Abstract
This study assessed the improvement in relationship quality, effectiveness and safety associated with vardenafil for the treatment of erectile dysfunction (ED). The study was conducted in 1433 centres across 21 countries and comprised a baseline patient visit and up to four follow-up visits during an observational period of 1 year. Relationship quality, happiness, satisfaction with vardenafil treatment, and safety and tolerability were assessed by physician interviews and patient and partner questionnaires. Overall, 7496 patients were enrolled in the study, of which 7430 were included in the safety analyses and 6470 in the effectiveness analyses. Relationship quality, assessed by a relationship questionnaire (partnerschaftsfragebogen [PFB]), was improved at last observation carried forward, compared with baseline, in both patients and partners and satisfaction with the effectiveness of vardenafil treatment was high. Vardenafil was well tolerated and adverse events were consistent with the known safety profile of phosphodiesterase type 5 inhibitors. These results confirm the well-established effectiveness and safety profiles of vardenafil. This study is the first to demonstrate improvements in relationship quality following vardenafil therapy, in both patients and partners, using the PFB questionnaire.
Introduction
Erectile dysfunction (ED) impacts both patients and their partners [Citation1]. Men with ED may have feelings of shame, embarrassment, fear of sexual failure, and experience withdrawal from their partner [Citation2]. Female partners may fear her partner has lost interest, or no longer finds her attractive, and may feel rejected, abandoned and experience low self-esteem [Citation2].
Untreated ED can have negative effects on the female partner, leading to decreased sexual satisfaction and impacting her relationship with her partner [Citation3–5]. Consequently, ED can cause substantial psychological and emotional distress in both members of the couple, and negatively impact the relationship, causing withdrawal of intimacy and affection [Citation6–8]. Communication between partners about ED, their sex lives, and relationship is important to maintain trust, intimacy and closeness [Citation2].
Successful treatment of ED can help restore relationship and sexual satisfaction for both members of the couple. Treatment with phosphodiesterase type 5 (PDE-5) inhibitors has been shown to improve sexual and relationship quality in patients, while also improving sexual satisfaction in untreated female partners [Citation9]. The FEMALES study demonstrated an improvement in the sexual experience in women whose partners were treated for ED [Citation3]. Similarly, PDE-5 inhibitor therapy can have beneficial effects on the sexual quality of life of the female partner, as well as improving relationship quality [Citation10].
The efficacy and safety profile of the PDE-5 inhibitor, vardenafil, in the treatment of ED is well established [Citation11–16]. Studies also show that vardenafil has beneficial effects on the sexual quality of life of both the treated man and his untreated female partner [Citation15,Citation17], and their satisfaction with the functional and emotional aspects of their sexual activity [Citation18].
The REal-life Perception of Efficacy, Attitude, satisfaction and safety of vardenafil Therapy (REPEAT) study assessed the improvement in relationship quality from both the patients’ and partners’ points of view, as well as the effectiveness and safety associated with vardenafil treatment in men with ED under real-life conditions.
Methods
Patients
REPEAT was a prospective, non-interventional, non-controlled, multicentre, observational, post-marketing study conducted between 28 March 2007 and April 26, 2010, in 1433 centres across 21 countries: China, Croatia, France, Germany, Hungary, Indonesia, Malaysia, the Middle East (Jordan, Kingdom of Saudi Arabia, Kuwait, Lebanon, Qatar and United Arab Emirates), Namibia, Poland, Republic of Korea, Singapore, South Africa, Spain, Sweden and Thailand.
This was a competitive recruitment study; all eligible patients were enrolled consecutively, a key element of the design for reducing selection bias. Men aged ≥18 years of age with a diagnosis of ED according to the National Institutes of Health Consensus Development Panel on Impotence [Citation19], treated with vardenafil, were eligible. Exclusion criteria were contraindications to vardenafil, as described in the local summary of product characteristics.
The study was conducted in accordance with the guidelines of the European Medicines Agency and applicable local laws and regulations. All patients were provided with full information about the study by the physician and provided verbal informed consent. If requested by local regulations, patients also signed informed consent forms prior to participating in the study.
