Abstract
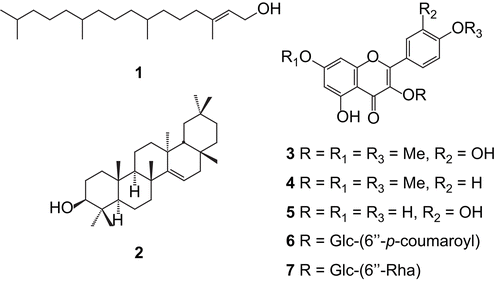
In this work, the total phenolic content and antioxidant activity of extracts and four flavonoids isolated from leaves of two Boraginaceae species (Cordia multispicata Cham. and Tournefortia bicolor Sw.) were evaluated using Folin-Ciocalteu reagent, DPPH free radical scavenging and inhibition of peroxidation of linoleic acid by FTC method. For comparison, ascorbic acid, α-tocopherol and BHT were used. In general, extracts from T. bicolor (68.8 ± 0.001 to > 1000 mg/g) showed higher phenolic content than C. multispicata (66.1 ± 0.009 to 231 ± 0.07 mg/g), and also scavenged radicals (IC50 12.8 ± 2.5 to 437 ± 3.5 mg/L) and inhibited lipid peroxide formation (IC50 51.2 ± 2.29 to 89 ± 0.59 mg/L). For these extracts a good correlation between the phenolic content and antioxidant activity was observed, suggesting that T. bicolor is richer in phenolic compounds and that it could serve as a new source of natural antioxidants or nutraceuticals with potential applications. Chromatographic procedures monitored by antioxidant assays afforded seven compounds, which were identified by spectral analyses (IR, MS and 1D and 2D NMR) and comparison with reported data as being trans-phytol (1), taraxerol (2), 3,7,4’-trimethoxyflavone (3), 5,3’-dihydroxy-3,7,4’-trimethoxyflavone (4), quercetin (5), tiliroside (6), and rutin (7). Compounds (4-7) were also evaluated and were effective as DPPH quenching (IC50 7.7 ± 3.6 to 79.3 ± 3.4 mg/L) and as inhibition of lipid peroxidation (IC50 80.1 ± 0.98 to 88.7 ± 3.62 mg/L). This is the first report on the total phenolic content, radical-scavenging and antioxidant activities of these species.
Introduction
In recent years there has been a global trend toward the use of natural substances present in vegetables and other edible plants as antioxidants. In plants extracts, this activity is often associated with the presence of polyphenol compounds, which have an important role in stabilizing lipid oxidation (CitationYen et al., 1993; CitationHuang et al., 2005). Phenolic natural substances such as flavonoids are of particular interest because of their antioxidant activity through scavenging oxygen radicals (CitationRice-Evans et al., 1995). In this regard, several in vitro methods (CitationPrior & Guohua, 1999) have been used to determining this activity in the various plant extracts and phenolic isolated compounds.
Boraginaceae constitute a large cosmopolitan family that comprises approximately 156 genera and 2500 species (CitationGe-Ling et al., 1995), of which only ten genera, including Cordia and Tournefortia, are native to Brazil (CitationScheel et al., 1996). The genus Cordia, consisting of about 325 species (CitationKuroyanagi et al., 2001), is probably the largest in the family and it is widely distributed in the warmer regions of the world, but is especially well represented in Central and South America (CitationNowicke & Ridgway, 1973). C. multispicata Cham., a Brazilian medicinal plant known as “carucaá”, is used as an expectorant and as a drug for contusion (CitationKuroyanagi et al., 2001). Previous phytochemical study from leaves of this species has yielded only ursane, oleanane and dammarane triterpenes containing anti-androgenic activity (CitationKuroyanagi et al., 2001, Citation2003). On the other hand, the genus Tournefortia possesses about 150 species of shrubs or wood vines found also in the warmer regions of the world, but centering in the neotropics (CitationNowicke & Skvarla, 1974). The fermented bark and stems of T. bicolor Sw. are sprayed in a home where someone is ill, to purify it against sickness and contagion; it also detoxifies the blood (CitationNowicke & Skvarla, 1974). No phytochemical or biological studies on this plant have previously been reported so far.
