Abstract
The traditional pharmacological treatment of patients with late onset hypogonadism (LOH) is represented by different formulations of testosterone (T) or alternatively by the extractive human chorionic gonadotropin (HCG). The hormone replacement treatment (HRT) is associated with the potential increase of hematocrit, serum concentrations of prostate-specific antigen (PSA) and prostate volume. Moreover, the gynecomastia represent a condition frequently associated with HRT. Recent evidences showed the role of leydig cells in the 25-hydroxylation of vitamin D and the elevated frequency of hypovitaminosis D among LOH patients. Finally, another important aspect of LOH is represented by the frequency of secondary infertility due to age or to traditional HRT. This study evaluated 40 LOH patients treated for 6 months with extractive HCG (n = 10 patients) and three different formulations of T: transdermal (n = 10 patients), undecaonate (n = 10 patients) and enantate (n = 10 patients). Hormonal, anthropometric, metabolic and sperm parameters were evaluated and compared. Moreover, the main safety parameters and the results of the main questionnaires were evaluated. After treatment, HCG group showed serum concentrations of 25-OH-vitamin D significantly higher (p < 0.05) and serum concentrations of oestrogens significantly lower (p < 0.05) compared with other groups. Moreover, they showed a mean value of hematocrit, PSA and prostate volume significantly lower (p < 0.05) compared with other groups. Finally, all the groups treated with T showed a significant reduction (p < 0.05) of sperm density and of percentage of spermatozoa with progressive motility compared with HCG group.
Introduction
Late onset hypogonadism (LOH) is an endocrine condition characterized by a progressive reduction in serum concentrations of androgens in middle-aged men [Citation1]. The frequency of this condition, as recently reported by the study European Male Aging Study (EMAS) is 2.1% [Citation2]. Though applying only biochemical criteria, the prevalence of LOH has been reported to be up to 15% in the general population [Citation3].
The pathogenic mechanisms responsible for LOH can be distinguished in a central (altered hypothalamic or pituitary function) and in a peripheral form (impaired testicular function). The two mechanisms can coexist in the same patient [Citation4]. The sexual dysfunction (in particular, the erectile dysfunction) represents the main symptom of LOH, with a prognostic significance. LOH is associated with an increased cardiovascular and metabolic risk [Citation5] and an increased risk of osteoporosis and depression [Citation6].
The traditional pharmacological treatment for LOH is represented by testosterone (T) in transdermal or intramuscular formulation. The use of T for replacement therapy requires the regular evaluation of some parameters, in particular: the serum concentrations of prostate-specific antigen (PSA), prostate volume and the hematocrit. The gynecomastia represent a potential condition associated with this treatment. The presence of obstructive sleep apnoea represents a contraindication for the treatment [Citation7].
Another important aspect is represented by patients with LOH and concomitant condition of secondary infertility (inability to induce a subsequent pregnancy) [Citation8]. It is estimated that in USA ∼11% of couples has this problem. LOH is one of the causes of secondary infertility, along with others such as varicocele, urogenital infection, metabolic diseases and use of drugs [Citation8].
Based on these premises, and in particular on the basis of recent data that suggest that the pathogenesis of LOH is mainly due to central mechanisms [Citation4], the present study evaluated the differences of a different pharmacological treatment of LOH, evaluating the results of a treatment with extractive human chorionic gonadotropin (HCG), with pharmacological action similar to luteinizing hormone (LH) [Citation9] compared with traditional therapy with T.
Materials and methods
Forty patients’ median age 50 years, interquartile range (IQR): 45–53, with diagnosis of LOH formulated in accordance with international guidelines were consecutively enrolled [Citation10]. At the enrolment, all the patients had serum concentrations of total testosterone (TT) <230 ng/dl [Citation10] and normal-low levels of gonadotropins. Patients were randomized into four treatment groups for a total duration of 6 months:
Group A: Extractive HCG (Gonasi HP® 2000 IU): one intramuscular injection twice a week;
Group B: T gel formulation (Tostrex® gel 60 mg): six transdermal applications per day;
Group C: T-undecanoate (Nebid® 1000 mg): one intramuscular injection every 12 weeks and
Group D: T-enanthate (Testoviron® 250 mg): one intramuscular injection every 28 days.
