ABSTRACT
Background: Reproductive health and pregnancy outcomes may be improved if the reproductive risk assessment is moved from the antenatal to the preconception period. Primary care has been highlighted as an ideal setting to offer preconception assessment, yet the effectiveness in this setting is still unclear.
Objectives: To evaluate the effectiveness of preconception interventions on improving reproductive health and pregnancy outcomes in primary care.
Methods: MEDLINE, CINAHL, EMBASE and PsycINFO databases were searched from July 1999 to the end of July 2015. Only interventional studies with a comparator were included, analysed and appraised systematically, taking into consideration the similarities and differences of the participants, the nature of interventions and settings.
Results: Eight randomized controlled trials were eligible. Preconception interventions involved multifactorial or single reproductive health risk assessment, education and counselling and the intensity ranged from brief, involving a single session within a day to intensive, involving more than one session over several weeks. Five studies recruited women planning a pregnancy. Four studies involved multifactorial risks interventions; two were brief and the others were intensive. Four studies involved single risk intervention, addressing folate or alcohol. There was some evidence that both multifactorial and single risk interventions improved maternal knowledge; self-efficacy and health locus of control; and risk behaviour, irrespective of whether brief or intensive. There was no evidence to support reduced adverse pregnancy outcomes. One study reported no undue anxiety. The quality of the studies was moderate to poor.
Conclusion: The evidence from eligible studies is limited to inform future practice in primary care. Nevertheless, this review has highlighted that women who received preconception education and counselling were more likely to have improved knowledge, self-efficacy and health locus of control and risk behaviour. More studies are needed to evaluate the effects on adverse pregnancy outcomes.
Preconception care is recognized to improve reproductive and pregnancy outcomes.
There is limited evidence about the effectiveness of preconception care in primary care.
This review identified encouraging benefits of preconception education and counselling on maternal knowledge, self-efficacy and health locus of control, and risk behaviour. The effects on adverse pregnancy outcomes remain unclear.
KEY MESSAGE
Introduction
In the UK, since the inception of British general practice, antenatal care has been an integral aspect of the specialty in reproductive health. In recent years, general practitioners’ involvement in the care of pregnant women has diminished; it could disappear completely unless future role and responsibilities can be defined (Citation1). There is potential opportunity for primary care practitioners or general practitioners to provide care to women prior to pregnancy or preconception care. Effective preconception care interventions could be offered to improve the health of women, infants and families (Citation2–5). The potential role of primary care in delivering preconception care is recognized (Citation5–11). Primary care practitioners acknowledged that preconception care can improve reproductive health and pregnancy outcomes (Citation5,Citation6,Citation8,Citation9). Women have expressed interest in discussing preconception issues with primary care practitioners (Citation10,Citation12,Citation13). Despite this, preconception care has not become part of routine practice in the primary care settings (Citation14). Barriers to implementation include the dilemmas whether preconception interventions provided in primary care truly link with improved pregnancy outcomes as well as concerns over the cost–benefit of the interventions.
In Hungary, an observational study involving comprehensive preconception care, using trained primary care-based nurses over a 20-year period, appeared to have reduced rate of major congenital abnormalities and infectious diseases, and improved detection of genetic risk (Citation15). Public health campaigns recommending folate intake in women of childbearing age have demonstrated improved awareness and reduced risk of neural tube defects (Citation16,Citation17). A review by Korenbrot et al. between 1990 and 1999, found evidence supporting preconception interventions involving single reproductive health risk in reducing adverse outcomes; for example, the prevalence of congenital abnormalities reduced with folate supplementation, appropriate management of hyperglycaemia and phenylalanine dietary restriction (Citation18). These studies were mainly in the secondary care (Citation18). A Cochrane review found encouraging benefits of preconception health education. However, the review was limited to women with no pre-existing medical, obstetric or genetic risks (Citation19).
The aim of this systematic review is to examine the effectiveness of preconception intervention strategies in primary care on improving reproductive health and pregnancy outcomes. This review aims to include a wider spectrum of women of reproductive age; planned and unplanned pregnancies, and women with existing medical, obstetric or genetic risk.