Study design
Patients attended an initial visit (baseline) and up to four follow-up visits, approximately every 3 months, during the observation period of 12 months. The physician collected data at baseline and at each subsequent visit. Patients and partners were asked to voluntarily complete a patient and/or partner questionnaire at baseline and a relationship questionnaire (Partnerschaftsfragebogen [PFB]) [Citation20,Citation21] at each follow-up visit.
The PFB was designed and validated in German [Citation20]. Validated English, French, Afrikaans, South African English and Spanish translations of the PFB were used in this study. Other PFB language versions were not validated.
There were local differences in study methods: in the Middle-East region, patients and partners were not asked to complete questionnaires. In China, the observation period was 3–6 months (1–2 follow-up visits).
Study medication
An initially prescribed dose of vardenafil could be titrated within the observation period according to physician recommendation and labelling instructions. All decisions regarding therapy, dose and duration were made by the attending physician.
Relationship quality assessments
Relationship quality and happiness were assessed in the set of patients and partners who completed PFB questionnaires. Patients and partners were asked an identical set of questions measuring marital quality. The PFB questionnaire comprised three sub-scales: tenderness, togetherness/communication, and quarrelling, each containing ten questions [Citation20,Citation21]. Each question was scored between 0 (“never/very rarely”) and 3 (“very often”). For the tenderness and togetherness/communication sub-scale, 0 was the lowest score and 30 was the highest score, and vice versa on the quarrelling sub-scale. The overall score ranged from 0 to 90 and was calculated as follows:
Additionally, patient and partner happiness was evaluated using the question “How happy would you rate your partnership at the moment?” with the following responses: “very happy”, “happy”, “somewhat happy”, “somewhat unhappy”, “unhappy” and “very unhappy”. The first three options, and latter three options, comprised the “happy” and “unhappy” subject groups, respectively.
To account for the varying number of follow-up questionnaires completed, change in happiness and relationship quality was analysed from baseline to the last visit documented/last observation carried forward (LOCF). Intra-couple comparisons were conducted only if valid information was available for both patient and partner.
Effectiveness assessments
Effectiveness assessments were conducted by physician interview and included patient-rated satisfaction with vardenafil treatment covering the following aspects: effectiveness, onset of action, duration of action, hardness of erection, ease of penetration, maintenance of erection and whether the patient would continue to take vardenafil.
Safety assessments
Safety assessments included the patient-rated tolerability of vardenafil (assessed by physician interview), and the nature and incidence of adverse events (AEs). All AEs were recorded by the physician in case report forms (CRFs), regardless of possible causal relationship to vardenafil treatment. Safety was assessed for up to 1 year.
Data management and statistical methods
CRFs were screened to clarify incomplete data and identify any hidden AEs. CRFs were entered into an electronic database, and the data were verified by an error analysis.
Incidences of AEs were calculated for a safety dataset, which included patients who took at least one dose of vardenafil, and who had sufficient information regarding whether or not they experienced an AE at any time point during the study, regardless of whether they were later lost to follow-up. The effectiveness population (per-protocol), which included all patients who took at least one dose of study medication and had any information regarding efficacy of vardenafil, was used to analyse relationship quality and effectiveness.
Basic quantitative statistics were calculated and p-values were based on Student’s t-tests. Available case analysis was used for missing values.
Results
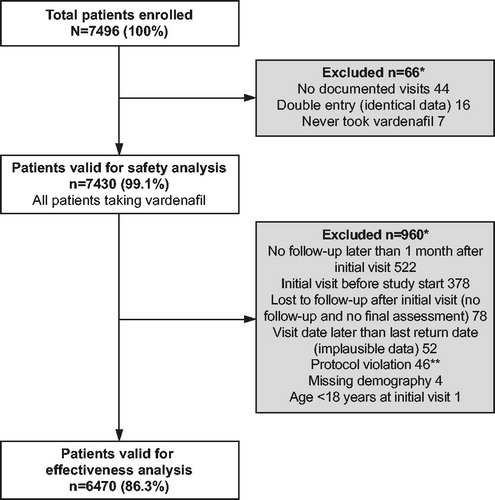
Overall, 7496 patients were enrolled in the study, of which 7430 and 6470 were valid for safety and effectiveness analysis, respectively. Reasons for exclusion from the safety and effectiveness populations are detailed in . Similar proportions of the population were present in Europe (32.0%), Asia-Pacific (32.9%) and the Middle East (35.1%).