In this work, the total phenolic content by Folin-Ciocalteu phenol reagent, DPPH staining, DPPH free radical scavenging and total antioxidant activity by FTC method of extracts and some isolated compounds 1-7 () from leaves of C. multispicata and T. bicolor are described.
Materials and methods
General experimental procedures
Acetone, chloroform, ethanol, ethyl acetate, hexane, and methanol analytical grade were purchased from Quimex (F. Maia Indústria e Comércio, Mogi das Cruzes/SP, Brazil) or Vetec (Vetec Química Final, Rio de Janeiro, Brazil) while ascorbic acid, butylated hydroxytoluene (BHT), (+)-catechin, 1,1-diphenyl-2-picryl-hydrazyl (DPPH) radical, linoleic acid, polyoxyethylenesorbitan monolaurate (Tween 20), and α-tocopherol were purchased from Sigma (St. Louis, MO). All other chemicals used were of analytical grade supplied by Fluka, Merck or Acros Organics. Thin layer chromatographic plates (Merck, Darmstadt, Germany) Kieselgel 60 F254 were used for TLC assays. Silica gel (70-230 and 230-400 mesh, Merck) and Sephadex LH-20 (Pharmacia) were used for column chromatography. The NMR spectra were recorded either on a Bruker Avance DRX-500 or Mercury-Varian 200 operating for 1H at 500 and 200 MHz, respectively, and for 13C at 125 and 50.3 MHz, respectively, in CDCl3, CD3OD or mixture of both solutions with TMS as internal standard. IR spectra were obtained on an FT-IR 1750 Perkin-Elmer spectrometer. APCIMS spectrum was acquired in positive ion mode, using an LC/MS-Shimadzu 2010A instrument, and EIMS was measured at 70 eV on a GCMS Shimadzu, QP 5050A spectrometer, and UV analyses were recorded using a Shimadzu UV Mini-1240 and Biospectro spectrophotometer SP 220, using a polystyrene cuvette of 1 mL and 10 mm optical light path.
Plant material
Leaves of C. multispicata (Voucher MAC-14.284) and T. bicolor (Voucher MAC-14.904) were collected in November 2001, in the área de Proteção Ambiental de Murici, Marechal Deodoro, Alagoas, Brazil, and were identified by Rosangela P. Lyra Lemos of the Instituto do Meio Ambiente do Estado de Alagoas, where voucher specimens were deposited.
Extraction and isolation
The air-dried and powdered leaves from C. multispicata (1050 g) and T. bicolor (1700 g) were extracted at room temperature, during seven days, with acetone (C. multispicata and T. bicolor) 90% ethanol (EtOH) (T. bicolor). After removal of solvents under vacuum, the acetone (C. multispicata: 55.1 g and T. bicolor: 86.2 g) and EtOH (T. bicolor: 63.2 g) extracts were individually dissolved in MeOH-H2O (3:2) solution and extracted successively with hexane and CHCl3. After that, the MeOH solvent was removed from each hydro-alcoholic solution and the remaining aqueous solutions were extracted with EtOAc. The acetone and EtOH extracts, as well as the fractions proceeding from partition (C. multispicata (hexane, 20.9 g), (CHCl3, 24.2 g), (EtOAc, 0.7 g), and (MeOH-H2O, 1.0 g); T. bicolor acetone (hexane, 45.1 g), (CHCl3, 24.2 g), (EtOAc, 2.6 g), and (MeOH-H2O, 12.2 g); T. bicolor EtOH (hexane, 37.5 g), (CHCl3, 3.8 g), (EtOAc, 5.8 g), and (MeOH-H2O, 16.1 g)) were evaluated for their total phenolic content, DPPH free radical scavenging and total antioxidant activities.