Methods
Search strategy
Searches from MEDLINE, CINAHL, EMBASE and PsycINFO were undertaken from July 1999 to the end of July 2015. The start date was selected following the end of search of an earlier review by Korenbrot et al. (Citation18). The search strategy was created with the help of an expert in the library and information science. The main search terms were ‘preconception care,’ ‘preconception assessment,’ ‘preconception health,’ ‘pre-pregnancy care,’ ‘pre-pregnancy assessment,’ ‘pre-pregnancy health,’ ‘primary care,’ ‘primary healthcare,’ ‘family practice,’ ‘general practice,’ ‘community health services,’ community health centres,’ ‘community clinics,’ ‘outpatient clinics,’ ‘ambulatory care’ and ‘ambulatory care facility.’ The limits were applied to the year, English language and humans. Reference lists of eligible studies were screened for relevant records.
Study selection
The following criteria were applied for eligibility:
Studies clearly stated the interventions and had comparator arms described as ‘usual care’ or ‘alternative care’ or ‘not involving preconception care.’
Interventions encompassed both primary and secondary prevention before pregnancy; the former includes advice on nutrition, lifestyle, folate intake, smoking or alcohol; and the latter includes screening for genetic disorders or diabetes.
All women between 18 and 45 years old, including those with established medical, obstetric and genetic risk.
Studies conducted in the primary care settings described as family or general practices, community health centres or services, community or outpatient clinics and ambulatory care services.
Study designs employed were randomized controlled trials and quasi-randomized controlled trials, specifically controlled before and after intervention.
Study identification
Title and abstract of each study potentially meeting the inclusion criteria was screened by one author and checked independently by a second author. Full-text papers retrieved from potentially relevant studies were assessed independently by two authors for inclusion. Disagreement was resolved through discussion or by consulting a third author. One author manually searched the reference lists of included papers.
Data extraction and assessment of risk of bias
For study description, the characteristics for extraction was guided by previous reviews (Citation18,Citation19) which were: study design; study population; inclusion and exclusion criteria; details of interventions and comparator; duration of intervention and follow-up period (if applicable); settings; and outcomes. Studies were assessed for risk of bias as outlined in the Cochrane Handbook for Systematic Reviews of Intervention (Citation20). The criteria assessed were; sequence generation; allocation concealment; blinding of participants; study personnel and outcome assessors; incomplete outcome data; and selective reporting bias, and classified as ‘high,’ ‘low’ or ‘unclear’ risk of bias.
Data synthesis
Because of presumed clinical heterogeneity, no meta-analysis was carried out. Each study was presented separately (Citation20). Eligible studies were analysed and appraised systematically, taking into consideration the similarities and differences of the participants, the nature of interventions and settings. Effect sizes of each study are presented as reported; for dichotomous data, odds ratio with 95% confidence intervals; and continuous data was reported either as absolute difference (total or proportion of outcome in the intervention minus control group), mean difference (before and after in the intervention and control group), or change in total score; and the P value.
Results
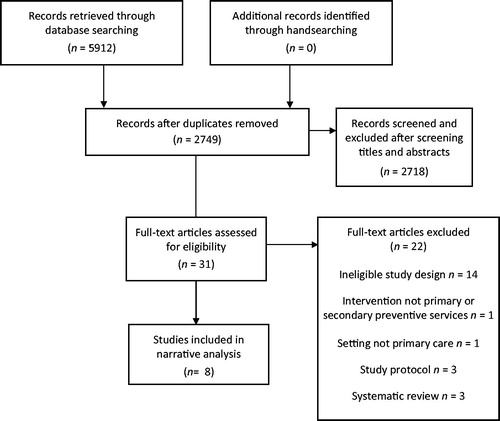
There were 5912 references retrieved from all databases, 3163 duplicates were removed. After screening titles and abstracts, only 31 full-text papers were assessed for eligibility. Only eight studies met the inclusion criteria (). Two of the included studies were from one larger study (Citation21,Citation22).
summarized the characteristics of eligible studies involving multifactorial and single health risk interventions. The summaries of results are presented in . All studies identified were randomized controlled trials. Both the nature of interventions and the outcome measures varied across the eight studies.
Table 1(a). Characteristics of included studies involving multifactorial reproductive health risks interventions.
Table 1(b). Characteristics of included studies involving single reproductive health risk interventions.
Table 2(a). Summary of results of included studies involving multifactorial reproductive health risks interventions on maternal knowledge; self-efficacy and health locus of control; risk behaviour change; adverse pregnancy outcomes and psychological stress.