Figure 1. Disposition of patients enrolled in the study. *Multiple responses possible. **Written consent not obtained prior to the first documented visit.
Demographic and baseline characteristics
Most participants were Asian (43.8%) or white (42.0%), and aged ≥45 years (68.1%; ). On average, partners were 4.6 years younger than patients, with a mean age of 45.2 ± 12.0 years (range: 18–81 years). Overall, 74.6% of patients had moderate or severe ED at baseline and the majority of men (76.7%) had experienced ED for ≥6 months. Over half of patients (55.2%) were taking at least one concomitant medication. Common underlying conditions included cardiovascular disease (defined as the presence of either cardiac or vascular disorders, including hypertension) (29.9%), hypertension (23.3%), diabetes mellitus (20.1%) and lipid metabolic disorders (11.1%). Approximately, one-third (30.5%) of patients had received prior treatment for ED, of which 59.5%, 25.0% and 9.3% had used sildenafil, tadalafil or vardenafil, respectively. Demographics and baseline characteristics for the subset of patients included in the relationship quality/happiness analysis were comparable with those of the total population.
Table 1. Patient demographics and baseline characteristics.
Vardenafil utilisation
A total of 21.6% (n = 1395), 44.2% (n = 2861), 9.4% (n = 605) and 24.9% (n = 1609) of patients attended one, two, three or four follow-up visits, respectively. The mean (±standard deviation [SD]) duration from baseline until first follow-up visit was 13.4 ± 8.5 weeks, and until the fourth follow-up visit was 45.2 ± 13.1 weeks. The mean observation period (defined as the time interval between the first visit and last documented follow-up visit/LOCF) was 29.8 ± 15.8 weeks. The majority of doses prescribed at baseline were for vardenafil 20 mg (66.7%). For most patients, the dose remained unchanged at last follow-up visit/LOCF (80.0%), and was up- or down-titrated in 8.5% and 7.4% of patients, respectively.
Patient and partner conceptions of ED
Over 40% of patients and partners believed that general or sexual relationship problems were the reason for the man’s ED. Approximately two-thirds of patients and partners believed the man’s ED to be temporary, and the majority were confident that his ED could be successfully treated ().
Table 2. Comparison of patient/partner questionnaire responses for self-assessment of ED, and attitudes and expectations of ED and its treatment.
Patient satisfaction with effectiveness
Almost all patients (94.6%) were very satisfied or satisfied with the effectiveness of vardenafil, while 4.7% were unsatisfied. Most patients (>90%) were very satisfied or satisfied with the onset and duration of action, ease of penetration, and hardness and maintenance of erection with vardenafil therapy. At last follow-up visit/LOCF, 84.6% of patients intended to continue vardenafil use.
Relationship quality and patient happiness
Overall, 3698 patients and 1886 partners completed a baseline patient/partner questionnaire, of which 2556 patients and 1266 partners also completed at least one follow-up patient/partner PFB relationship questionnaire.
At baseline, the mean scores for patients on the “tenderness”, “togetherness/communication” and “quarrelling” sub-scales were 14.7 ± 5.6, 16.2 ± 5.3 and 9.7 ± 5.8, respectively. The mean overall score was 51.1 ± 13.3. The mean PFB questionnaire scores for each sub-domain were similar in partners and patients (). A breakdown of patients’ and partners’ responses at baseline, to the questions on each PFB sub-scale, is shown in .
Figure 2. PFB relationship questionnaire scores in patients and partners at baseline and last follow-up visit/LOCF (patient/partner relationship population). ***p < 0.0001 for comparison of last follow-up visit/LOCF versus baseline. LOCF, last observation carried forward; PFB, relationship questionnaire (“Partnerschaftsfragebogen”); SD, standard deviation.