The following parameters were examined before and after 6 months of pharmacological treatment:
Hormonal parameters: serum TT, follicle-stimulating hormone (FSH), LH, 17β-estradiol (E2), prolactin and 25-hydroxy-vitamin D (met. chemiluminescence);
Anthropometric and metabolic parameters: weight, height, waist circumference body mass index, blood glucose and fasting insulin levels to calculate homeostasis model assessment (HOMA) index, percentage of lean (muscle) and fat mass (met. impedance), total and high-density lipoprotein (HDL) cholesterol, triglycerides;
Sperm parameters: density, normal forms, progressive motility, volume and leukocytes (met. WHO, 2010 V Edition) [Citation11];
Safety parameters: hematocrit, PSA and prostate volume (estimated by ultrasound examination) and
Questionnaires: IPSS (International Prostate Symptoms Score) [Citation12], IIEF-5 (International Index of Erectile Function) [Citation13] and AMS (Aging Male Symptom Score) [Citation14].
Hormonal measurements
Blood sampling was performed at 8.00 am, after at least 8 h of sleep. Determination of prolactin was repeated at a distance of 30 min. LH, FSH, E2, TT and prolactin evaluation was performed by electrochemiluminescence immunoassay (ECLIA) with Cobas equipment, while the quantitative determination of total 25-hydroxy-vitamin D was performed by competitive electrochemiluminescence protein binding assay with Cobas equipment. Normal values were LH = 1.7–8.6 mIU/ml, FSH = 1.5–12.4 mIU/ml, 17β-estradiol = 25.8–60.7 pg/ml, TT = 2.49–8.36 ng/ml, prolactin = 4.04–15.2 ng/ml, 25-hydroxy-vitamin D = 12.3–107 nmol/l and thyroid-stimulating hormone (TSH) = 0.3–4.5 mIU/ml.
Transrectal colour Doppler ultrasound
The prostate-vesicular region was evaluated at transrectal ultrasonography through transverse, longitudinal and oblique scans with patients placed in the left lateral decubitus. A transrectal biplanar probe (i.e. linear transducer 7.5 MHz; convex transducer 6.5 MHz) with an “end fire” transducer was used. Prostate volume was calculated through the following formula: width × height × length) × 0.523.
Exclusion criteria [Citation10]:
High levels of gonadotropins;
Prostate or breast cancer;
High risk of developing prostate cancer;
Significant erythrocytosis (hematocrit >52%);
Untreated obstructive sleep apnoea and
Untreated severe congestive heart failure.
The protocol was approved by the internal Institutional Review Board and an informed written consent was obtained from each patients.
Statistical analysis
All the statistical analyses were completed using SPSS v. 19 software (SPSS Inc, IBM Corp, Somers, NY). The continuous variables, presented as median, were tested by Mann–Whitney U-test. Changes from baseline to end of therapy were analyzed using ranked one-way analysis of variance (ANOVA) with a term for treatment group. Treatment group differences for end points were determined using post hoc analysis. Data were reported as median (IQR) and nominal p values were presented. A two-sided p value <0.05 was considered statistically significant in all the tests used.
Results
shows the baseline characteristics of the patients enrolled. Treatment groups were well balanced with regard to demographic and clinical characteristics at baseline. Of all the subjects, median age was 50 years (IQR: 45–53), median TT was 1.8 (1.62–2.0) and median of vitamin D was 12 (8.0–18.75). shows the median differences from baseline to final visit among groups with intergroup analysis when considering all the variables.
Table 1. Baseline characteristics of groups.
Table 2. Median differences from baseline to final visit among groups with intergroup analysis.
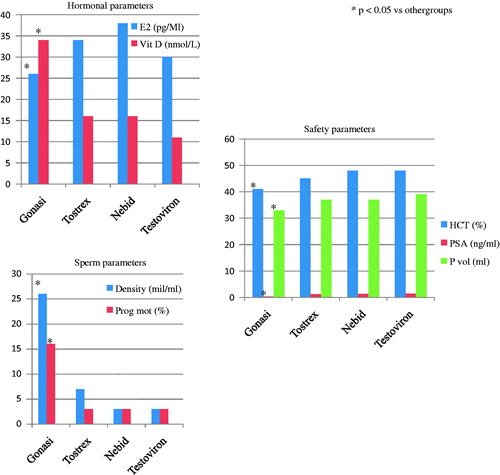
The increase of TT was significantly lower in Group 1 versus Group 3 (p < 0.05), and between Group 3 versus Group 2 (p < 0.05) and Group 4 (p < 0.05) while the increase of vitamin D was greater in Group 1 versus each other group (all p < 0.05) (). Serum concentrations of E2 after treatment in Group 1 were significantly lower (p < 0.05) compared with other groups ().