Table 2(b). Summary of results of included studies involving single reproductive health risk interventions on maternal knowledge; self-efficacy and health locus of control; risk behaviour change; adverse pregnancy outcomes and psychological stress.
Intervention. The interventions are divided into two main groups: four studies involved multifactorial reproductive health risks, such as addressing nutrition, lifestyle, vaccination, infection prevention and genetic conditions (Citation22–25); and in the remaining four studies involved single reproductive health risk, whereby the interventions were specifically focussed on folate supplementation or alcohol consumption (Citation26–29). The interventions comprised of preconception health assessment, education and counselling; and delivered by various health professionals: general practitioners (Citation21,Citation22); gynaecologists (Citation29); midwives or nurses (Citation25,Citation28); nutrition educators (Citation27); and trained facilitators (Citation23,Citation24,Citation28) with computer-assisted counselling applied in one study (Citation26).
Intensity of intervention. Intensity of intervention is grouped into two categories: brief, involving a single session within a day; and intensive, which involved more than one session over several weeks. The interventions were brief in five studies: from 30–60 s (Citation29); 15 min (Citation26); to two hours of education and counselling over a single day (Citation23,Citation25,Citation27). In three studies interventions were intensive involving risk assessment followed by general practitioners’ consultation on risk factors for adverse pregnancy outcomes (Citation21,Citation22); two-hour group sessions on preconception health services, stress management, physical activity, smoking, gynaecological infection, nutrition, and healthy eating demonstration over a 12-week period (Citation24); and counselling sessions on alcohol moderation over a 14-week period (Citation28). In three studies, the interventions involved one to one counselling sessions (Citation21,Citation22,Citation26,Citation29) while the other studies involved groups (Citation23,Citation24,Citation27,Citation28).
Follow-up. The follow-up interval varied between two weeks and nine months (Citation23,Citation24,Citation26–29) or follow-up ended on the birth of subsequent pregnancy (Citation21,Citation22,Citation25).
Setting. The primary care settings were general practices (Citation21,Citation22,Citation28), urgent care clinics (Citation26), gynaecology outpatient clinics (Citation29), premarital counselling clinics (Citation23), community-based health centres, such as women-infant-children clinics (Citation24,Citation27) and home visits by midwives (Citation25). Participants recruited were limited to women planning pregnancy in five studies (Citation21–24,Citation26,Citation28).
Outcomes. Five broad categories of outcomes were identified after examining all the studies: improvement in maternal knowledge; self-efficacy and health locus of control; maternal risk behaviour; adverse pregnancy outcomes; and psychological stress due to receiving information.
Improvement in knowledge
Two studies reported an improvement in knowledge (Citation21,Citation26). The intervention used in the study by Schwarz et al. was brief and on a single health risk; a 15-min computerized-assisted counselling on preconception folate supplementation with a bottle of free folate tablets. This led to improved women’s knowledge that folate can prevent birth defects (OR: 4.19; 95% CI: 1.98–8.85) and folate is important in the first few weeks of pregnancy (OR: 2.7; 95% CI: 1.75–4.26) (Citation26). The study by Elsinga et al., was intensive involving preconception counselling on multifactorial health risks (Citation21). This study reported a significant difference in total knowledge score on pregnancy-related risks and prevention between the intervention and control group in both women who had never been pregnant (difference in score: 11.3; 95% CI: 4.6–18.0) and those who had been pregnant (difference in score 3.0; 95% CI: 1.2–4.84) (Citation21).
Improvement in self-efficacy and health locus of control
Two studies reported on self-efficacy and health locus of control (Citation23,Citation24). Health locus of control (HLOC) relates to the idea that women’s reinforcement towards preconception health is related to their behaviour, for example in physical activity (Citation23). Interventions in both studies involved preconception education addressing multifactorial reproductive health risks. The study by Hillemeier et al. reported a significant improvement on self-efficacy; eating healthier food (OR: 1.757, P = 0.008), physically active (OR: 2.185, P = 0.0001) and perceived higher preconception control of birth outcomes (OR: 1.916, P = 0.031) (Citation24). The study by Bastani et al. reported significant improvement in exercise self-efficacy (difference in score 15.4; 95% CI: 13.74 - 17.06) and internal health locus of control (HLOC) scores (difference in score 6.4; 95% CI: 5.41–7.39) (Citation23).