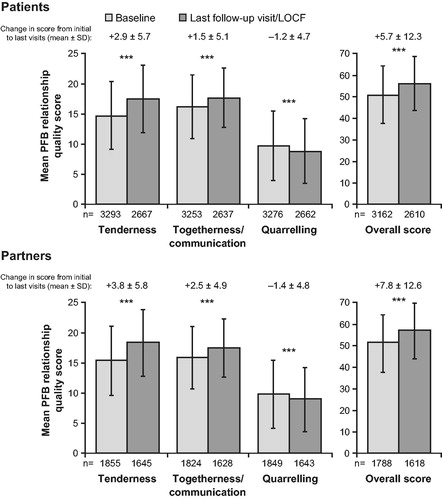
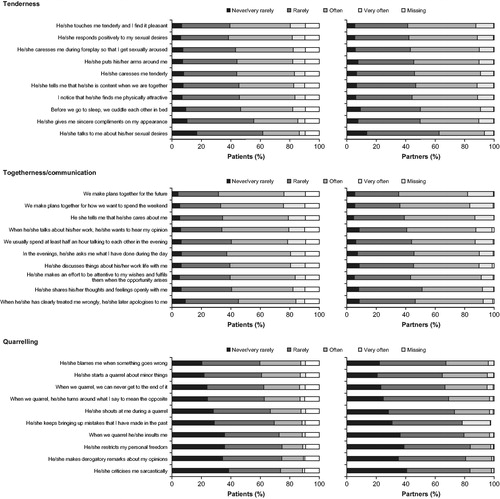
Figure 3. Patients’ and partners’ responses at baseline to the questions on the sub-scales of the PFB questionnaire (patient/partner relationship population). PFB, relationship questionnaire (“Partnerschaftsfragebogen”).
Overall, relationship quality was improved at last follow-up visit/LOCF, compared with baseline, in both patients and partners (mean PFB score 56.3 ± 12.7 versus 51.1 ± 13.3 [patients] and 57.1 ± 12.8 versus 51.5 ± 13.1 [partners]). Mean improvements in overall PFB scores for patients and partners were +5.7 ± 12.3 (p < 0.0001) and +7.8 ± 12.6 (p < 0.0001), respectively (). In patients, the greatest improvement was observed in the tenderness sub-scale (mean difference +2.9 ± 5.7; p < 0.0001), with smaller improvements in the togetherness/communication (mean difference +1.5 ± 5.1; p < 0.0001) and quarrelling (mean difference −1.2 ± 4.7; p < 0.0001) sub-scales. A clinically relevant improvement (a sub-scale change of four points) [Citation22–24] was observed in 38.6% (95% confidence interval [CI]: 36.7–40.5) of patients for the tenderness sub-scale, 27.2% (95% CI: 25.4–29.0) of patients for the togetherness/communication sub-scale and 24.2% (95% CI: 22.6–26.0) of patients for the quarrelling sub-scale. Similar statistically significant (p < 0.0001) improvements were observed in all partners’ PFB scores, with partners showing a slightly greater improvement than patients on all sub-scales (). A clinically relevant improvement was observed in 43.4% (95% CI: 40.7–46.2), 32.5% (95% CI: 29.9–35.1) and 27.2% (95% CI: 24.8–29.7) of partners for the tenderness, togetherness/communication and quarrelling sub-scales, respectively. Patients’ and partners’ PFB scores were positively correlated at baseline and last follow-up visit/LOCF (Pearson correlation coefficients at each time point, respectively, for tenderness: 0.759, 0.793; togetherness/communication: 0.770, 0.793; quarrelling: 0.796, 0.826; and overall: 0.828, 0.859).