Isolation of the constituents from leaves of C. multispicata
The hexane fraction (20.9 g), proceeding from partition of acetone extract, although found to be inactive as a radical scavenger as well as antioxidant (), was treated with NaOH 4%, and the neutral portion (13.2 g) was fractioned on a silica gel column (70-230 mesh) using hexane containing increasing amounts of EtOAc. Sub-fractions 35-45 (0.1 g) and 88-92 (0.2 g), after gel permeation (Sephadex LH-20 with MeOH) and successive recrystallizations from MeOH and acetone afforded 1 (72 mg) and 2 (20 mg), respectively. The CHCl3 fraction (24.2 g), proceeding from partition of acetone extract, possessing good antioxidant activity (50.5 ± 1.94 mg/L), was submitted to chromatography on silica gel column using hexane with increasing amounts of EtOAc. Sub-fractions 35-42 (0.5 g) after successive recrystallizations with MeOH afforded 3 (15 mg) and 4 (211 mg). The EtOAc fraction (0.7 g), proceeding from partitioning the acetone extract, also exhibited good antioxidant activity (51.6 ± 4.54 mg/L), was fractioned on silica gel column (70-230 mesh) using CHCl3 with increasing amounts of EtOAc. The sub-fractions 30-38 (0.2 g) after gel filtration (Sephadex LH-20 with MeOH) and recrystallization from acetone afforded 5 (10 mg).
Table 1. Total phenolic (TP) contents, DPPH free radical scavenging (FRS) and total antioxidant (TA) activities of extracts, isolated compounds and standards used.
Isolation of the constituents from leaves of T. bicolor
The EtOAc fraction (5.2 g), proceeding from EtOH extract, had a high phenolic content (691.8 ± 0.02 mg/g) and showed significant free radical scavenging (30.1 ± 3.2 mg/L) and antioxidant (78.6 ± 3.43 mg/L) activities. Thus, this fraction was fractionated on silica gel column (70-230 mesh) with CH2Cl2 (0.2 g), CH2Cl2-EtOAc 1:1 (1.8 g), EtOAc (1.2 g) and MeOH (1.7 g). These sub-fractions were also evaluated and the CH2Cl2-EtOAc 1:1 sub-fraction (1.8 g), containing the highest phenolic content, was the most active DPPH free radical scavenger (12.8 ± 2.5 mg/L), as well as antioxidant (81.8 ± 1.72 mg/L). This sub-fraction, after chromatographic fractionations (silica gel (230-400 mesh, CHCl3 with increasing amounts of MeOH) and Sephadex LH-20 (MeOH)) resulted in the isolation of 5 (15 mg) and 6 (25 mg). EtOAc sub-fraction (1.2 g), also active on the anti-radical assay (35.5 ± 2.3 mg/L) and as antioxidant (88.8 ± 0.41 mg/L), was also fractioned on silica gel (230-400 mesh) with hexane increasing amounts of EtOAc. The sub-fractions 70-88 (0.25 g) after recrystallization with acetone afforded an additional amount of 6 (25 mg).
The EtOAc fraction (2.0 g), proceeding from acetone extract by partitioning, contained a high phenolic content, also showed good DPPH free radical scavenging (101.0 ± 3.5 mg/L) and antioxidant activities (88.8 ± 3.2 mg/L), was chromatographed on silica gel column (230-400 mesh) using hexane with increasing amounts of EtOAc. The remaining sub-fractions after gel permeation (Sephadex LH-20 with MeOH) and recrystallizations from MeOH afforded 6 (30 mg) and 7 (30 mg).
Screening for free radical scavenging activity ( qualitative assay)
The qualitative assays of crude extracts, fractions and isolated compounds were carried out according to the procedure described by CitationSoler-Rivas et al. (2000). An aliquot of each sample (3 mg/mL) and standard ((+)-catechin, 1 mg/mL), dissolved in chloroform or methanol, were applied in duplicate on TLC plates and developed with appropriate solvent systems. After drying of the plates at room temperature for a few minutes, the plates were immersed upside down for 10 s in a 0.4 mM DPPH radical solution methanol. The negative control was pure solvents used for dissolving the extracts and isolated compounds. The stained silica layer gave a purple background with yellowish spots at the location of those compounds that showed free radical scavenger capacity. Samples that showed positive results in this assay were then evaluated for their total phenolic content.