Serum concentrations of LH after treatment in Group 2 were significantly higher (p < 0.05) compared with other groups ().
As concerning the variation of hematocrit, the increase in Group 1 was significantly lower compared with other groups (all p < 0.05) and lower in Group 2 compared with Group 3 (p < 0.05) and Group 4 (p < 0.05). The increases of PSA and prostate volume were significantly lower in Group 1 when compared with other groups (all p < 0.05). No significant differences were observed in term of anthropometric, glucose and lipidic assessment changes ().
We observed significantly changes from baseline to final visit when considering IIEF-5 and AMS for all the groups without significant differences at intergroup analysis. No change was observed in terms of IPSS changes ().
The decreases of sperm density and progressive motility were significantly greater in Group 2, Group 3 and Group 4 when compared with Group 1 (all p < 0.05). No differences were observed in terms of normal forms and ejaculate volume.
shows the mean values of the main modified parameters in the different groups after the pharmacological treatment.
Discussion
Patients treated with HCG after 6 months of therapy had serum concentrations of 25-OH-vitamin D significantly higher and serum concentrations of E2 significantly lower compared with the three groups treated with different formulations of T. Moreover, after treatment, HCG group showed an increase of hematocrit, PSA and prostate volume significantly lower compared all the groups treated with T. Finally, all the groups treated with T showed a significant reduction of sperm concentration and of percentage of spermatozoa with progressive motility.
On the basis of these results, HCG treatment could be preferable for the hormonal aspects (correction of hypovitaminosis D and lower concentrations of E2 after treatment) and for the safety, in particular, about the main parameters that must be controlled during hormone replacement therapy: hematocrit for risk of coronary disease [Citation15], PSA and prostate volume for the risk of obstructive urinary disorders. Finally, the impact of HCG treatment on sperm parameters could be less severe, preserving fertility of these patients.
There are few studies in the literature on the treatment of LOH with HCG. We analyze the main differences between the present study and these evidences.
In 1991, Kaufman et al. [Citation16] have shown that the attenuated response of LH levels to antiopioids and antioestrogens, as well as, the reduced number of spontaneous high amplitude of LH pulses in elderly men may be the consequence of the release of a decreased mass of (LH releasing hormone) LHRH at each pulse, suggesting a possible altered central mechanism as cause of LOH.
In 1995, Turek et al. [Citation17] described the recovery of spermatogenesis and induction of pregnancy after 3 months of treatment with HCG in a patient with iatrogenic azoospermia.
In 2002, Liu et al. evaluated the efficacy and the safety of 3 months of treatment with recombinant hCG (r-hCG, Ovidrel) on muscle mass, strength, mobility and physical activity in ambulant, community-dwelling men >60 year old with partial androgen deficiency. There were no significant changes in PSA, and the IPSS score did not change. The authors concluded that 3 months of treatment with twice weekly r-hCG demonstrates sustained androgenic effects on hormones and muscle mass but has no effect on muscle strength or physical functioning [Citation18].
In 2002, Depenbusch et al. evaluated 13 patients with hypogonadotropic hypogonadism (a different clinical model than the present study) demonstrated that treatment with HCG alone is able to maintain spermatogenesis, after it was induced by a combined treatment with gonadotropin-releasing hormone (GnRH) or HCG/human menopausal gonadotropin (hCG/hMG). In this study, patients were treated with HCG for 24 months (longer duration of treatment than the present study). After 12 months, the sperm count was present but was reduced gradually. The serum concentrations of FSH and LH were suppressed [Citation19].
In 2005, Coviello et al. assessed the quantitative relationship between administered dose of HCG and concentrations of intratesticular testosterone (ITT). The study evaluated 25 patients treated with T enanthate 200 mg every week in combination with saline or increasing doses of HCG (125, 250 and 500 IU) every day in the following 3 weeks (different clinical model and different duration of treatment than the present study). ITT was suppressed by 94% in the T enanthate/placebo group. Post-treatment ITT was 25% lower compared with baseline in the 125-IU HCG group, 7% lower compared with baseline in the 250-IU HCG group and 26% greater compared with baseline in the 500-IU HCG group. These results demonstrate that low dose of HCG Maintains ITT within the normal range in healthy men with gonadotropin suppression [Citation20].
