Abstract
Purpose: To prospectively evaluate 5α-reductase inhibitors (5αRIs) for benign prostatic hyperplasia (BPH) patients with a large prostate (>80 mL) after transurethral resection of the prostate (TURP).
Materials and methods: Eighty-seven patients were recruited from January 2007 to October 2014. Patients were randomized into a trial and a control group. The trial group was treated with 5αRIs for 3 years after TURP, while the control group received a placebo. We evaluated the indicators before, peri and after TURP.
Results: There were no significant differences in the indicators before and peri-TURP. Six months later, there were significant differences in PSA and hematuria (HU). Three years after TURP, there were significant differences in prostate volume (PV), level of prostate-specific antigen (PSA), the maximum flow rate (Qm), and HU between the trial and control groups. Additionally, there were significant differences in the PV, PSA, international prostate symptom score (IPSS), patient quality of life (QoL) in the trial group alone between those treated with finasteride and those treated with dutasteride.
Conclusions: After TURP for large BPH, administration of 5αRIs for 3 years improved PV, PSA, Qm and HU. Additionally, dutasteride produced superior improvements in PV, PSA, IPSS and QoL compared with finasteride.
Introduction
Benign prostatic hyperplasia (BPH) is a common urological disease. Its prevalence is approximately 40% for men in their fifties, and reaches 90% for men in their nineties [Citation1]. It should be noted that only 4% of prostates in men >70 years of age can reach sizes >100 g [Citation2]. Due to ongoing improvements in surgical technology, large BPH (>80 mL) patients have been treated with transurethral resection of the prostate (TURP) method as well as other micro-invasive procedures [Citation3–13]. After TURP, however, patients with large BPH may still suffer from lower urinary tract symptoms (LUTS), and even require retreatment with TURP (re-TURP). Therefore, it is important for these patients to take medicines in order to relieve symptoms associated with TURP and reduce the possibility of re-TURP.
5α-reductase inhibitors (5αRIs) are commonly used to treat patients with BPH, and can reduce prostate volumes (PVs) by 20–30% [Citation14], thereby relieving obstructive symptoms. While it is clear that 5αRIs can delay the progression of BPH before an operation, the clinical benefits of continued 5αRI administration after TURP have not been well explored. Therefore, we conducted a prospective, randomized and controlled study from three clinical centers from January 2007 to October 2014, and observed the efficacy of 5αRIs for large BPH patients (>80 mL) after TURP.
Subjects and methods
This study was performed in three clinical centers: the Urology Department at Xinhua Hospital affiliated to Shanghai Jiaotong University School of Medicine; the Urology Department at Ninth Hospital affiliated to Shanghai Jiaotong University School of Medicine; and the Urology Department at Tenth Hospital affiliated to Shanghai Tongji University School of Medicine. The study was approved by the responsible ethics committee of the Chinese Clinical Trial Register. All patients completed the written informed consent process.
Participants
There were 120 patients with large BPH (>80 mL) included in the study from January 2007 to October 2014. The mean age was 71.1 ± 7.8 years (range, 65–81 years). By the end of the 3-year follow-up period, 87 patients completed the randomized clinical trial (RCT). Patients in the trial group included 45 cases, 28 treated with finasteride and 17 treated with dutasteride. The patients in the control group included 42 cases treated with a placebo. Among them, there were 78 patients (89.6%), who received medical treatment (including 5α-reductase inhibitors, α receptor blockers or antimuscarinics) from 3 months to 3 years before the operation.
PV (calculated as PV = 0.52 × transverse × anteroposterior × cephalocaudal diameter) was measured by transrectal ultrasound. Post-void residual volume (PVR) was measured by transabdomen ultrasound. The maximum urine flow rate (Qm) and pressure of detrusor at Qm (PdetQm) were measured by urodynamic examination. Voiding symptoms and quality of life (QoL) were graded according to the International Prostate Symptom Score (IPSS) and the QoL assessment index. In addition, we assessed levels of prostate-specific antigen (PSA), acute urinary retention (AUR), hematuria (HU), bladder stone and urinary tract infection (UTI) history with the result of BPH.