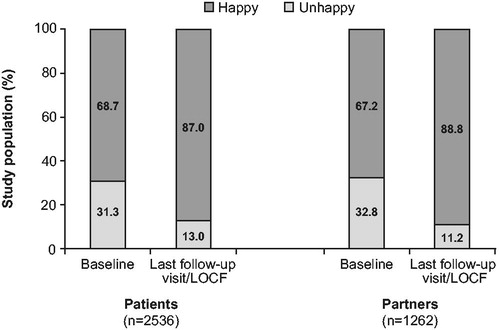
At baseline, over two-thirds of patients and partners reported being happy with their relationship (). At last follow-up visit/LOCF, there was an increase in the proportion of both patients (+18.3%) and partners (+21.6%) reporting happiness. The proportion of patients assessing themselves as happy increased from 68.7% at baseline to 87.0% at last follow-up visit/LOCF. Similar improvements were reported by the partners.
Safety and tolerability
AEs were reported by 344 patients (4.63%), and the incidence of treatment-related AEs was 4.28%. Two serious AEs occurred, but were not considered to be related to vardenafil (). The most frequently reported treatment-related AEs were headache (2.10%), flushing (0.90%) and nasal congestion (0.39%; ). Overall, 94.6% of patients were very satisfied or satisfied with the tolerability of vardenafil.
Table 3. Incidence of adverse events during the study (safety population, n = 7430).
Discussion
This study investigated improvement in relationship quality, effectiveness and safety of vardenafil for the treatment of ED under real-life conditions. The study population was representative of men receiving ED therapy in the real world. The prevalence of underlying conditions in the study population was comparable with that in previous studies [Citation25–27]. Importantly, this study also included patients taking concomitant medications for these conditions, who may be excluded from some clinical trials.
This is the first study to assess the effect of vardenafil on relationship quality, from both the patients’ and partners’ points of view, using the PFB questionnaire. The PFB questionnaire was developed for diagnosis and evaluation in conjoint marital therapy [Citation20], and it has been cross-validated using normal and distressed couples. The PFB was first used in ED patients in a cross-sectional study, which compared PFB scores for sildenafil-treated patients and their female partners, with those in untreated patients and their female partners [Citation24]. Erectile function correlated highly with tenderness and togetherness in both male patients and their female partners [Citation24]. The REPEAT study, however, examined the longitudinal change of PFB scores before and during treatment with vardenafil.
Patients and partners in the REPEAT study demonstrated agreement in their perceptions of the man’s ED, and their beliefs about ED therapy, supporting the findings of the earlier FEMALES study [Citation1]. However, studies examining the impact of PDE-5 inhibitor treatment on patient–partner relationships are limited [Citation1,Citation3,Citation28]. Improvements in relationship quality were found across the international and multicultural REPEAT study population, in both patients and partners, for each aspect of the PFB questionnaire. However, care should be taken when interpreting these results, as the number of partners who completed baseline PFB questionnaires was relatively small compared with the overall number of patients. However, the absolute number of participating partners makes REPEAT the largest prospective relationship quality study where the male partner has ED. Similar improvements in relationship quality were described in a previous study in couples (n = 96), in which treatment of the man’s ED with tadalafil or sildenafil was associated with an improvement in the female partner’s perception of their relationship quality [Citation10]. Successful pharmacological treatment of ED may thus benefit and support non-medical approaches to addressing relationship problems, such as relationship counselling and psychosexual therapy.
Vardenafil was well tolerated. The incidence of AEs (4.6%) was comparable with that seen in a large observational study (REALISE study) in 73 946 patients (3.6%) [Citation27]. The most frequently reported treatment-related AEs were headache, flushing and nasal congestion, consistent with the most frequently reported AEs from the REALISE study [Citation27], and the known safety profile of PDE-5 inhibitors.
Differences in drug responses between Asian and other ethnicities (e.g. Caucasian, African-American) may exist [Citation29]. In the present study, which contained a high percentage of Asian subjects (43.8%), vardenafil treatment was associated with favourable outcomes for effectiveness, safety and relationship quality. This echoes the findings of a previous study, which demonstrated the excellent efficacy and tolerability of vardenafil in a population of exclusively East Asian men [Citation30].