Improvement in maternal risk behaviour
Six studies reported improvement in maternal risk behaviour. Three were brief interventions (Citation26,Citation27,Citation29) and the remaining were intensive (Citation21,Citation24,Citation28). All brief interventions focussed on folate supplementation. In the intensive group, two studies involved multifactorial reproductive risks intervention (Citation21,Citation24) whilst another study involved only on alcohol intervention (Citation28).
There was a statistically significant improvement in self-reported folate intake before pregnancy in two studies involving multifactorial reproductive health risks intervention (Citation21,Citation24) and one study that adopted the single risk intervention (Citation27). The study by Robbins et al. reported a significant increase in weekly folate intake but not daily intake (Citation29). The study by Schwarz et al., involving computerized counselling on folate did not show significant increase in folate intake (OR: 1.55; 95% CI: 0.88–2.72) (Citation26). Maternal alcohol consumption appeared to improve significantly in one study following five counselling sessions over a 14-week period (Citation28). Reduced risky drinking was reported at three points: three months (OR: 1.79; 95% CI: 1.28–2.51); six months (OR: 1.64; 95% CI: 1.15–2.33); and nine months (OR: 1.54; 95% CI: 1.09–2.18). More women who received preconception counselling by general practitioners stopped smoking before pregnancy but this was not statistically significant (OR: 3.04; 95% CI: 0.95–9.69) (Citation21).
Improvement in adverse pregnancy outcomes
Reported adverse pregnancy outcomes include low birth weight, preterm delivery, congenital abnormalities and prenatal death. Two studies involving multifactorial reproductive health risks intervention reported adverse pregnancy outcomes (Citation21,Citation25). In the study by Elsinga et al., women were followed-up until two months after subsequent delivery, reported fewer total adverse pregnancy outcomes; 16.2% in the intervention group versus 20.2% in the control group, however, this was not statistically significant (OR: 0.77; 95% CI: 0.48–1.22) (Citation21). The study by Lumley and Donohue also did not produce statistically significant findings but reported more infants born in subsequent pregnancy in the intervention group had low birth weight (less than 2500 g: OR: 1.14; 95% CI: 0.55–2.38) and preterm (less than 37 weeks: OR: 1.44; 95% CI: 0.73–2.91) (Citation25).
Psychological stress due to receiving information
Only one study reported psychological outcome which was anxiety following preconception counselling by general practitioners (Citation22). Here, Spielberger State-Trait Anxiety Inventory (STAI) score was used. It is a four-point scale of six-item statement referring to calm, tense, upset, relaxed, content or worried. In this study, anxiety was assessed at baseline (STAI-1), immediately following intervention (STAI-2) and after delivery of subsequent pregnancy (STAI-3). The (STAI-3) scores were based on anxiety experienced during the first trimester of pregnancy. The STAI-2 scores appeared lower immediately after the intervention when compared to baseline (-3.6 points, 95% CI: 2.4–4.8). There was no significant difference in STAI-3 scores; there was only a small difference in mean between the intervention and the control group.
Risk of bias of included studies
The methodological quality of the studies was moderate to poor. The studies involving multifactorial reproductive health risks intervention had a higher risk of bias compared to single risk intervention. Allocation concealment was only described in two studies (Citation26,Citation28). Blinding of the participants and personnel who delivered the intervention was not possible as interventions involved education and counselling. Documentation of participants who were lost to or refused follow-up was incomplete and excluded from analyses (Citation24,Citation25). In one study, 47% of women from the intervention group and 50% from the control group did not attend the follow-up (Citation24). In another study, 43% of women from the intervention group who were lost to follow-up or did not become pregnant were excluded (Citation25). One study reported only between two and three per cent of the recruited women attended during the study period (Citation21,Citation22). Results of these studies with high levels of attrition should be interpreted with caution. Selective reporting was minimal in studies involving single reproductive health risk intervention; most data for pre-specified outcomes was reported (Citation26–29). In a study involving multifactorial reproductive risks intervention, results were mainly reported on the intervention group and insufficient data on the control group (Citation24). Reporting bias is possible in studies where outcome measures were self-reported such as anxiety score (Citation22) and studies assessing risk behaviour (Citation21,Citation24,Citation26,Citation28,Citation29). Assessment of risk of bias is summarized in .
