessed when a constrained joint is assumed. More specifically, the sometimes substantial contre-coup lesion remains undetected when this assumption is applied. A completely nonconstrained approach does not seem appropriate either, as several studies have demonstrated a “suction effect” in the hip joint, i.e., the resistance generated to distraction of the head from the acetabular socket. This sealing function not only enhances joint stability, but is thought to more uniformly distribute compressive loads applied to the articular surfaces, thereby reducing peak cartilage stresses during weight-bearing Citation[10]. We calculated the direction vector of this stabilizing force as the sum of the normals of the faces representing the acetabular joint area. Translational joint movement in the case of cam engagement was then allowed according to the normal of the colliding vertices, restricted to a plane tangential to the previously defined direction vector. The maximal total translation in this plane was constrained not to exceed the average joint space distance. In the case of a cam-type morphology, therefore, a patient's ROM was defined by the ROM at which this translational limit was reached (). The acetabular cartilage area at risk for shear damage was then evaluated at the end-ROM by means of a normalized distance map of the internal joint, allowing evaluation of both the impingement area and the contre-coup area ()
Figure 2. (A) Description of femoral and acetabular contact points in relation to the location and size of the cam lesion, and (B) identification of the intra-articular collision area and contre-coup area for evaluation of cartilage at risk for shear damage during specific motions (red areas). This particular example represents a simulation for flexion in a clinical case of FAI.
Detection of pincer-type lesions
Once the morphology of the proximal femur and the size and location of a possible cam lesion were defined, the potential presence of a pincer-type lesion at the acetabular side was evaluated using an approach similar to the penetration depth method described by Arbabi et al Citation[4].
To evaluate the anatomy of the acetabulum and the potential presence of a pincer-type lesion independent of any structural abnormalities on the femoral side, we used for the collision analysis the reshaped femur anatomy as obtained by the previously described morphological analysis of the femur. A pincer lesion - if present - was then defined by the acetabular area to be penetrated in order to achieve normal ROM in flexion, abduction and internal rotation in 90° of flexion. As shown previously Citation[11], all three motions are involved in FAI, with their respective contributions to the impingement problem varying between patients depending on the size and location of the cam lesion as well as on the patient's hip anatomy (e.g., varus position or retroversion of the femoral neck) ().
Computer aided planning and surgery
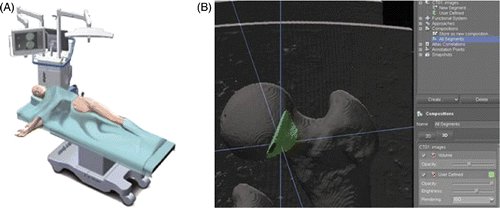
Both methods for the detection of cam- and pincer-type morphology provide a three-dimensional (3D) volumetric description of the lesions. The surface geometry of the detected lesions was imported as STL files into the Mimics® R 14.1 medical image-processing software (Materialise NV, Heverlee, Belgium). The 3D description of the cam and/or pincer lesions was matched onto the patient's original imaging files and exported in DICOM format. The modified DICOM files thus incorporated both the patient's anatomy and the previously defined femoral and acetabular areas requiring surgical resection. A classical navigation system allowing for segmentation of the cam and pincer lesions to be corrected was then used.
An in vitro test on 3 sawbone models was performed to evaluate the feasibility and accuracy of the technique. As sawbones with specific cam-type morphology are not commercially available, a virtual lesion was created at the femoral head-neck junction. The Stryker OrthoMap 3D navigation and planning software (Stryker Orthopaedics, Mahwah, NJ) was used (). The OrthoMap 3D navigation system requires DICOM image sets as a basis for navigation. The planning software provides segmentation tools that allow the user to accentuate anatomical structures of interest, such as tumors, bone surfaces and vessels. In our case, a cam lesion was accentuated (). Following digitization, the models were correlated with the sawbone by using a surface-surface matching algorithm. Finally, a universal tracker was attached to a calibrated surgical drill and the lesion was resected under computer guidance.
Results and discussion
The performance of the algorithm for detecting cam-type lesions has previously been assessed in an in vitro study on 102 dry femurs. Sensitivity in detecting aspherical portions at the head-neck junction was calculated to be close to the actual pixel size Citation[12]. Diagnostic agreement with the alpha angle, the most commonly used radiographic measurement for assessing the risk of FAI, was found to be 0.96 (95% confidence interval, 0.94-0.97).
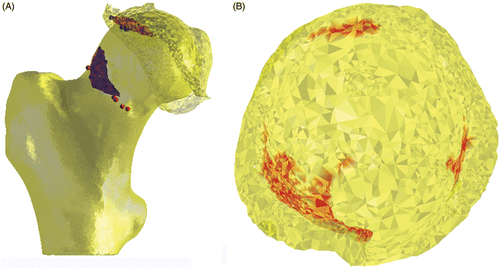
The accuracy of the surface-to-surface matching of the digitized sawbone models and their CT imaging data was 0.4 mm. The navigated chondroplasty resulted in a complete resection of the planned and segmented lesions in all cases (). The surgical accuracy in performing the planned osteochondroplasty, defined as the distance between the cam lesion to be resected and the corresponding postoperative femoral surface, was 0.14 mm. These data support the further exploration of this technique in clinical applications, and a clinical study has been initiated to assess the accuracy and feasibility of the technique in FAI patients.