There are some limitations to this study. A competitive recruitment method was used in this study to facilitate recruitment of a large population of patients. All patients who met the inclusion criteria and did not have any contraindications to vardenafil were eligible for enrolment. This method does not allow for exclusion of PDE-5 inhibitor non-responders from the study population. Additionally, the 522 patients lost to follow-up and excluded from the effectiveness population might have discontinued due to various reasons, including dissatisfaction with treatment. These two factors may have influenced the effectiveness of vardenafil treatment observed in this study. This study was non-interventional and the objectives were assessed within the context of routine treatment. ED diagnosis and severity were determined by the physician, and all effectiveness assessments, apart from the PFB, were patient-rated. All documentation had to be made without interfering with treatment. Therefore, no additional diagnostic methods could be used, and all documentation forms were optional and voluntarily self-completed. The assessment of relationship quality before, during and at the end of the study was particularly challenging under these conditions. It should be noted that the number of partners who completed PFB questionnaires was relatively small compared with the overall number of patients. The PFB questionnaire was initially designed for use in marital behavioural therapy [Citation20], but has since been used in clinical trials and observational ED studies [Citation24]. The results of this study do not attempt to explain how individual sexual or marital behaviours change due to ED treatment, but rather provide descriptive quantitative information as a foundation for further research.
Although the REPEAT study included men across 21 countries, the results of the study might not be universally applicable to all couples in all countries. Assessments of relationship quality can vary from country to country, presumably due to cultural differences. Therefore, it will be beneficial to analyse regional subgroup populations (e.g. Europe and Asia-Pacific) to verify and extend the findings of the REPEAT study.
Conclusions
The results from the REPEAT study confirm the established effectiveness and safety profile of vardenafil under real-life conditions. The significant improvements in all dimensions of relationship quality in both members of the couple further demonstrate the benefits conferred by vardenafil treatment, for both patients with ED, and their partners.
Declaration of interest
U. H. has been a clinical trial investigator for Bayer HealthCare, and a consultant to Astellas, Boehringer Ingelheim, Eli Lilly and Company, Pfizer and Procter & Gamble; J. U. H. and A. M. are employees of Bayer HealthCare. Editorial support was provided by Joanne Mulcahy, PhD, of Fishawack Communications Ltd.
Financial support for this study was provided by Bayer HealthCare.
References
- Fisher WA, Eardley I, McCabe M, Sand M. Erectile dysfunction (ED) is a shared sexual concern of couples I: couple conceptions of ED. J Sex Med 2009;6:2746–60
- DiMeo PJ. Psychosocial and relationship issues in men with erectile dysfunction. Urol Nurs 2006;26:442–6, 53; quiz 447
- Fisher WA, Rosen RC, Eardley I, et al. Sexual experience of female partners of men with erectile dysfunction: the female experience of men's attitudes to life events and sexuality (FEMALES) study. J Sex Med 2005;2:675–84
- Conaglen HM, Conaglen JV. The impact of erectile dysfunction on female partners: a qualitative investigation. Sex Rel Ther 2008;23:147–56
- O’Connor EJ, McCabe MP, Conaglen HM, Conaglen JP. Attitudes and experiences: qualitative perspectives on erectile dysfunction from the female partner. J Health Psychol 2012;17:3–13
- Dunn ME. Restoration of couple's intimacy and relationship vital to reestablishing erectile function. J Am Osteopath Assoc 2004;104:S6–10
- Ehrensaft MK, Condra M, Morales A, Heaton J. Communication patterns in patients with erectile dysfunction and their partners. Int J Impot Res 1994;6:25–32
- McCabe MP. Intimacy and quality of life among sexually dysfunctional men and women. J Sex Marital Ther 1997;23:276–90