Patient inclusion criteria were: (1) PV > 80 mL; (2) IPSS ≥ 13, QoL ≥ 3; (3) PVR ≥ 200 mL; (4) Qm < 15 mL/s; (5) age ≥ 60 years; (6) refractory HU history; (7) bladder stone history; (8) AUR history; (9) refractory UTI history.
Patient exclusion criteria were: (1) prostate cancer or bladder cancer, (2) urethral stenosis, (3) neurogenic bladder, (4) refractory diabetes and (5) urological system tuberculosis.
Therapy methods
A total of 87 patients were randomized into the trial and control groups according to a computer-generated randomization schedule at study entry. All the operations were monopolar TURP and were conducted by the deputy chief urologist with an experience of over 100 cases. All operations were performed under general or spinal anesthesia using a standard technique (ACMI of America or Storz of Germany) and the electro surgical instrument system at a setting of 180 W for cutting and 65 W for coagulation. During TURP, continuous irrigation was performed using a 5% mannitol solution in 3000 mL bags. The assistants weighed the removed prostate tissue. After TURP, all the patients were retained on an F24 catheter with 60 mL normal saline in the balloon, and were exposed to routine irrigation using normal saline in 3000 mL bags. After 2–5 days, the catheter was removed and discharged the patients.
On the first day after TURP, patients in the trial group were administered finasteride (5 mg, qN) or dutasteride (0.5 mg, qN) for at least 3 years according to a computer-generated randomization schedule. Patients in the control group were administered a placebo. All patients were followed-up at six months and 3 years.
Observation indicators
Before TURP observation, the indicators included age, PV, PVR, PSA, IPSS, QoL, Qm, PdetQm, AUR history, HU history and UTI history. Peri-TURP observation indicators included the operation minutes (OM), the resection ratio [RR; calculated as RR = weight of resected prostate tissue/(1.05 × PV)], catheterization days after operation (CDAO), and hospitalized days after operation (HDAO). After TURP (6 months and 3-year follow-ups) observation indicators included PV, PVR, PSA, IPSS, QoL, Qm, PdetQm, AUR history, HU history, UTI history and re-TURP history.
Statistical analysis
Statistical analyses were carried out using SPSS® statistical software, version 18.0.1 (SPSS, Armonk, NY) for Windows®. Differences between the two treatment groups were compared using Student’s t-test, and the incidence of adverse events was analyzed using either Pearson’s χ2-test or the Fisher exact test. A p value <0.05 was considered to be statistically significant.
Results
There were 120 patients with large BPH included in our study. By the end of the 3 years follow-up, there were 87 patients who had completed the RCT. The lost to follow-up rate was approximately 27.5%. There were 45 and 42 patients in trial and control groups, respectively. The baseline characteristics of all patients in both groups are shown in . The mean ages were 70.1 ± 8.8 and 72.3 ± 5.8 years, respectively. PV was 105.1 ± 15.6 and 104.2 ± 17.9 mL, respectively. PSA was 7.3 ± 0.7 and 7.4 ± 1.0 ng/mL, respectively. Qm was 5.8 ± 3.0 and 6.2 ± 3.1 mL/s, respectively. PdetQm was 74.6 ± 30.1 and 75.1 ± 39.2 cm H2O, respectively. IPSS was 24.2 ± 4.5 and 23.3 ± 5.8, respectively. QoL was 4.7 ± 1.0 and 5.0 ± 0.9, respectively. PVR was 262.1 ± 20.3 and 262.7 ± 24.7 mL, respectively. The rates of AUR, HU and UTI history in the trial and control groups before TURP were 91.1 and 88.0%, 31.1 and 33.3%, and 26.7 and 26.1%, respectively. There were no significant differences in these variables between the trial and control groups (p > 0.05).