In 2013, Hsieh et al. assessed the quality of sperm parameters in patients treated with exogenous T and low daily doses of HCG (500 IU) (a different clinical model than the present study). A total of 26 patients with a mean age of 35.9 years were included in the study. About 19 patients were treated with intramuscular T and 7 patients with transdermal formulation of T gel. After 1 year of follow up, none of the sperm conventional parameters has been significantly modified. There have been no cases of azoospermia. There were no differences between groups. None of the patients were there during the follow-induced pregnancy [Citation21].
The significant increase in serum concentrations of 25-OH-vitamin D obtained after treatment with HCG could be explained by recent evidence regarding the ability of the testes, in particular, the Leydig cells to contribute to the 25-hydroxylation of vitamin D, therefore, the pharmacological stimulation with HCG stimulate this function [Citation22].
After the treatment, the serum concentrations of TT in HCG group was significant lower compared with patients treated with uncecanoate T. No difference about the serum concentrations of TT compared with patients treated with T gel and T enanthate. The increase of serum concentrations of E2 after the treatment was significantly lower in patients treated with HCG than the other groups treated with T.
The pharmacological correction of the hypovitaminosis D and the lower concentrations of E2 obtained after the treatment could further explain the maintenance of the sperm parameters in HCG treated patients that along with the best safety profile represent the more important result of this study.
The recent study of Hammoud et al. [Citation23] showed that high and low serum levels of vitamin D can be negatively associated with the quality of sperm parameters. In men, vitamin D is positively associated with androgen status [Citation24]. However, on this topic, there are conflicting data and evidence that low serum concentrations of vitamin D is not considered a risk factor for the alteration of sperm parameters [Citation25].
The increase of oestrogens in the experimental model represent an important mechanism for the idiopathic infertility, in fact, it is associated with the alteration of functional systems involved in the mechanism of apoptosis, causing an increased ratio of proapoptotic/antiapoptotic proteins and the augmented activity of caspase-3 [Citation26]. High levels of oestrogens are also associated with increased oxidative response in semen and sperm DNA fragmentation. In fact, in the clinical practice, antioestrogens are used as antioxidants [Citation27]. However, there are conflicting data regarding the adverse effects of oestrogens on sperm parameters. In fact, some evidence suggests an important role of oestrogens for the control of spermatogenesis as well as the damage associated with the use of aromatase inhibitors [Citation28].
The study has some important limitations and deserves further study. The number of examined patients is small, and it will be important to acquire the results of more large populations. Another important aspect is represented by the duration of the treatment, in order to verify if these results are confirmed even after a more prolonged therapy.
Moreover, LH levels significantly higher after treatment in patients treated with T gel will be re-evaluated after a longer duration of treatment. This aspect in fact is in contrast with the sperm quality of this group that was worse than patients treated with HCG. Finally, the methodological limitations of the determination of serum levels of oestrogen assessed by chemiluminescence represent another potential problem [Citation29].
Declaration of interest
The authors report no conflicts of interest.
References
- Lunenfeld B, Mskhalaya G, Zitzmann M, et al. Recommendations on the diagnosis, treatment and monitoring of hypogonadism in men. Aging Male 2015;18:5–15
- Tajar A, Huhtaniemi IT, O’Neill TW, et al. Characteristics of androgen deficiency in late-onset hypogonadism: results from the European Male Aging Study (EMAS). J Clin Endocrinol Metab 2012;97:1508–16
- Buvat J, Maggi M, Guay A, Torres LO. Testosterone deficiency in men: systematic review and standard operating procedures for diagnosis and treatment. J Sex Med 2013;10:245–84
- Huhtaniemi I. Late-onset hypogonadism: current concepts and controversies of pathogenesis, diagnosis and treatment. Asian J Androl 2014;16:192–202