Table 3. Assessment of risk of bias: Review authors’ judgements about each methodological quality item for each included studies of multifactorial and single reproductive health risk interventions.
Discussion
Main findings
This review identified eight randomized controlled trials. Two of the studies conducted in Netherlands were from one large study (Citation21,Citation22). The quality of the studies was moderate to poor. There was variation in the characteristics of interventions and results were heterogeneous. Four studies involving multifactorial reproductive health risk interventions assessing maternal knowledge; self-efficacy and health locus of control; risk behaviour; adverse pregnancy; and psychological outcomes (Citation21–25). Four studies involving single reproductive health risk intervention reported on maternal knowledge and risk behaviour (Citation26–29). Three interventions were intensive and five were brief (Citation21–29). Irrespective of the duration of intervention, the studies indicated that both multifactorial and single reproductive risk interventions could improve maternal knowledge (Citation21,Citation26), self-efficacy and health locus of control (Citation23,Citation24) and risk behaviour (Citation21,Citation23,Citation24,Citation26). The evidence for effects of multifactorial risk interventions on adverse pregnancy outcomes was insignificant (Citation21,Citation25). With regards to psychological impact, one study reported no untoward short-term anxiety but the evidence is less clear for a longer-term effect (Citation22). In five studies, the target participants were of women planning pregnancy (Citation21–24,Citation26,Citation28).
Comparison with existing literature
This review has included recent relevant trials and, therefore, has added value to the existing evidence. Following the systematic review published in 1999 by Korenbrot et al., eight more studies were identified (Citation18). Compared to the Cochrane review published in 2009, this review has identified four more studies (Citation19,Citation21–23,Citation26,Citation27). Consistent with earlier reviews, the evidence for adverse pregnancy outcomes was reported (Citation18). However, the evidence in this review was not statistically significant. In contrast, the intervention in this review reported on adverse pregnancy outcomes involved multifactorial reproductive health risks whereas Korenbrot mainly reported interventions involving a single reproductive health risk such as folate supplementation in reducing neural tube defects (Citation21,Citation30,Citation31) and preconception control of hyperglycaemia in women with pre-existing diabetes was associated with reduced risk of congenital abnormalities (Citation32). Furthermore, studies included in the review by Korenbrot et al. were mainly carried out in hospital settings (Citation18).
A recent Cochrane review did not demonstrate that routine preconception health promotion results in reduced adverse pregnancy outcomes (Citation19). Similar to this review, there was positive evidence on maternal risk behaviour involving alcohol and folate. However, Whitworth et al. excluded trials where interventions were aimed specifically at women with existing medical, obstetric or genetic risk. Unlike the previous reviews, our review includes interventions that involved all women of reproductive age irrespective of whether they have pre-existing health risks, and in primary care settings.
Strengths and limitations
To our knowledge, this is the first review to examine the effects of preconception intervention in women recruited from primary care settings. However, as the results of this review were heterogeneous, meta-analysis was not possible to conclude precise estimate of the effect of the interventions (Citation20). In two-thirds of the studies, participants were restricted to women planning pregnancy (Citation21–24,Citation26,Citation28); thus, the findings may not apply to all women including those with unplanned pregnancy. It is important to evaluate the benefits of the intervention to all women, as unplanned pregnancies contribute to at least half of all pregnancies in England and Scotland (Citation33,Citation34). The overall quality of the evidence was moderate to poor, particularly in studies involving multifactorial risks intervention requiring caution in interpreting their effectiveness. Studies reporting on adverse pregnancy outcomes were underpowered, leading to inconclusive results. Evidence from self-reported information on maternal risk behaviour may not be reliable; participants who attended preconception counselling may be motivated and thus contributed to positive outcomes.
Implications for future practice and research
National and international policy document such as the UK Human Genetics Commission; Centers for Disease Control and Prevention; and Health Council of Netherlands, have highlighted the benefits of preconception care and agreed on the need for multidisciplinary care including primary care (Citation35–37). However, there remains lack of quality of evidence to support providing preconception care in primary care. The studies were of various formats; this may raise potential barriers to developing future preconception interventions in existing health infrastructure. More studies should aim to evaluate on adverse pregnancy outcomes, and this should include not just examining new-born endpoints (low birth weight, preterm) but maternal health endpoints such as maternal mortality and morbidity. As most women in the review were those planning pregnancy, future studies should capture women with unplanned pregnancy. One possible suggestion is to examine intervention involving delivery of preconception care in family planning or pre-marital clinics. Future studies should also recruit other primary care providers such as nurses or midwives.