Table 1. Comparison of baseline characteristics between the trial and control groups before TURP.
All patients underwent TURP successfully without mortality. The comparison of peri-TURP variables in both groups is shown in . The OM was 88.1 ± 23.4 and 86.0 ± 32.1 minutes, respectively. The RR was 43.1 ± 3.2 and 42.3 ± 4.7%, respectively. The DCAO were 3.1 ± 1.0 and 3.2 ± 1.2 days, respectively. The HDAO were 4.5 ± 1.0 and 4.7 ± 1.3 days, respectively. There were no significant differences in these variables between the trial and control groups (p > 0.05).
Table 2. Comparison between trial and control group peri-TURP variables.
The comparisons of variables measured at the six month follow-up after TURP between the trial and control groups are shown in . PV was 60.2 ± 7.8 and 62.4 ± 5.4 mL, respectively. PSA was 3.9 ± 0.5 and 6.0 ± 0.7 ng/mL, respectively. Qm was 18.4 ± 3.2 and 17.3 ± 4.0 mL/s, respectively. IPSS was 8.1 ± 3.7 and 8.5 ± 2.9, respectively. QoL was 2.8 ± 0.6 and 2.6 ± 0.8, respectively. PVR was 14.2 ± 5.6 and 15.7 ± 4.9 mL, respectively. HU incidence after operation was 4.4 and 23.8%, respectively. UTI incidence after operation was 8.9 and 16.7%, respectively. Re-TURP incidence after operation was 2.2 and 2.4%, respectively. In the six months after TURP, there were significant differences in PSA and HU between the trial and control groups (p < 0.05). However, there were no significant differences between the trial and control groups in the indicators of PV, Qm, IPSS, QoL, PVR, UTI or re-TURP incidence (p > 0.05).
Table 3. Comparison between the trial and control groups at the six-month follow-up after TURP.
Comparisons between finasteride-treated and dutastride-treated patients in the trial group at the six-month follow-up after TURP are shown in . PV was 61.0 ± 5.8 and 59.4 ± 5.1 mL, respectively. PSA was 4.0 ± 0.6 and 3.8 ± 0.4 ng/mL, respectively. Qm was 18.9 ± 3.2 and 17.6 ± 4.2 mL/s, respectively. IPSS was 6.9 ± 3.7 and 7.3 ± 2.3, respectively. QoL was 2.5 ± 0.2 and 2.7 ± 0.8, respectively. PVR was 13.2 ± 5.1 and 15.3 ± 4.2 mL, respectively. HU incidence after operation was 3.45 and 6.25%, respectively. UTI incidence after operation was 10.34 and 6.25%, respectively. Re-TURP incidence after operation was 3.45 and 0.0%, respectively. In the six months after TURP, there were no significant differences in any of the comparisons between the finasteride-treated and dutastride-treated patients in the trial group (p > 0.05).
Table 4. Comparison between finasteride-treated and dutastride-treated patients in the trial group at the six-month follow-up after TURP.