Determination of total phenolic content by Folin-Ciocalteu’s phenol reagent
The total phenolic content of each extract that showed free radical scavenging activity in the qualitative assays was determined using Folin-Ciocalteu’s phenol reagent (CitationSoong & Barlow, 2004). To 2.76 mL of double distilled water, 0.035 mL of each sample and 0.175 mL Folin-Ciocalteu reagent was mixed. After 5 min at room temperature, 0.525 mL Na2CO3 (15%) was added and the solution was then centrifuged for 10 min at 10,000 rpm. After incubation for 1 h at 25°C, the absorbance was measured at 765 nm in triplicate and the phenolic content was calculated with a gallic acid standard and expressed as mg of gallic acid equivalent (GAE) per g of dry weight (dw). The values shown in the text and represent the average values of three measurements. Analysis of variance (ANOVA) was used to determine the standard deviation.
1,1-Diphenyl-2-picryl-hydrazyl (DPPH) free radical scavenging activity
All assays for radical scavenging ability were conducted in triplicate, according to the methods described by CitationSánchez-Moreno et al. (1998) and CitationBrand-Williams et al. (1995). The free radical scavenging activity of each extract, isolated compounds and standards (ascorbic acid (12.5 to 75 mg/L), α-tocopherol and BHT (50 to 200 mg/L) was measured at 515 nm, using at least five concentrations (12.5 to 100 mg/L), against a blank (a cuvette of 1 mL containing all of the reaction components, except the samples). In a cuvette, 0.1 mL of the solution of the samples was mixed with 0.9 mL DPPH• dissolved in methanol HPLC grade (40 mg/L) and the absorbance was monitored for 1 h (0, 15, 30, 45, and 60 min). Lower absorbance of the reaction mixture indicates higher free radical scavenging activity that was expressed as the percentage of DPPH by the equation:
IC50 values in the text and , determined in mg/L, mean the effective concentration at which the DPPH was scavenged by 50% and were obtained by a simple linear regression analysis established using Origin™ version 7.0 (Micronal, Northampton, MA). Data were analyzed by ANOVA followed by Tukey’s test (p < 0.05).
Total antioxidant activity by ferric thiocyanate (FTC) method
The antioxidant activity of extracts, isolated compounds and standards (ascorbic acid and α-tocopherol) was determined by a modified thiocyanate method described by CitationGülçin et al. (2006). A solution (2 mL) of each sample at five different concentrations (ranging from 12.5 to 100 mg/L) was mixed with 2 mL of 0.02 M linoleic acid emulsion at pH 7 and 4 mL of 0.2 M phosphate buffer (pH 7). The reaction mixture was incubated at 37°C for 6 days. To 0.1 mL of the reaction mixture at 24h intervals was added EtOH 75% (2.4 mL), ammonium thiocyanate 30% (0.1 mL), 0.02 M ferrous chloride in 3.5% HCl (0.1 mL). The degree of inhibition of linoleic acid peroxidation was determined by reading the absorbance at 500 nm and compared with those of standards. The control was subjected to the same procedures as the sample except that only the solvent was added. The IC50 values in the text and , determined in mg/L, mean the effective concentration at which the inhibition of linoleic acid peroxidation by each sample was 50% and was obtained by a simple linear regression analysis established using Origin™ version 7.0.
Statistical analysis
The results in the text and are the average of five concentrations for each sample and for each of three measurements were made. The data were analyzed by ANOVA followed by Tukey test and the difference was considered to be statistically significant when p <0.05.
Results and discussion
In order to determine the number of extracts with probable phenolic content and antioxidant activity, crude extracts and fractions from partition and chromatographic fractionation from leaves of both species, as well as some isolated compounds (4-7) (), were initially screened for their free radical scavenging capacity by DPPH staining of the silica plate (CitationSoler-Rivas et al., 2000). presents all extracts and fractions that showed yellowish spots at the location of the Retention Factors (Rfs) of compounds possessing probable radical scavenger capacity, when compared with standard used [(+)-catechin]. As described previously by CitationSánchez-Moreno et al. (1998), the more polar extracts (acetone and EtOH) and fractions (EtOAc and MeOH-H2O) gave a fast and strong positive reaction, while all non-polar fractions (hexane and CHCl3) were slow and weak. According to CitationSánchez-Moreno et al. (1998) and CitationChoi et al. (2002), this behavior is related to the nature and concentration of the antioxidant compounds present in the samples.