Conclusion
This review has demonstrated that women who received preconception education and counselling were more likely to improve their knowledge and positive health behaviour. The evidence for adverse pregnancy outcomes still needs to be addressed. Although there is relatively limited evidence, this should not preclude recommending preconception care as a component of improving reproductive health. Interventions involving multifactorial reproductive risks seem plausible strategy in primary care.
Acknowledgements
The authors thank Professor Dr Ng Chirk Jenn (University of Malaya, Malaysia) for his invaluable feedback on the content of this review. The authors also thank Wendy Stanton (Greenfield Medical Library Information Scientist, University of Nottingham) for her assistance in the search strategy and Manjo Doug (Research Fellow) for assisting in data extraction.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Smith A, Shakespeare J, Dixon A. The role of GPs in maternity care—what does the future hold? An inquiry into the quality of general practice in England. London: The King's Fund; 2010. Available at: http://www.kingsfund.org.uk/current_projects/gp_inquiry/dimensions_of_care/maternity_care_in.html (accessed 15 August 2013).
- Centers for Disease Control and Prevention. Surveillance of preconception health indicators among women delivering live-born infants—Oklahoma, 2000–2003. MMWR Morb Mortal Wkly Rep 2007;56:631–4.
- Cefalo RC, Bowes WA, Moos M. Preconception care: A means of prevention. Baillieres Clin Obstet Gynaecol 1995;9:403–16.
- Cikot R, Gaytant M, Steegers E, Braspenning J. Dutch GPs acknowledge the need for preconceptual health care. Br J Gen Pract 1999;49:314–5.
- Wallace M, Hurwitz B. Preconception care: Who needs it, who wants it, and how should it be provided? Br J Gen Pract 1998;48:963–6.
- Watson EK, Shickle D, Qureshi N, Emery J, Austoker J. The ‘new genetics’ and primary care: GPs' views on their role and their educational needs. Fam Pract 1999;16:420–5.
- Gaytant MA, Cikot RJ, Braspenning JC, Grol RP, Merkus JM, Steegers EA. Preconception counseling in family practice; a survey of 100 family physicians. Ned Tijdschr Geneeskd 1998;142:1206–10.
- March of Dimes Birth Defect Foundation. March of Dimes updates: Is early prenatal care too late? Contemp Ob/Gyn 2002;54–72.
- Heyes T, Long S, Mathers N. Preconception care: Practice and beliefs of primary care workers. Fam Pract 2004;21:22–7.
- Poppelaars FAM, Cornel MC, Ten Kate LP. Current practice and future interest of GPs and prospective parents in pre-conception care in The Netherlands. Fam Pract 2004;21:307–9.
- Morgan MA, Hawks D, Zinberg S, Schulkin J. What obstetrician-gynecologists think of preconception care. Matern Child Health J 2006;10:59–65.
- de Jong-Potjer LC, de Bock GH, Zaadstra BM, de Jong ORW, Verloove-Vanhorick SP, Springer MP. Women’s interest in GP-initiated pre-conception counselling in the Netherlands. Fam Pract 2003;20:142–6.
- Mazza D, Chapman A. Improving the uptake of preconception care and periconceptional folate supplementation: What do women think? BMC Public Health 2010;10:786.
- Dunlop AL, Jack B, Frey K. National recommendations for preconception care: The essential role of the family physician. J Am Board Fam Med 2007;20:81–4.
- Czeizel AE. Ten years of experience in periconceptional care. Eur J Obstet Gynecol Reprod Biol 1999;84:43–9.
- Berry RJ, Li Z, Erickson JD, Li S, Moore CA, Wang H, et al. Prevention of neural-tube defects with folic acid in China. China—U.S. collaborative project for neural tube defect prevention. N Engl J Med 1999;341:1485–90.
- Watson M, Watson L, Bell R, Halliday J. The increasing knowledge of the role of periconceptional folate in Victorian women of child-bearing age: Follow-up of a randomised community intervention trial. Aust NZ J Public Health 2001;25:389–95.