- La Vignera S, Condorelli R, Vicari E, et al. Original immunophenotype of blood endothelial progenitor cells and microparticles in patients with isolated arterial erectile dysfunction and late onset hypogonadism: effects of androgen replacement therapy. Aging Male 2011;14:183–9
- Almehmadi Y1, Yassin DJ, Yassin AA. Erectile dysfunction is a prognostic indicator of comorbidities in men with late onset hypogonadism. Aging Male 2015;1:1–9. DOI:10.3109/13685538.2015.1046044
- Leung KM, Alrabeeah K, Carrier S. Update on testosterone replacement therapy in hypogonadal men. Curr Urol Rep 2015;16:523
- Katib AA, Al-Hawsawi K, Motair W, Bawa AM. Secondary infertility and the aging male, overview. Cent Eur J Urol 2014;67:184–8
- McCullough A. Alternatives to testosterone replacement: testosterone restoration. Asian J Androl 2015;17:201–5
- Wang C, Nieschlag E, Swerdloff R, et al. Investigation, treatment and monitoring of late-onset hypogonadism in males: ISA, ISSAM, EAU, EAA and ASA recommendations. Eur J Endocrinol 2008;159:507–14
- World Health Organization. WHO Laboratory Manual for the Examination and processing of human semen. 5th ed. Cambridge: Cambridge University Press; 2010
- Barry MJ, Fowler FJ Jr, O’Leary MP, et al. The American Urological Association symptom index for benign prostatic hyperplasia. The Measurement Committee of the American Urological Association. J Urol 1992;148:1549–57
- Rosen RC, Cappelleri JC, Smith MD, et al. Development and evaluation of an abridged, 5-item version of the International Index of Erectile Function (IIEF-5) as a diagnostic tool for erectile dysfunction. Int J Impot Res 1999;11:319–26
- Heinemann LA, Saad F, Zimmermann T, et al. The Aging Males’ Symptoms (AMS) scale: update and compilation of international versions. Health Qual Life Outcomes 2003;1:15
- Bilgi M, Güllü H, Kozanoğlu İ, et al. Evaluation of blood rheology in patients with coronary slow flow or non-obstructive coronary artery disease. Clin Hemorheol Microcirc 2013;53:317–26
- Kaufman JM, Giri M, Deslypere JM, et al. Influence of age on the responsiveness of the gonadotrophs to luteinizing hormone-releasing hormone in males. J Clin Endocrinol Metab 1991;72:1255–60
- Turek PJ, Williams RH, Gilbaugh JH III, Lipshultz LI. The reversibility of anabolic steroid-induced azoospermia. J Urol 1995;153:1628–30
- Liu PY, Wishart SM, Handelsman DJ. A double-blind, placebo-controlled, randomized clinical trial of recombinant human chorionic gonadotropin on muscle strength and physical function and activity in older men with partial age-related androgen deficiency. J Clin Endocrinol Metab 2002;87:3125–35
- Depenbusch M, von Eckardstein S, Simoni M, Nieschlag E. Maintenance of spermatogenesis in hypogonadotropic hypogonadal men with human chorionic gonadotropin alone. Eur J Endocrinol 2002;147:617–24
- Coviello AD1, Matsumoto AM, Bremner WJ, et al. Low-dose human chorionic gonadotropin maintains intratesticular testosterone in normal men with testosterone-induced gonadotropin suppression. J Clin Endocrinol Metab 2005;90:2595–602
- Hsieh TC, Pastuszak AW, Hwang K, Lipshultz LI. Concomitant intramuscular human chorionic gonadotropin preserves spermatogenesis in men undergoing testosterone replacement therapy. J Urol 2013;189:647–50
- Foresta C, Strapazzon G, De Toni L, et al. Bone mineral density and testicular failure: evidence for a role of vitamin D 25-hydroxylase in human testis. J Clin Endocrinol Metab 2011;96:E646–52
- Hammoud AO, Meikle AW, Peterson CM, et al. Association of 25-hydroxy-vitamin D levels with semen and hormonal parameters. Asian J Androl 2012;14:855–9
- Lerchbaum E, Obermayer-Pietsch B. Vitamin D and fertility: a systematic review. Eur J Endocrinol 2012;166:765–78
- Ramlau-Hansen CH, Moeller UK, Bonde JP, et al. Are serum levels of vitamin D associated with semen quality? Results from a cross-sectional study in young healthy men. Fertil Steril 2011;95:1000–4
- Correia S, Alves MR, Cavaco JE, et al. Estrogenic regulation of testicular expression of stem cell factor and c-kit: implications in germ cell survival and male fertility. Fertil Steril 2014;102:299–306
- Mancini A, Raimondo S, Persano M, et al. Estrogens as antioxidant modulators in human fertility. Int J Endocrinol 2013;2013:607939
- Finkelstein JS, Yu EW, Burnett-Bowie SA. Gonadal steroids and body composition, strength, and sexual function in men. N Engl J Med 2013;369:1011–22
- Bryant C, Moore J, Curry TE Jr. Determination of serum estradiol levels by radiometric and chemiluminescent techniques. Methods Mol Biol 2009;590:21–32