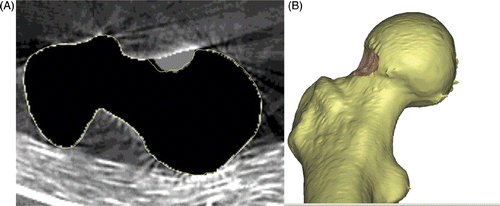
Figure 5. In vitro evaluation of computer-assisted surgical accuracy. (A) Axial CT reconstruction of the planned (grey area) versus actual (yellow outline) resection of a cam-type lesion in a sawbone model; and (B) 3D reconstruction of the postoperative result.
Brunner et al. Citation[13] have already evaluated a CT-based navigation protocol for improving the accuracy of arthroscopic osteochondroplasty in impingement patients. Although the navigational system itself is highly accurate, the authors concluded that it does not improve the rate of insufficient alpha angle corrections after hip arthroscopy (24%). The major weakness of the tested navigation system is that the software only displays the position of the surgical instrument in relation to the femur neck; it does not allow for preoperative planning, nor does it highlight the impingement zone or display the amount of bone to be resected. Without this important information, any precise navigational system will fail to improve surgical accuracy Citation[14].
The technique presented in this paper could theoretically improve on these results, as it enables 3D virtual simulation of the impingement process, preoperative planning of the localization and extent of femoral reshaping, and postoperative evaluation of the accuracy of offset restoration. Further validation in a clinical environment is obviously mandatory to substantiate such claims.
Conclusion
This paper presents a new method for three-dimensional evaluation and computer aided treatment of FAI. The methodology described offers a number of advantages compared to currently used techniques for the diagnosis and evaluation of hip impingement, and has direct orthopaedic applications as it allows for computer aided planning and surgery. Further validation in a clinical environment is clearly necessary and has been initiated.
Declaration of interest: The presented work was supported in part by Materialise NV, the Institute for the Promotion of Innovation through Science and Technology in Flanders (IWT-Vlaanderen), and Stryker.
References
- Gosvig KK, Jacobsen S, Sonne-Holm S, Gebuhr P. The prevalence of cam-type deformity of the hip joint: A survey of 4151 subjects of the Copenhagen Osteoarthritis Study. Acta Radiol 2008; 49(4)436–441
- Beck M, Kalhor M, Leunig M, Ganz R. Hip morphology influences the pattern of damage to the acetabular cartilage: Femoroacetabular impingement as a cause of early osteoarthritis of the hip. J Bone Joint Surg Br 2005; 87(7)1012–1018
- Chegini S, Beck M, Ferguson SJ. The effects of impingement and dysplasia on stress distributions in the hip joint during sitting and walking: A finite element analysis. J Orthop Res 2007; 27(2)195–201
- Arbabi E, Chegini S, Boulic R, Tannast M, Ferguson SJ, Thalmann D. Penetration depth method – novel real-time strategy for evaluating femoroacetabular impingement. J Orthop Res 2010; 28(7)880–886
- Johnston TL, Schenker ML, Briggs KK, Philippon MJ. Relationship between offset angle alpha and hip chondral injury in femoroacetabular impingement. Arthroscopy 2008; 24(6)669–675
- Zebaze RM, Jones A, Welsh A, Knackstedt M, Seeman E. Femoral neck shape and the spatial distribution of its mineral mass varies with its size: Clinical and biomechanical implications. Bone 2005; 37(2)243–252
- Howe WG. Two-sided tolerance limits for normal populations – some improvements. J Am Statist Assoc 1969; 64: 610–620
- Thornberry RL, Hogan AJ. The combined use of simulation and navigation to demonstrate hip kinematics. J Bone Joint Surg Am 2009; 91(Suppl 1)144–152
- Arbabi E, Boulic R, Thalmann D. Fast collision detection methods for joint surfaces. J Biomech 2009; 42(2)91–99
- Crawford MJ, Dy CJ, Alexander JW, Thompson M, Schroder SJ, Vega CE, Patel RV, Miller AR, McCarthy JC, Lowe WR, et al. The 2007 Frank Stinchfield Award. The biomechanics of the hip labrum and the stability of the hip. Clin Orthop Relat Res 2007; 465: 16–22
- Audenaert EA, Mahieu P, Pattyn C. Three-dimensional assessment of cam engagement in femoroacetabular impingement. Arthroscopy 2010;Oct 15 [Epub ahead of print].
- Audenaert EA, Baelde N, Huysse W, Vigneron L, Pattyn C. Development of a three-dimensional detection method of cam deformities in femoroacetabular impingement. Skeletal Radiol 2010;Aug 18 [Epub ahead of print].
- Brunner A, Horisberger M, Herzog RF. Evaluation of a computed tomography-based navigation system prototype for hip arthroscopy in the treatment of femoroacetabular cam impingement. Arthroscopy 2009; 25(4)382–391
- Musahl V, Plakseychuk A, Fu FH. Current opinion on computer-aided surgical navigation and robotics: Role in the treatment of sports-related injuries. Sports Med 2002; 32(13)809–818