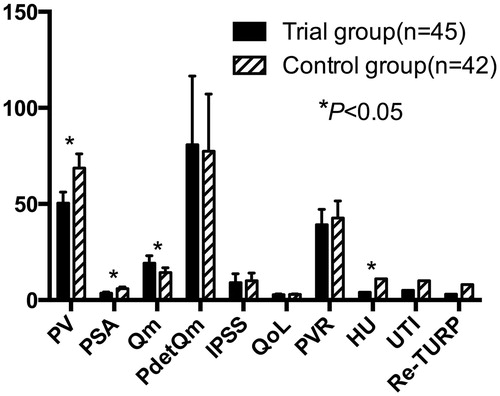
Comparisons between the trial and control groups at the 3-year follow-up after TURP are shown in . PV was 50.4 ± 5.8 and 68.7 ± 7.4 mL, respectively. PSA was 3.6 ± 0.6 and 5.9 ± 0.9 ng/mL, respectively. Qm was 19.2 ± 3.8 and 14 ± 2.5 mL/s, respectively. IPSS was 9.0 ± 4.7 and 10.1 ± 3.9, respectively. PdetQm was 80.8 ± 35.8 and 77.4 ± 29.8 cmH2O, respectively. QoL was 2.8 ± 0.4 and 2.9 ± 0.3, respectively. PVR was 39.2 ± 8.0 and 42.7 ± 8.9 mL, respectively. HU incidence after operation was 8.9 and 26.2%, respectively. UTI incidence after operation was 13.3 and 23.8%, respectively. Re-TURP incidence after operation was 6.7 and 19.0%, respectively. Three years after TURP, there were significant differences in PV, PSA, Qm and HU between the trial and control groups (p < 0.05), and there were no significant differences in PdetQm, IPSS, QoL, PVR, UTI or re-TURP between the trial and control groups (p > 0.05).
Figure 1. Comparison between the trial and control groups at the 3-year follow-up after TURP. PV: prostate volume, PSA: prostate-specific antigen, Qm: maximum urine flow rate, PdetQm: pressure of detrusor at Qm, IPSS: International Prostate Symptom Score, QoL: quality of life, PVR: post-void residual volume, HU: hematuria, UTI: urinary tract infection, Re-TURP: retreatment with TURP.
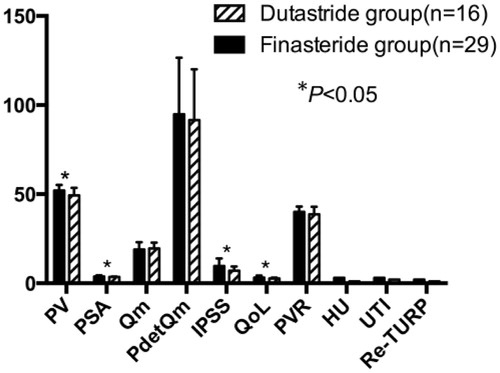
Comparisons between finasteride-treated and dutastride-treated patients in the trial group at the 3-year follow-up after TURP are shown in . PV was 52.1 ± 3.1 and 49.3 ± 4.2 mL, respectively. PSA was 3.8 ± 0.6 and 3.5 ± 0.3 ng/mL, respectively. Qm was 18.9 ± 4.2 and 19.5 ± 3.2 mL/s, respectively. PdetQm was 94.8 ± 31.7 and 91.5 ± 28.5 cmH2O, respectively. IPSS was 9.7 ± 4.2 and 7.1 ± 2.3, respectively. QoL was 3.2 ± 1.1 and 2.7 ± 0.5, respectively. PVR was 40.0 ± 3.1 and 38.7 ± 4.2 mL, respectively. HU incidence after operation was 10.34 and 6.25%, respectively. UTI incidence after operation was 13.79 and 12.50%, respectively. Re-TURP incidence after operation was 6.9 and 6.25%, respectively. Three years after TURP, there were significant differences in PV, PSA, IPSS and QoL between finasteride-treated and dutastride-treated patients in the trial group (p < 0.05). However, there were no significant differences in Qm, PdetQm, HU, UTI, PVR or re-TURP between these two groups (p > 0.05).
Figure 2. Comparison between finasteride-treated and dutastride-treated patients in the trial group at the 3-year follow-up after TURP. PV: prostate volume, PSA: prostate-specific antigen, Qm: maximum urine flow rate, PdetQm: pressure of detrusor at Qm, IPSS: the International Prostate Symptom Score, QoL: quality of life, PVR: post-void residual volume, HU: hematuria, UTI: urinary tract infection, Re-TURP: retreatment with TURP.