presents the results of total phenolic content, DPPH free radical scavenging and total antioxidant activity of extracts, fractions and isolated compounds from leaves of both species. In accordance to the preliminary DPPH assay, the higher phenolic contents were also observed with the more polar extracts (EtOH and acetone), fractions proceeding from partition (EtOAc and MeOH-H2O) and from fractionation on silica gel column (CH2Cl2-EtOAc 1:1, EtOAc and MeOH). In general, crude extracts from T. bicolor [EtOH (865 ± 0.032 mg/g dw) > acetone (367 ± 0.03 mg/g dw)] showed higher phenolic content than C. multispicata [acetone (231 ± 0.07 mg/g dw)]. According to these results it is possible to suggest that T. bicolor is richer in phenolic compounds than C. multispicata.
DPPH radical scavenging assay is one of the analyses used to prove the ability of the phenolic components present in the extracts of plants to act as donors of hydrogen atoms (CitationStoilova et al., 2007). IC50 values determined in this assay are considered to be a good measure of their antioxidant efficiency (CitationYen et al., 1993). According to the IC50 values displayed in , with exception of C. multispicata where all extracts tested were considered ineffective (IC50 > 225.0 ± 3.4 mg/L), the same crude extracts from T. bicolor that showed a high phenolic content also showed IC50 values best or comparable to standards used. Among them are the EtOH extract and two of its partition fractions (EtOAc, as well as three of its sub-fractions: CH2Cl2-EtOAc 1:1 (12.8 ± 2.5 mg/L) - from which compounds 5 and 6 were isolated, EtOAc (35.5 ± 2.3 mg/L) - of which compound 6 was also obtained, MeOH, and MeOH-H2O); acetone extract from T. bicolor and two of its partition fractions [EtOAc (101.0 ± 3.5 mg/L) - of which compounds 6 and 7 were isolated, and MeOH-H2O]. For all extracts the IC50 values were with statistical significance (p < 0.05) and showed a good correlation (R = -0.81) with the total phenolic contents. Moreover, they showed a kinetic behavior comparable to BHT (slow). These results suggest that the activity observed for T. bicolor might be in part attributed to the phenolic compounds present in the extracts.
In the FTC method, the peroxide reacts with ferrous chloride to form a reddish ferric chloride pigment, where the concentration of peroxide decreases as the antioxidant activity increases. In this assay, inhibition of the oxidation of linoleic acid by the samples was observed on day 5 of incubation at 37°C. shows the IC50 values obtained with different extracts from C. multispicata and T. bicolor and standards used. In general, when compared to standards (ascorbic acid and α-tocopherol), extracts from T. bicolor that showed a high phenolic content and a good free radical scavenging activity also showed significant antioxidant activity. Among them are mainly the acetone extract and two of its partition fractions [EtOAc (IC50 88.8 ± 3.20 mg/L) - of which compounds 6 and 7 were isolated, and MeOH-H2O], EtOH extract, two of its partition fractions (EtOAc and MeOH-H2O) and three of the EtOAc fractionating sub-fractions (CH2Cl2-EtOAc 1:1) (IC50 81.8 ± 1.72 mg/L - of which compounds 5 and 6 were isolated), EtOAc (IC50 88.8 ± 0.41 mg/L - of which compound 6 was also obtained) and MeOH. Although all extracts tested from C. multispicata have been considered ineffective for DPPH radical scavenging capacity (IC50 225 ± 3.4 to 437 ± 5.4 mg/L), they were quite significant for antioxidant activity (IC50 ranging from 33 ± 2.06 to 67.2 ± 1.23 mg/L). These results showed that compared to the less polar extracts the more polar exert greater antioxidant activity. This difference may be explained by the fact that many of the active compounds, probably flavonoids and other phenolic compounds containing hydroxyl groups are dissolved more easily in polar solvents. Moreover, considering that FTC method is used to determine at the initial stage of lipid peroxidation the amount of peroxide produced by the oxidation of linoleic acid; this may suggest that the amount of peroxide in this stage of lipid peroxidation is high.