- Korenbrot CC, Steinberg A, Bender C, Newberry S. Preconception care: A systematic review. Matern Child Health J 2002;6:75–88.
- Whitworth M, Dowswell T. Routine pre-pregnancy health promotion for improving pregnancy outcomes. In: Cochrane Database of Systematic Reviews 2009. Available at: http://doi.10.1002/14651858.CD007536.pub2 (accessed 15 Aug 2012).
- Higgins J, Green S. Cochrane handbook for systematic reviews of interventions version 5.1.0 (updated March 2011). The Cochrane Collaboration. 2011. Available at: http://www.cochrane-handbook.org (accessed 15 Aug 2013).
- Elsinga J, de Jong-Potjer LC, van der Pal-de Bruin KM, le Cessie S, Assendelft WJJ, Buitendijk SE. The effect of preconception counselling on lifestyle and other behaviour before and during pregnancy. Womens Health Iss 2008;18:117–25.
- de Jong-Potjer LC, Elsinga J, le Cessie S, van der Pal-de Bruin KM, Neven AK, Buitendijk SE, et al. GP-initiated preconception counselling in a randomised controlled trial does not induce anxiety. BMC Fam Pract 2006;7:66.
- Bastani F, Hashemi S, Bastani N, Haghani H. Impact of preconception health education on health locus of control and self-efficacy in women. East Mediterr Health J 2010;16:396–401.
- Hillemeier MM, Downs DS, Feinberg ME, Weisman CS, Chuang CH, Parrott R, et al. Improving women’s preconceptional health: Findings from a randomized trial of the Strong Healthy Women intervention in the Central Pennsylvania women’s health study. Womens Health Iss 2008;18:87–96.
- Lumley J, Donohue L. Aiming to increase birth weight: A randomised trial of pre-pregnancy information, advice and counselling in inner-urban Melbourne. BMC Public Health 2006;6:299.
- Schwarz EB, Sobota M, Gonzales R, Gerbert B. Computerized counseling for folate knowledge and use: A randomized controlled trial. Am J Prev Med 2008;35:568–71.
- Cena ER, Joy AB, Heneman K, Epinosa-Hall G, Garcia L, Schneider C, et al. Learner-centred nutrition education improves folate intake and food-related behaviors in nonpregnant, low-income women of childbearing age. J Am Diet Assoc 2008;108:1627–35.
- Floyd RL, Sobell M, Velasquez MM, Ingersoll K, Nettleman M, Sobell L, et al. Preventing alcohol-exposed pregnancies: A randomized controlled trial. Am J Prev Med 2007;32:1–10.
- Robbins JM, Cleves MA, Collins HB, Andrews N, Smith LN, Hobbs CA. Randomized trial of a physician-based intervention to increase the use of folic acid supplements among women. Am J Obstet Gynecol 2005;192:1126–32.
- Medical Research Council Vitamin Study Research Group. Prevention of neural tube defects: Results of the Medical Research Council vitamin study—MRC Vitamin Study Research Group. Lancet 1991;338:131–7.
- Czeizel AE, Dudás I. Prevention of the first occurrence of neural-tube defects by periconceptional vitamin supplementation. N Engl J Med 1992;327:1832–5.
- Kitzmiller JL, Gavin LA, Gin GD, Jovanovicpeterson L, Main EK, Zigrang WD. Preconception care of diabetes—glycemic control prevents congenital anomalies. J Am Med Assoc 1991;265:731–6.
- Rowlands S. Contraception and abortion. J R Soc Med 2007;100:465–8.
- Schünmann C, Glasier A. Measuring pregnancy intention and its relationship with contraceptive use among women undergoing therapeutic abortion. Contraception 2006;73:520–4.
- Human Genetics Commission. Increasing options, informing choice: A report on preconception genetic testing and screening. London: Human Genetics Commission; 2011. Available at: http://www.hgc.gov.uk (accessed 3 February 2013).
- Johnson K, Posner SF, Biermann J, Cordero JF, Atrash HK, Parker CS, et al. Recommendations to improve preconception health and health care—United States. A report of the CDC/ATSDR preconception care work group and the select panel on preconception care. MMWR Recomm Rep 2006;55:1–23.
- Health Council of the Netherlands. Preconception care: A good beginning. The Hague: Health Council of the Netherlands. 2007. Available at: http://www.gr.nl (accessed 3 February 2013).