Discussion
The incidence of BPH, a disease that progresses with age, was approximately 40% for men in their fifties and reaches 90% for men in their nineties [Citation1]. Approximately 4% of the prostates in men >70 years of age could reach sizes >100 g [Citation2]. Although TURP remained the gold standard for patients with BPH [Citation15], the cut-off point set by the European Association of Urology guidelines was 80 mL [Citation16]. As for large BPH, many other micro-invasive procedures [Citation7–13], such as photoselective vaporization of the prostate (PVP), holmium laser enucleation of the prostate (HoLEP), and plasma kinetic resection of the prostate (PKRP) are available. A systematic meta analysis conducted by Gupta [Citation7] reported that surgical management of large prostates should be individualized based on the patient's comorbidities and the surgeon's expertise. Tasci et al. [Citation8] conducted a prospective non-randomized bi-center trial with 2 years of follow-up to compare PVP versus TURP for large prostates, and concluded that the post-operative micturition improvement was significant, lasting and was equivalent in both groups. The rate of late complications was equally low for both procedures. Krambeck et al. [Citation10] conducted HoLEP for prostates larger than 175 g and concluded that HoLEP provided a satisfactory outcome with low morbidity, even in large prostate glands. It should be noted that HoLEP was the only endoscopic technique that allowed for tissue removal comparable to that of open prostatectomy for such patients. Long et al. [Citation13] compared the safety and efficacy of PKRP versus transvesical prostatectomy (TVP) in the treatment of large volume BPH (100–149 mL), and concluded that PKRP had the advantage over TVP of being minimally invasive in the treatment of large volume BPH, while achieving the same post-operative outcomes.
Additional literature [Citation8,Citation17,Citation18] regarding TURP for large volume BPH reported that it could solve bladder outlet obstructions effectively. Mehmet Yucel et al. [Citation17] conducted conventional monopolar TURP in patients with large prostates (≥80 g), and concluded that conventional monopolar TURP could be performed effectively in those with large prostates based on technological improvements. After TURP, however, many patients still experienced frequent urination, urgency, and even re-occurrence of AUR. Additionally, the post-operative IPSS and QoL were not satisfying. Han et al. [Citation19] assessed which medications were used after TURP and found that many patients had persistent voiding dysfunction after surgical treatment for LUTS/BPH. Older age, history of diabetes, history of cerebrovascular accidents and pre-operative antimuscarinic drug use were determined to be possible risk factors.
The androgen dihydrotestosterone (DHT) can stimulate prostate development following conversion from testosterone by the action of 5αR in prostate stromal cells. The primary concern associated with 5αRIs in the past was that treatment of BPH would decrease the PV before TURP [Citation20]. Additionally, it was reported [Citation21] that the use of 5αARIs could reduce PVs by 20–30%, with the result of relieving obstructive symptoms in BPH patients. Some scholars, such as Kavanagh et al. [Citation22] and Hagerty et al. [Citation23] reported that finasteride should be routinely administered preoperatively for TURP, as it could reduce bleeding either during or after TURP. Other scholars, such as Zaitsu et al. [Citation24] reported that finasteride could rapidly reduce prostatic vascularity within two weeks. However, therapy with 5αARIs for LUTS after TURP and its efficacy have rarely been reported.
Age, serum PSA, Qm, the volume of the prostate, and histological inflammation are all established risk factors for BPH progression. Our study focused on large BPH, which represents one of the high-risk factors for progression. Although some urologists have expressed a belief that medicines for BPH is not necessary after TURP, the incidence of LUTS was found to be higher after TURP, necessitating prescriptions for those patients with large BPH. From January 2007 to October 2014, we conducted a prospective, randomized and controlled study. The outcomes of our study showed that these two sets of preoperative and perioperative indicators were comparable (p > 0.05). In a short amount of time (six months), 5αRIs significantly improved postoperative HU incidence, which may have been the result of reducing vascularity and prostatic tissue volume. Because the ability of 5αRIs to reduce PV shown to be slow, there were no significant differences in PV, Qm, PVR, IPSS or QoL in the short term. In the long term (3 years), however, PV, Qm, PSA and HU started to show significant differences between the trial and control groups (p < 0.05).