In an effort to identify the compounds responsible for the anti-radical and antioxidant activities, the promising fractions (chloroform and ethyl acetate), proceeding from acetone and EtOH extracts of leaves of both species were separated by guided chromatographic fractionation into their main constituents (1-7) (). These compounds were identified on the basis of their spectral data (Infrared, Mass Spectrometry and Nuclear Magnetic Resonance, including Attached Proton Test, Distortioless Enhacement by Polarization Transfer, Correlated Spectroscopy, Heteronuclear Single Quantum Coherence, Heteronuclear Multiple Quantum Coherence, and Heteronuclear Multiple Bond Coherence experiments) and by comparison with reported data as trans-phytol (1) (CitationSims & Pettus, 1976), taraxerol (2) (CitationNobuko et al., 1987), 3’,5-dihydroxy-3,4’,7-trimethoxyflavone (3) (CitationAgrawal et al., 1989), 5-hydroxy-3,4’,7-trimethoxyflavone (4) (CitationPaula et al., 2006), quercetin (5) (CitationAgrawal et al., 1989), tiliroside (6) (CitationKaouadji, 1990), and rutin (7) (CitationKazuma et al., 2003). Some these compounds (4-7) were also evaluated for their DPPH free radical scavenging and antioxidant activities and compared with standards used (). The results showed that all evaluated compounds scavenged hydroxyl radicals. Among them, quercetin was the most effective (5, IC50 7.7 ± 3.6 mg/L), followed by rutin (7, IC50 21.8 ± 0.33 mg/L) and tiliroside (6, IC50 50.4 ± 4.2 mg/L), while compound 5-hydroxy-3,4’,7-trimethoxyflavone was the least effective (4, IC50 79.3 ± 0.34 mg/L). These results seem to suggest that radical-scavenging activity was influenced by the presence of hydroxyl groups on the carbon skeleton and by the substitution type at C-3. So, flavonoids containing hydroxyl groups at C-5 and C-7 and o-dihydroxyl system at C-3’ and C-4’, such as compounds 5-7, could be good radicals scavenger. From the other side, all isolated compounds effectively inhibited the formation of peroxide (ranging from 80.1 ± 0.98 to 88.7 ± 3.62 mg/L). Although compound 4 had showed lower effect on radical scavenging, it showed significant effect on lipid peroxidation (IC50 80.1 ± 0.98 mg/L). As the extracts of which were isolated, all the compounds evaluated showed a kinetic behavior comparable to BHT (slow).
Conclusions
In this study the results showed that crude extracts and fractions from T. bicolor were most effective as inhibitors of the formation of radicals and peroxide produced during linoleic acid peroxidation than C. multispicata, suggesting that T. bicolor could serve as a new source of natural antioxidants or nutraceuticals with potential applications to reducing the level of oxidative stress and to related health benefits. In general, for many of these extracts, a good correlation between the phenolic content and radical scavenging was observed. Considering that this activity in the plant extracts is often associated with the polyphenolic compounds, this activity might be related to the flavonoids isolated and other phenolic compounds present. In fact, four of the flavonoids isolated from promising extracts (5-hydroxy-3,7, 4’-trimethoxyflavone, quercetin, tiliroside, and rutin) showed activity better or comparable to the BHT, commercially available synthetic phenolic compound. According to these results, our study suggests that these compounds and other unknown but present in these extracts seem to be responsible for the antioxidant activity observed in some of the extracts.
Declaration of interest: The authors are grateful to CNPq, FAPEAL, MCT-IMSEAR and BNB-RENORBIO for financial support and research fellowships, to Vicente Carlos C. Oliveira (LTF, Universidade Federal da Paraíba), Dr Edilberto R. Silveira and Daniel E. A. Uchoa (CENAUREMN, Universidade Federal do Ceará), and Dr Jorge M. David (Instituto de Química, Universidade Federal da Bahia) for the NMR and APCIMS spectra. The authors alone are responsible for the content and writing of the paper.
References
- Agrawal PK, Thakur RS, Bansal MC (1989): Flavonoids, in: Agrawal PK, ed., Studies in Organic Chemistry: Carbon-13 NMR of Flavonoids. New York, Elsevier, pp. 95–182.