- Seftel AD, Buvat J, Althof SE, et al. Improvements in confidence, sexual relationship and satisfaction measures: results of a randomized trial of tadalafil 5 mg taken once daily. Int J Impot Res 2009;21:240–8
- McCabe MP, O’Connor EJ, Conaglen JV, Conaglen HM. The impact of oral ED medication on female partners' relationship satisfaction. J Sex Med 2011;8:479–83
- Cheng E. Real-life safety and efficacy of vardenafil in the treatment of erectile dysfunction-results from 30,010 U.S. patients. J Sex Med 2007;4:432–9
- Hatzichristou D, Montorsi F, Buvat J, et al. The efficacy and safety of flexible-dose vardenafil (Levitra®) in a broad population of European men. Eur Urol 2004;45:634–41; discussion 641
- Hellstrom WJ, Gittelman M, Karlin G, et al. Vardenafil for treatment of men with erectile dysfunction: efficacy and safety in a randomized, double-blind, placebo-controlled trial. J Androl 2002;23:763–71
- Mirone V, Palmieri A, Cucinotta D, et al. Flexible-dose vardenafil in a community-based population of men affected by erectile dysfunction: a 12-week open-label, multicenter trial. J Sex Med 2005;2:842–7
- Ralph D, Eardley I, Kell P, et al. Improvement in erectile function on vardenafil treatment correlates with treatment satisfaction in both patients and their partners. BJU Int 2007;100:130–6
- Stief C, Porst H, Saenz De Tejada I, et al. Sustained efficacy and tolerability with vardenafil over 2 years of treatment in men with erectile dysfunction. Int J Clin Pract 2004;58:230–9
- Martin-Morales A, Graziottin A, Jaoude GB, et al. Improvement in sexual quality of life of the female partner following vardenafil treatment of men with erectile dysfunction: a randomized, double-blind, placebo-controlled study. J Sex Med 2011;8:2831–40
- Edwards D, Hackett G, Collins O, Curram J. Vardenafil improves sexual function and treatment satisfaction in couples affected by erectile dysfunction (ED): a randomized, double-blind, placebo-controlled trial in PDE5 inhibitor-naive men with ED and their partners. J Sex Med 2006;3:1028–36
- NIH Consensus Conference. Impotence. NIH Consensus Development Panel on Impotence. JAMA 1993;270:83–90
- Hahlweg K. Konstruktion und Validierung des Partnerschaftsfragebogens PFB [Construction and validation of the partnership questionnaire]. Zeitschrift für Klinische Psychologie 1979;8:17–40
- Hinz A, Strobel-Richter Y, Brahler E. Der Partnerschaftsfragebogen (PFB): Normierung und soziodemographische Einflussgröβen auf die Partnerschaftsqualität. Diagnostica 2001;47:132–41
- Hahlweg K. Partnership questionnaire PFB. In: Hersen M, Bellack AS, eds. Partnership questionnaire PFB. Dictionary of behavioral assessment devices. New York (NY): Pergamon; 1988
- Hahlweg K. Fragebogen zur Partnerscaftsdiagnostik (FDP). PFB, PL und FLP. Göttingen: Hogrefe, 1996
- Muller MJ, Ruof J, Graf-Morgenstern M, et al. Quality of partnership in patients with erectile dysfunction after sildenafil treatment. Pharmacopsychiatry 2001;34:91–5
- Braun M, Wassmer G, Klotz T, et al. Epidemiology of erectile dysfunction: results of the ‘Cologne Male Survey'. Int J Impot Res 2000;12:305–11
- Rosen RC, Fisher WA, Eardley I, et al. The multinational Men's Attitudes to Life Events and Sexuality (MALES) study: I. Prevalence of erectile dysfunction and related health concerns in the general population. Curr Med Res Opin 2004;20:607–17
- Van Ahlen H, Zumbe J, Stauch K, Hanisch JU. The real-life safety and efficacy of vardenafil (REALISE) study: results in men from Europe and overseas with erectile dysfunction and cardiovascular or metabolic conditions. J Sex Med 2010;7:3161–9
- Fisher WA, Eardley I, McCabe M, Sand M. Erectile dysfunction (ED) is a shared sexual concern of couples II: association of female partner characteristics with male partner ED treatment seeking and phosphodiesterase type 5 inhibitor utilization. J Sex Med 2009;6:3111–24
- Chowbay B, Zhou S, Lee EJ. An interethnic comparison of polymorphisms of the genes encoding drug-metabolizing enzymes and drug transporters: experience in Singapore. Drug Metab Rev 2005;37:327–78
- Chen KK, Paick JS, Ishii N. The efficacy and safety of vardenafil in East Asian men with erectile dysfunction. J Sex Med 2007;4:753–61