Because 5αRIs (mainly including finasteride and dutasteride) can reduce serum DHT, they are first-line therapy drugs to treat BPH. Dutasteride inhibits both isoforms of 5αRs (types I and II), and has a 45-fold greater affinity for 5αR type I and a 2.5-fold greater affinity for 5αR type II than finasteride, which results in significantly greater reductions in serum DHT than does finasteride therapy [Citation25]. As a result of this higher affinity, dutasteride effectively inhibits DHT much more rapidly than finasteride. Dutasteride was found to be well tolerated and efficacious at 48 months, suggesting that it is an effective long-term treatment for men with symptomatic BPH [Citation26]. In our research, we found no significant differences between finasteride- and dutasteride-treated patients in the short term (six months), but observed significant differences in PV, PSA, IPSS and QoL between the two groups over the long term (3 years). Therefore, our results are in alignment with those reported by Claus [Citation26] and Nickel [Citation25].
Because residual prostate tissue can still enlarge, some patients may suffer from re-TURP in the long term. Within six months after TURP, there were two patients (2.3%) who suffered from re-TURP in our study, with no difference (2.2 versus 2.4%) shown between the trial and control groups. Within 3 years after TURP, three patients in the trial group (6.7%) and eight patients in the control group (19.0%) were found to be suffering from re-TURP. Before TURP, therefore, we regularly told patients with large BPH that the re-TURP incidence rate was fairly high and that they may expect re-TURP in 3 years in order to reduce medical quarrels between patients and urologists. Although the difference between 6.7 and 19.0% was not statistically significant, we thought it was necessary to administer 5αRIs continuously after TURP, which had the benefit of reducing the incidence of re-TURP.
However, because the sample size was small (only 87 patients), the longest follow-up period was only 3 years, and the study was not double blinded, our results may not be sufficient to explain the outcomes and drug treatment characteristics of large BPH patients after TURP. Confirmation of our findings will require a larger sample size and a longer-term follow-up study. Additionally, many aspects of our present study design could be improved in order to achieve more accurate results in the future.
Conclusions
After TURP for large BPH, administration of 5αRIs for 3 years improved PV, PSA, Qm and HU. Additionally, dutasteride administration was found to be superior to finasteride administration in improving PV, PSA, IPSS and QoL.
Declaration of interest
The authors have no conflicts of interest to declare in relation to this article.
This study is sponsored by Shanghai Science and Technology Commission funds (No. 134119a0600, No. 14430720800 and No. 2134y038).