- Brand-Williams W, Cuvelier ME, Berset C (1995): Use of a radical method to evaluate antioxidant activity. LWT-Food Sci Technol 28: 25–30.
- Choi CW, Kim SC, Hwang SS, Choi BK, Ahn HJ, Lee MY, Park SH, Kim SK (2002): Antioxidant activity and free radical scavenging capacity between Korean medicinal plants and flavonoids by assay-guided comparison. Plant Sci 163: 1161–1168.
- Ge-Ling Z, Riedel H, Kamelim R (1995): Boraginaceae. Flora of China 16: 397–427.
- Gülçin I, Mshvildadze V, Gepdiremen A, Elias R (2006): Screening of antiradical and antioxidant activity of monodesmosides and crude extracts from Leontice smirnowii tuber. Phytomedicine 13: 343–351.
- Huang D-J, Chen H-J, Lin C-D, Lin Y-H (2005): Antioxidant and antiproliferative activities of water spinach (Ipomoea aquatica Forsk) constituents. Bot Bull Acad Sin 46: 99–106.
- Kaouadji M (1990): Acylated and nonacylated kaempferol monoglycosides from Platanus acerifolia buds. Phytochemistry 29: 2295–2297.
- Kazuma K, Noda N, Suzuki M (2003): Malonylated flavonol glycosides from the petals of Clitoria ternatea. Phytochemistry 62: 229–237.
- Kuroyanagi M, Seki T, Hayashi T, Nagashima Y, Kawahara N, Sekita S, Satake M (2001): Anti-androgenic triterpenoids from the Brazilian medicinal plants Cordia multispicata. Chem Pharm Bull 49: 954–957.
- Kuroyanagi M, Kawahara N, Sekita S, Satake M, Hayashi T, Takase Y, Masuda K (2003): Dammarane-type triterpenes from the Brazilian medicinal plant Cordia multispicata. J Nat Prod 66: 1307–1312.
- Nobuko S, Yaguchi Y, Inoue T (1987): Triterpenoids from Myrica rubra. Phytochemistry 26: 217–219.
- Nowicke JW, Skvarla JJ (1974): A palynological investigation of the genus Tournefortia (Boraginaceae). Amer J Bot 61: 1021–1036.
- Nowicke JW, Ridgway JE (1973): Pollen studies in the genus Cordia (Boraginaceae). Amer J Bot 60: 584–591.
- Paula NF, Cruz MP, Barbosa LCA (2006): Constituintes químicos de Bombacopsis glabra (Bombacaceae). Quím Nova 29: 213–215.
- Prior RL, Guohua C (1999): In vivo total antioxidant capacity: Comparison of different analytical methods. Free Rad Biol Med 27: 1173–1181.
- Rice-Evans CA, Muller NJ, Bolwell PG, Bramley PM, Pridham JB (1995): The relative antioxidant activities of plant-derived polyphenolic flavonoids. Free Rad Res 22: 375–383.
- Sánchez-Moreno C, Larrauri JA, Saura-Calixto FA (1998): A procedure to measure the antiradical efficiency of polyphenols. J Sci Food Agric 76: 270–276.
- Scheel R, Ybert J-P, Barth OM (1996): Pollen morphology of the Boraginaceae from Santa Catarina State (southern Brazil), with comments on the taxonomy of the family. Grana 35: 138–153.
- Sims JJ, Pettus JA Jr (1976): Isolation of free cis and trans-phytol from the red alga Gracilaria andersoniana. Phytochemistry 15: 1076–1077.
- Soler-Rivas C, Espín JC, Wichers HJ (2000): An easy and fast test to compare total free radical scavenger capacity of foodstuffs. Phytochemical Anal 11: 1–9.
- Soong YY, Barlow PJ (2004): Antioxidant activity and phenolic content of selected fruit seeds. Food Chem 88: 411–417.
- Stoilova I, Krastanov A, Stoyanova A, Denev P, Gargova S (2007): Antioxidant activity of a ginger extract (Zingiber officinale). Food Chem 102: 764–770.
- Yen GC, Duh PD, Tsai CI (1993): Relationship between antioxidant activity and maturity of peanut hulls. J Agric Food Chem 41: 67–70.