References
- Berry SJ, Isaacs JT. Comparative aspects of prostatic growth and androgen metabolism with aging in the dog versus the rat. Endocrinology 1984;114:511–20
- Berry SJ, Coffey DS, Walsh PC, et al. The development of human benign prostatic hyperplasia with age. J Urol 1984;132:474–9
- Yucel M, Aras B, Yalcinkaya S, et al. Conventional monopolar transurethral resection of prostate in patients with large prostate (≥80 grams). Cent Eur J Urol 2013;66:303–8
- Persu C, Georgescu D, Arabagiu I, et al. TURP for BPH. How large is too large? J Med Life 2010;3:376–80
- Chen Q, Zhang L, Liu YJ, et al. Bipolar transurethral resection in saline system versus traditional monopolar resection system in treating large-volume benign prostatic hyperplasia. Urol Int 2009;83:55–9
- Muzzonigro G, Milanese G, Minardi D, et al. Safety and efficacy of transurethral resection of prostate glands up to 60 ml: a prospective comparative study with 1 year of followup. J Urol 2004;172:611–15
- Gupta NP, Nayyar R. Management of large prostatic adenoma: lasers versus bipolar transurethral resection of prostate. Indian J Urol 2013;29:225–35
- Tasci AI, Tugcu V, Sahin S, et al. Rapid communication: photoselective vaporization of the prostate versus transurethral resection of the prostate for the large prostate: a prospective nonrandomized bicenter trial with 2-year follow-up. J Endourol 2008;22:347–53
- Alivizatos G, Skolarikos A, Chalikopoulos D, et al. Transurethral photoselective vaporization versus transvesical open enucleation for prostatic adenomas >80 ml: 12-mo results of a randomized prospective study. Eur Urol 2008;54:427–37
- Krambeck AE, Handa SE, Lingeman JE. Holmium laser enucleation of the prostate for prostates larger than 175 grams. J Endourol 2010;24:433–7
- Naspro R, Suardi N, Salonia A, et al. Holmium laser enucleation of the prostate versus open prostatectomy for prostates >70 g: 24-month follow-up. Eur Urol 2006;50:563–8
- Wei H, Shao Y, Sun F, et al. Thulium laser resection versus plasmakinetic resection of prostates larger than 80 ml. World J Urol 2014;32:1077–85
- Long Z, Zhang YC, He LY, et al. Comparison of transurethral plasmakinetic and transvesical prostatectomy in treatment of 100–149 mL benign prostatic hyperplasia. Asian J Surg 2014;37:58–64
- McConnell JD, Wilson JD, George FW, et al. Finasteride, an inhibitor of 5 alpha-reductase, suppresses prostatic dihydrotestosterone in men with benign prostatic hyperplasia. J Clin Endocrinol Metab 1992;74:505–8
- Rassweiler J, Teber D, Kuntz R, Hofmann R. Complications of transurethral resection of the prostate (TURP) – incidence, management, and prevention. Eur Urol 2006;50:969–79
- C Persu, D Georgescu, I Arabagiu, et al. TURP for BPH. How large is too large? J Med Life 2010;3:376–80
- Yucel M, Aras B, Yalcinkaya S, et al. Conventional monopolar transurethral resection of prostate in patients with large prostate (≥80 grams). Cent European J Urol 2013;66:303–8
- Michielsen DP, Coomans D, Peeters I, et al. Conventional monopolar resection or bipolar resection in saline for the management of large (>60 g) benign prostatic hyperplasia: an evaluation of morbidity. Minim Invasive Ther Allied Technol 2010;19:207–13
- Han HH, Ko WJ, Yoo TK, et al. Factors associated with continuing medical therapy after transurethral resection of prostate. Urology 2014;84:675–80
- Bartsch G, Rittmaster RS, Klocker H. Dihydrotestosterone and the concept of alpha reductase inhibition in human benign prostatic hyperplasia. Eur Urol 2000;37:367–80
- Feneley MR, Span PN, Schalken JA, et al. A prospective randomized trial evaluating tissue effects of finasteride therapy in benign prostatic hyperplasia. Prostatic Cancer Prostatic Dis 1999;2:277–81
- Kavanagh LE, Jack GS, Lawrentschuk N. Prevention and management of TURP-related hemorrhage. Nat Rev Urol 2011;8:504–14
- Hagerty JA, Ginsberg PC, Harmon JD, et al. Pretreatment with finasteride decreases perioperative bleeding associated with transurethral resection of the prostate. Urology 2000;55:684–9
- Zaitsu M, Tonooka A, Mikami K, et al. A dual 5α-reductase inhibitor dutasteride caused reductions in vascular density and area in benign prostatic hyperplasia. ISRN Urol 2013;2013:863489
- Nickel JC. Comparison of clinical trials with finasteride and dutasteride. Rev Urol 2004;6:S31–9
- Claus GR. 5α-Reductase inhibition in the treatment of LUTS and BPH: update and importance of dual inhibition of types 1 and 2. Rev Urol 2004;6:S1–2
